Abstract
The responses of inhibitory neurons/synapses to motoneuron injury in the cranial nervous system remain to be elucidated. In this study, we analyzed GABAA receptor (GABAAR) and GABAergic neurons at the protein level in the transected rat facial nucleus. Immunoblotting revealed that the GABAARα1 protein levels in the axotomized facial nucleus decreased significantly 5–14 days post-insult, and these levels remained low for 5 weeks. Immunohistochemical analysis indicated that the GABAARα1-expressing cells were motoneurons. We next examined the specific components of GABAergic neurons, including glutamate decarboxylase (GAD), vesicular GABA transporter (VGAT) and GABA transporter-1 (GAT-1). Immunoblotting indicated that the protein levels of GAD, VGAT and GAT-1 decreased transiently in the transected facial nucleus from 5 to 14 days post-insult, but returned to the control levels at 5 weeks post-insult. Although GABAARα1 protein levels in the transected nucleus did not return to their control levels for 5 weeks post-insult, the administration of glial cell line—derived neurotrophic factor at the cut site significantly ameliorated the reductions. Through these findings, we verified that the injured facial motoneurons suppressed the levels of GABAARα1 protein over the 5 weeks post-insult, presumably due to the deprivation of neurotrophic factor. On the other hand, the levels of the GAD, VGAT and GAT-1 proteins in GABAergic neurons were transiently reduced in the axotomized facial nucleus at 5–14 days post-insult, but recovered at 4–5 weeks post-insult.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Facial motoneurons are cranial neurons that serve to contract the facial expression muscles [1]. The axotomy of the facial nerve has been used to study the process of neuronal cell death/survival, regeneration, and glial reactions as an experimental model [2, 3]. Thus far, several groups have analyzed what happens in axotomized rat facial motoneurons, and their findings demonstrated that some functional molecules are downregulated [4,5,6] while others are upregulated [7,8,9] in injured facial motoneurons. We have also reported that the levels of choline acetyltransferase (ChAT) and vesicular acetylcholine transporter (VAchT) in motoneurons were significantly reduced at 3–14 days post-insult, but were restored at 3–5 weeks post-insult [10]. These studies thus provided new and notable information in terms of the injury/regeneration of motoneurons, but at the same time they raised a question about the response of inhibitory neurons that function coordinately with the excitatory neurons to control motoneurons [11, 12].
Gamma-aminobutyric acidergic (GABAergic) neurons are inhibitory neurons that have been found to produce GABA by a specific enzyme, glutamate decarboxylase (GAD) [13], and to pack it into the vesicles by means of a vesicular GABA transporter (VGAT) [14, 15]. When an electric signal arrives at an axon terminal in a GABAergic neuron, the vesicles containing GABA are fused to the pre-synaptic membrane and GABA is released into the synaptic cleft. Some of the released GABA binds to a specific receptor on the plasma membrane of motoneurons [16], leading to hyperpolarization, which blocks the formation of action potential [17, 18]. The other, extra GABA is taken back up by GABA transporter-1 (GAT-1) on the plasma membrane. There are two classes of GABA receptors (GABAR). GABAAR are ligand-gated ion channels (ionotropic receptors) and GABABR are metabotropic receptors (G protein-coupled receptors) [19, 20].
In fact, the inhibitory GABAergic system has been recognized in the facial motonucleus. Vassias et al. [21] reported that the mRNA levels of GABAAR subunits (α1, β2, and γ2) and the immunoreactivity of antibodies against α1/γ2 subunits were downregulated in axotomized facial motoneurons. They also clarified that the mRNA levels of GABABR subunits (B1B and B2) and the immunoreactivity of an antibody against the B2 subunit were decreased in injured motoneurons [21]. However, it is still uncertain how GABA synthesis/package/reuptake systems are influenced in the injury model.
In this study, we analyzed the functional constituents of the GABAergic system at the protein level, but not the mRNA level in the injured rat facial nucleus. Notably, we found that the levels of the GABAARα1 and GAD/VGAT/GAT-1 proteins showed different patterns of change over the 5 weeks post-insult.
Materials and Methods
Reagents and Antibodies
Glial cell line—derived neurotrophic factor (GDNF) from rats (G1401), and Dulbecco’s phosphate-buffered saline (PBS) (D8537) were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Cresyl violet (22963-1A) for Nissl staining was obtained from Kanto Chemical (Tokyo, Japan).
Anti-GABAA receptor α1 (GABAARα1) antibody (AB5592), anti-vesicular GABA transporter (VGAT) (AB5062P, AB5855), anti-GABA transporter-1 (GAT-1) antibody (AB1570W) and anti-m2 muscarinic acetylcholine receptor (m2MAchR) antibody (AB5166) were purchased from Millipore (Temecula, CA). Anti-GABAARα1 (sc-7348) antibody for immunohistochemistry, anti-glutamate decarboxylase65/67 (GAD65/67) antibody (sc-365180) and anti-actin antibody (sc-1615) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody against NMDA receptor 3B subunit (NR3B) (ab35677) was supplied by Abcam (Cambridge, UK). Anti-human GDNF antibody (G8035) that can detect rat GDNF was purchased from Sigma-Aldrich Japan (Tokyo, Japan).
Horseradish peroxidase (HRP)-conjugated anti-mouse IgG (sc-2055), HRP-conjugated anti-rabbit IgG (sc-2374) and HRP-conjugated anti-goat IgG (sc-2020) were purchased from Santa Cruz Biotechnology. Alexa Fluor 488-conjugated anti-goat IgG (A11055), Alexa Fluor 488-conjugated anti-mouse IgG (A21042), Alexa Fluor 488-conjugated anti-rabbit IgG (A11008), Alexa Fluor 568-conjugated anti-rabbit IgG (A11036) and Alexa Fluor 568-conjugated anti-mouse IgG (A11004) were obtained from Invitrogen (Carlsbad, CA, USA).
Animals and Operation
Wistar rats (8 week-old, female and male) were purchased from Clea Japan (Tokyo), and the progeny were obtained by home breeding. Male littermates were kept on a 12-h daylight cycle with food and water, and at 8 weeks of age they were subjected to the operation. In some cases, 8- and 13-week-old male rats were used without any treatments. In total, we used 186 male rats in this study. The number of animals used in each experiment is stated in the figure legends.
Animal experiments were carried out in accordance with the guidelines laid down by the NIH regarding the care and use of animals, and were approved by the ethics committee of Soka University (approval code: 17002). We also made an effort to minimize the number of animals used in this study.
Right facial nerves of adult rats were transected at the stylomastoid foramen under diethylether anesthesia, as described previously [10, 22]. Left facial nerves were left without any treatment. The rats were reared for 1, 3, 5, 7 or 14 days, or for 3, 4 or 5 weeks, and decapitated under anesthesia. The whole brains were removed, frozen on dry ice and stored at − 80 °C until the facial nuclei were cut out.
To investigate the effect of GDNF on the levels of GABAARα1 in the axotomized facial nucleus, both right and left facial nerves were cut at the stylomastoid foramen under diethylether anesthesia. At the right cut site, a piece of Gelfoam (2 mm3; Pfizer, Berlin, Germany) soaked in 15 µl of 20 ng/µL GDNF/PBS was placed, and at the left cut site a piece of Gelfoam soaked in 15 µL PBS (as vehicle) was placed as a control. After 5 days post-insult, the whole brains were removed, frozen on dry ice and stored at − 80 °C.
Immunoblotting
The contralateral and ipsilateral facial nuclei were carefully cut from the frozen brainstem. The cut facial nuclei were solubilized with nonreducing sample buffer [62.5 mM Tris–HCl (pH 6.8), 2% sodium dodecyl sulfate and 5% glycerol] and centrifuged at 100,000×g for 30 min. The supernatant of each facial nucleus was recovered as tissue extract. The amounts of protein in the tissue extract were determined by the method of Lowry et al. [23]. The resultant tissue extract was prepared to contain 10% 2-mercaptoethanol, then subjected to immunoblotting for GABAARα1 (1:1000), actin (1:2000), GAD65/67 (1:3000), VGAT (1:1000), GAT-1 (1:1000) and GDNF (1:2000). The staining methods were described previously [10].
Histochemistry and Immunohistochemistry
The brainstem was cut into 20-µm-thick sections with a cryostat (Leica CM1510; Leica Biosystems, Nussloch, Germany) at the level of the facial nuclei, and the sections were frozen at − 80 °C until staining. Experiments were usually performed using three sections per brainstem. The number of sections used in the experiments is stated in each figure legend.
For Nissl staining, the brainstem sections were dehydrated and rehydrated, then stained with 0.5% cresyl violet/1M acetate buffer (pH 3.9) using Nissl staining methods [24].
For immunohistochemistry, the cryosections were fixed in 3.7% paraformaldehyde/PBS and treated sequentially with 50, 100 and 50% acetone for 2, 3 and 2 min, respectively. The sections were then blocked with blocking solution containing 2% skim milk/PBS.
For dual staining with fluorescence, the cryosections were first incubated with anti-GABAARα1 antibody (1:100) for 16 h and then with anti-NR3B antibody (1:100) for 16 h at 4 °C. Subsequently, these sections were incubated with Alexa Fluor 488-conjugated anti-goat IgG (1:200) and Alexa Fluor 568-conjugated anti-rabbit IgG (1:200) for 3 h at room temperature. For single staining, the cryosections were first incubated with antibodies against GABAARα1 (1:100), GAD65/67 (1:200), m2MAchR (1:500), VGAT (1:200) and GAT-1 (1:200) for 16 h. They were then rinsed and incubated with Alexa Fluor 488-conjugated anti-mouse IgG (1:200) or Alexa Fluor 568-conjugated anti-rabbit IgG (1:200) for 3 h at room temperature.
The sections stained by the fluorescence method were dehydrated, mounted and observed by a fluorescent microscope (Eclipse TS100; Nikon, Tokyo) with a stand-alone microscope camera controller (Digital Sight DS-L3; Nikon).
Statistical Analysis
The densities of protein bands (GABAARα1, actin, GAD, VGAT, GAT-1 and GDNF) in immunoblotting were measured by densitometry using ImageJ software (NIH, Bethesda, MD). The Nissl-stained cells in the facial nucleus were counted. These densities and cell numbers were expressed as the means ± SDs of three separate experiments. Differences between the contralateral and ipsilateral nuclei were assessed via Student’s t-test. In all cases, P values less than 0.05 were considered significant (*P < 0.05, **P < 0.01).
Results
Response of GABAAR to Motoneuron Injury
We first investigated the response of an ion channel-type GABA receptor, GABAAR, to axotomy of facial motoneurons. Immunoblotting indicated that GABAARα1 protein levels in the injured nucleus decreased at 5–14 days post-insult (Fig. 1a). On the basis of the quantified results, we estimated that the levels of GABAARα1 protein in the axotomized facial nucleus decreased to 104.4 ± 3.5%, 71.6 ± 12.4%, 24.5 ± 8.2%, 19.8 ± 4.5% and 11.8 ± 3.1% at 1, 3, 5, 7 and 14 days post-insult, respectively (Fig. 1b). In the 14-day period after transection, levels of GABAARα1 protein in the ipsilateral nucleus did not recover.
Response of GABAAR to motoneuron insult. a Changes in GABAARα1 levels over the 1–14 days after insult. Sets of control (left side: L) and injured (right side: R) facial nuclei recovered at 1, 3, 5, 7 and 14 days after transection were immunoblotted for GABAARα1 and actin (Actin). The result shown is representative of experiments performed in triplicate using five rats. b Quantification of GABAARα1 levels. The intensities of the GABAARα1 bands in panel (a) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant, **P < 0.01). c Changes in GABAARα1 levels over the 2–5 weeks after insult. Sets of contralateral (left side: L) and ipsilateral (right side: R) facial nuclei recovered at 2, 3, 4 and 5 weeks after axotomy were immunoblotted for GABAARα1 and actin (Actin). The result shown is representative of experiments performed in triplicate using four rats. d Quantification of GABAARα1 levels. The intensities of the bands in panel (c) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). The data shown are means ± SDs from three independent experiments (**P < 0.01). e Quantification of actin levels 1–14 days after insult. The intensities of the actin bands in panel (a) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant). f Quantification of actin levels 2–5 weeks after insult. The intensities of the actin bands in panel (c) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant). g GABAARα1 levels in the uninjured nucleus and control nucleus. Sets of left (L) and right (R) facial nuclei of 8 week-old rats (8w-0d), and sets of control (L) and injured (R) facial nuclei of 8 week-old rats recovered at 5 days post-insult (8w-5d) were immunoblotted for GABAARα1 and actin (Actin). The result shown is representative of experiments performed in triplicate using two rats. h Quantification of GABAARα1 levels in panel (g). The intensities of the GABAARα1 bands in (g) were determined by a densitometer, and the values for R of 8w-0d and L of 8w-5d were expressed relative to that for L of 8w-0d (defined as 100%). The value for R of 8w-5d was expressed relative to that for L of 8w-5d (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant; **P < 0.01). i GABAARα1 levels in the uninjured nuclei of 8 and 13 week-old rats. Sets of left (L) and right (R) facial nuclei of 8 week-old (8w-0d) and 13 week-old (13w-0d) rats were immunoblotted for GABAARα1 and actin (Actin). The result shown is representative of experiments performed in triplicate using two rats. j Quantification of GABAARα1 levels in panel (i). The intensities of the GABAARα1 bands in (i) were determined by a densitometer, and the values for R of 8w-0d, and L and R of 13w-0d were expressed relative to that for L of 8w-0d (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant)
We further examined the levels of GABAARα1 protein from 2 to 5 weeks post-insult. As shown Fig. 1c, immunoblotting indicated that the GABAARα1 protein levels in the injured nucleus remained low during this time. The quantification showed that the levels of GABAARα1 protein in the injured nucleus were 16.5 ± 3.4%, 19.0 ± 7.0%, 12.2 ± 5.1% and 13.5 ± 2.2% at 2, 3, 4 and 5 weeks post-insult, respectively (Fig. 1d). We thus found that the levels of GABAARα1 protein in the axotomized facial nucleus were decreased at 5 days post-insult and remained low for 5 weeks post-insult.
The levels of actin protein were examined during 1–14 days post-insult (Fig. 1a). The quantification indicated that actin levels on the transected side were 104.3 ± 4.2%, 98.3 ± 3.6%, 102.3 ± 2.1%, 96.7 ± 4.3% and 98.1 ± 5.4% at 1, 3, 5, 7 and 14 days post-insult, respectively, compared to those on the control side (defined as 100%) (Fig. 1e). At 2, 3, 4 and 5 weeks post-insult, the levels of actin protein on the transected side relative to the control side (defined as 100%) were 99.7 ± 2.0%, 99.2 ± 6.9%, 99.4 ± 3.7% and 101.3 ± 2.7%, respectively (Fig. 1f). These results suggested that there were no significant difference in the levels of actin protein between the injured and control nuclei over the 5 weeks post-injury.
To investigate the basal levels of GABAARα1 protein in the uninjured facial nucleus, we compared the levels between the uninjured facial nucleus (L and R) of 8 week-old rats (8w-0d) and left control facial nucleus (L) of 8 week-old rats whose right facial nerve was transected 5 days previously (8w-5d) (Fig. 1g). When the level of GABAARα1 protein in L of 8w-0d was defined as 100%, the levels in R of 8w-0d and L of 8w-5d were 97.9 ± 6.5% and 106.3 ± 6.5%, respectively (Fig. 1h). The level in R of 8w-5d was significantly lower (39.1 ± 5.3%) compared to that in L of 8w-5d (Fig. 1h). These quantified results suggested that the levels of GABAARα1 protein in the control nucleus were not essentially modified by motoneuronal injury on the opposite side.
We further compared the levels of GABAARα1 protein between the uninjured nuclei of 8w-0d and 13w-0d rats (Fig. 1i). When the level in L of 8w-0d was defined as 100%, the levels in R of 8w-0d, and L and R of 13w-0d were 100.0 ± 6.9%, 101.3 ± 7.0% and 102.0 ± 6.1%, respectively (Fig. 1j), indicating that there was no significant difference in GABAARα1 protein levels between the nuclei in 8week-old rats and 13 week-old rats. These results suggest that the levels of GABAARα1 protein in the facial nucleus are not essentially changed over 8–13 weeks of age.
Immunohistochemical Study of GABAAR in the Axotomized Facial Nucleus
To confirm the phenomenon of decreased GABAARα1 protein levels in the axotomized facial nucleus, we performed immunohistochemistry for GABAARα1 protein using cryosections of facial nuclei taken at 5 days post-insult.
Visualization by the fluorescent method indicated that many cells were strongly stained by anti-GABAARα1 antibody in the contralateral nucleus, while in the ipsilateral nucleus weakly stained cells were observed (Fig. 2a). As supported by the results of immunoblotting (Fig. 1a, b), these results indicated that the level of the GABAARα1 protein was significantly downregulated in the injured nucleus.
Immunohistochemical analysis of GABAAR in the facial nucleus. a Comparison of GABAARα1 levels in both facial nuclei. Brainstem sections obtained at 5 days after transection were immunohistochemically stained with anti-GABAARα1 antibody according to the fluorescence method. A control nucleus (ct) and operated nucleus (op) are shown on the left and right sides, respectively. The scale bar represents 100 µm. Three sections were stained for each of three animals. b Dual staining of the facial nucleus with GABAARα1 and NR3B. Brainstem sections were dually stained with anti-GABAARα1 antibody and anti-NMDA receptor 3B subunit (NR3B) antibody according to fluorescence methods. In the control facial nucleus, anti-GABAARα1 antibody-positive cells (GABAARα1) and anti-NR3B antibody-positive cells (NR3B) were visualized by Alexa Fluor-488 (green) and Alexa Fluor-568 (red), respectively. The merged image is shown on the right-hand side. The scale bar represents 50 µm. Three sections were stained for each of three animals. (Color figure online)
To determine which cell type decreased the GABAARα1 protein in the axotomized facial nucleus, we next carried out fluorescent dual immunohistochemistry for GABAARα1 and NR3B (a marker of motoneurons) using cryosections prepared at 5 days post-insult. In the control facial nucleus, many large cells were stained by anti-GABAARα1 antibody (Fig. 2b, GABAARα1), and they were all anti-NR3B antibody-positive (Fig. 2b, NR3B), indicating that the GABAARα1 protein is expressed in motoneurons (Fig. 2b, merged).
Survival Rate of Injured Motoneurons in the Axotomized Facial Nucleus
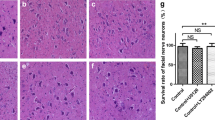
Our finding that the levels of GABAARα1 protein in the axotomized facial nucleus were decreased over the 5 weeks post-insult allowed us to anticipate the occurrence of metabolic changes of proteins or motoneuronal cell death. Here, to distinguish between protein metabolic change and motoneuronal cell death, we examined the survivability of injured motoneurons at 5 days, 14 days and 5 weeks post-insult. Nissl staining indicated that most motoneurons still survived at 5 days (Fig. 3a, b), 14 days (Fig. 3c, d) and 5 weeks (Fig. 3e, f) after the transection of the facial nerve.
Survival rate of injured motoneurons. a–f Nissl staining of the facial nucleus following insult. Brainstem sections recovered at 5 days (a, b), 14 days (c, d) and 5 weeks (e, f) after transection were subjected to Nissl staining. Control (a, c and e) and operated (b, d and f) sides are shown on the left and right, respectively. The scale bar represents 200 µm. The images are representative of experiments performed using three rats at each age, with three sections per animal. g Quantification of Nissl-stained motoneurons in facial nuclei. Three cryosections around the central position of the facial nuclei were stained with Nissl, and the number of Nissl-stained motoneurons was statistically compared between the control (ct) and injured facial nuclei (op) prepared at 5 days (g, 5D), 14 days (g, 14D) and 5 weeks (g, 5W) post-insult. Data shown are means ± SDs from an experiment using 3 rats. ns not significant
Next, we quantitatively compared the number of motoneurons stained with Nissl between the control and injured facial nucleus. In the facial nucleus 5 days post-injury, the average numbers on the control side and the injured side were 109.0 ± 3.2 cells/section and 112.7 ± 2.9 cells/section, respectively, indicating no significant difference (Figs. 3g, 5D). At 14 days post-injury, the average number on the control side was 120.7 ± 2.8 cells/section, while that on the injured side was 118.3 ± 3.5 cells/section, with no significant difference between them (Fig. 3g, 14D). There was no significant difference between the control (114.0 ± 4.0 cells/section) and injured (117.1 ± 4.6 cells/section) nucleus of rats at 5 weeks post-injury (Figs. 3g, 5 weeks). These results indicated that the decrease in GABAARα1 protein levels in the injured nucleus is not attributable to the cell death of motoneurons, but presumably due to the protein metabolic change in motoneurons.
Changes in the GABA-Synthesizing Enzyme Level
Since the above results suggested that the levels of the GABAergic components are affected by motoneuron lesions, we analyzed the levels of glutamate decarboxylase (GAD) protein in the injured facial nucleus. GAD is a GABA-synthesizing enzyme specific to GABAergic neurons. Immunoblotting indicated that the levels of GAD65/67 protein in the axotomized facial nucleus were decreased 5–14 days post-insult (Fig. 4a). The quantified results revealed that the levels of GAD65/67 in the operated nucleus were reduced to 97.7 ± 6.4%, 77.8 ± 16.9%, 22.6 ± 8.7%, 25.6 ± 8.1% and 33.2 ± 10.2% at 1, 3, 5, 7 and 14 days post-insult, respectively (Fig. 4b).
Response of GAD to motoneuron insult. a Changes in GAD levels over the 1–14 days after insult. Sets of contralateral (L) and ipsilateral (R) facial nuclei recovered at 1, 3, 5, 7 and 14 days after transection were immunoblotted for glutamate decarboxylase (GAD65/67) and actin (Actin). The result shown is representative of experiments performed in triplicate using five rats. b Quantitative analysis of GAD. The intensities of the GAD65/67 bands in panel (a) were determined by a densitometer and expressed as the value for the transected facial nucleus (R) relative to that for the control nucleus (L) (defined as 100%). Data shown are means ± SDs from three independent experiments (ns not significant, **P < 0.01). c Changes in GAD levels over the 3–5 weeks after insult. Sets of contralateral (left side: L) and ipsilateral (right side: R) facial nuclei recovered at 3, 4 and 5 weeks after axotomy were immunoblotted for GAD 65/67 and actin (Actin). The result shown is representative of experiments performed in triplicate using three rats. d Quantitative analysis of GAD. The intensities of the bands in panel (c) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant, *P < 0.05). e GAD65/67 levels in the uninjured nucleus and control nucleus. Sets of left (L) and right (R) facial nuclei of 8 week-old rats (8w-0d), and sets of control (L) and injured (R) facial nuclei of 8 week-old rats recovered at 5 days post-insult (8w-5d) were immunoblotted for GAD65/67 and actin (Actin). The result shown is representative of experiments performed in triplicate using two rats. f Quantification of GAD65/67 levels in panel (e). The intensities of the GAD65/67 bands in (e) were determined by a densitometer, and the value for R of 8w-0d and L of 8w-5d was expressed relative to that for L of 8w-0d (defined as 100%). The value for R of 8w-5d was expressed relative to that for L of 8w-5d (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant, **P < 0.01). g GAD65/67 levels in the uninjured nuclei of 8 and 13 week-old rats. Sets of left (L) and right (R) facial nuclei of 8 week-old (8w-0d) and 13 week-old (13w-0d) rats were immunoblotted for GAD65/67 and actin (Actin). The result shown is representative of experiments performed in triplicate using two rats. h Quantification of GAD65/67 levels in panel (g). The intensities of the GAD65/67 bands in (g) were determined by a densitometer, and the values for R of 8w-0d and L and R of 13w-0d were expressed relative to that for L of 8w-0d (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant). i Immunohistochemistry for GAD. The brainstem sections taken at 5 days post-insult were stained with anti-GAD65/67 antibody according to fluorescence methods. GAD65/67-expressing cells on the control nucleus (ct) and injured nucleus (op) were visualized by Alexa Fluor-488 (green). The scale bar represents 50 µm. Staining was carried out using three sections for each of three rats. j Motoneurons in the facial nucleus. The brainstem sections taken at 5 days post-insult were separately stained with anti-GABAARα1 antibody or anti-m2MAchR antibody. GABAARα1- and m2MAchR-expressing cells on the control nucleus were visualized by Alexa Fluor-488 (green) and Alexa Fluor-568 (red), respectively. The scale bar represents 50 µm. The results are representative of experiments performed using three rats, with three sections per animal. (Color figure online)
We further examined whether the levels of GAD65/67 protein in the lesion side recovered at 3–5 weeks after injury. The GAD65/67 protein levels on the lesion side appeared to recover to the control levels at 5 weeks post-insult (Fig. 4c). In the quantified results, the GAD65/67 protein levels in the injured nucleus returned to 59.5 ± 8.1%, 87.7 ± 5.7% and 99.3 ± 5.4% at 3, 4 and 5 weeks post-insult, respectively (Fig. 4d). Thus, GABAergic neurons transiently decreased the levels of GAD65/67 protein at 5–21 days post-insult, and these levels were recovered to the control levels at 4–5 weeks post-insult.
To examine the basal levels of GAD65/67 protein in the uninjured facial nucleus, we compared the levels between the uninjured facial nucleus (L and R) of 8 week-old rats (8w-0d) and the left control facial nucleus (L) of 8 week-old rats whose right facial nerve was transected 5 days earlier (8w-5d) (Fig. 4e). When the level of GAD65/67 protein in L of 8w-0d was defined as 100%, the levels in R of 8w-0d and L of 8w-5d were 95.7 ± 4.5% and 97.3 ± 4.0%, respectively (Fig. 4f). The level in R of 8w-5d was significantly lower (27.0 ± 9.6%) compared to that in L of 8w-5d (Fig. 4f). These quantified results suggested that the levels of GAD65/67 protein in the control nucleus are not essentially modified by motoneuronal injury on the operated side.
We further compared the levels of GAD65/67 protein between the uninjured nuclei of 8w-0d and 13w-0d rats (Fig. 4g). When the level in L of 8w-0d was defined as 100%, the levels in R of 8w-0d, and L and R of 13w-0d were 99.4 ± 10.1%, 103.3 ± 2.6% and 102.5 ± 9.1%, respectively (Fig. 4h), indicating that there was no significant difference in GAD65/67 protein levels between nuclei in 8 week-old rats and 13 week-old rats. These results suggest that the levels of GAD65/67 protein in the facial nucleus are not essentially changed over 8–13 weeks of age.
We carried out immunohistochemistry for GAD65/67-expressing cells in the injured facial nucleus taken at 5 days post-insult. In the control nucleus, many cells were stained with anti-GAD65/67 antibody (Fig. 4i, ct), but in the injured nucleus only weakly stained cells were observed (Fig. 4i, op). The GAD65/67-expressing cells were much smaller than the GABAARα1/m2MAchR-expressing motoneurons [10] (Fig. 4j), suggesting that the GAD65/67-expressing cells were GABAergic neurons, but not motoneurons. Therefore, these present and previous results suggested that the GABA synthesis of GABAergic neurons around injured motoneurons is transiently downregulated at 5 days to 3 weeks post-injury, but recovers to the control levels thereafter.
Change in the Vesicular GABA Transporter (VGAT) Level
VGAT is also a specific molecule for GABAergic neurons. Therefore, we next examined whether or not motoneuron injury affected the VGAT protein levels. Immunoblotting indicated that the VGAT protein levels in the axotomized facial nucleus were decreased over 3–14 days after transection compared to those in the control nucleus (Fig. 5a). The quantitative analysis indicated that the VGAT protein levels declined to 91.9 ± 4.2%, 75.8 ± 9.2%, 33.5 ± 11.2%, 36.7 ± 7.2% and 43.1 ± 7.3% at 1, 3, 5, 7 and 14 days post-insult, respectively (Fig. 5b). However, recovery to the control levels occurred at 4–5 weeks post-transection (Fig. 5c). In the quantified profile, the levels of VGAT protein in the injured nucleus were 44.8 ± 19.4%, 79.0 ± 9.5% and 103.0 ± 13.4% at 3, 4 and 5 weeks, respectively (Fig. 5d). The levels in the transected nucleus recovered after temporal downregulation.
Response of VGAT to motoneuron insult. a Changes in VGAT levels over the 1–14 days after insult. Sets of contralateral (L) and ipsilateral (R) facial nuclei recovered at 1, 3, 5, 7 and 14 days after transection were immunoblotted for vesicular GABA transporter (VGAT) and actin (Actin). The image shown is representative of experiments performed in triplicate using five rats. b Quantitative analysis of VGAT. The intensities of the bands in panel (a) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant, *P < 0.05, **P < 0.01). c Changes in VGAT levels over the 3–5 weeks after insult. Sets of contralateral (left side: L) and ipsilateral (right side: R) facial nuclei recovered at 3, 4 and 5 weeks after axotomy were immunoblotted for VGAT and actin (Actin). The result shown is representative of experiments performed in triplicate using three rats. d Quantitative analysis of VGAT. The intensities of the bands in panel (c) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant, *P < 0.05). e VGAT levels in the uninjured nucleus and control nucleus. Sets of left (L) and right (R) facial nuclei of 8 week-old rats (8w-0d), and sets of control (L) and injured (R) facial nuclei of 8 week-old rats recovered at 5 days post-insult (8w-5d) were immunoblotted for VGAT and actin (Actin). The result shown is representative of experiments performed in triplicate using two rats. f Quantification of VGAT levels in panel (e). The intensities of the VGAT bands in (e) were determined by a densitometer, and the values for R of 8w-0d and L of 8w-5d were expressed relative to that for L of 8w-0d (defined as 100%). The value for R of 8w-5d was expressed relative to that for L of 8w-5d (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant, **P < 0.01). g VGAT levels in uninjured nuclei of 8 and 13 week-old rats. Sets of left (L) and right (R) facial nuclei of 8 week-old (8w-0d) and 13week-old (13w-0d) rats were immunoblotted for VGAT and actin (Actin). The result shown is representative of experiments performed in triplicate using two rats. h Quantification of VGAT levels in panel (g). The intensities of the VGAT bands in (g) were determined by a densitometer, and the values for R of 8w-0d and L and R of 13w-0d were expressed relative to that for L of 8w-0d (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant). i Immunohistochemistry for VGAT. The brainstem sections recovered at 5 days post-insult were stained with anti-VGAT antibody according to fluorescence methods. VGAT-expressing cells on the control nucleus (ct) and injured nucleus (op) were visualized by Alexa Fluor-568 (red). The scale bar represents 50 µm. The images are representative of experiments performed using three rats, with three sections per animal. (Color figure online)
To examine the basal levels of the VGAT protein in the uninjured facial nucleus, we compared the levels between the uninjured facial nucleus (L and R) of 8 week-old rats (8w-0d) and left control facial nucleus (L) of 8 week-old rats whose right facial nerve was transected 5 days previously (8w-5d) (Fig. 5e). When the level of VGAT protein in L of 8w-0d was defined as 100%, the levels in R of 8w-0d and L of 8w-5d were 99.1 ± 6.4% and 97.8 ± 6.0%, respectively (Fig. 5f). The level in R of 8w-5d was significantly lower (32.7 ± 1.2%) than that in L of 8w-5d (Fig. 5f). These quantified results suggested that the levels of VGAT protein in the control nucleus are not essentially modified by motoneuronal injury on the operated side.
We further compared the levels of the VGAT protein between the uninjured nuclei of 8w-0d and 13w-0d rats (Fig. 5g). When the level in L of 8w-0d was defined as 100%, the levels in R of 8w-0d, and L and R of 13w-0d were 99.4 ± 2.0%, 97.6 ± 2.4% and 97.8 ± 4.4%, respectively (Fig. 5h), indicating that there was no significant difference in VGAT protein levels between the nuclei in 8 week-old rats and 13 week-old rats. These results suggest that the levels of VGAT protein in the facial nucleus are not essentially changed over 8–13 weeks of age.
Next, the change in VGAT protein levels in the facial nucleus measured at 5 days post-insult was investigated immunohistochemically. We observed that many small cells were stained with anti-VGAT antibody in the control nucleus (Fig. 5i, ct), but fewer small cells were stained in the injured nucleus (Fig. 5i, op). These results indicate that GABAergic neurons exhibit weakened GABA-packaging activity at 3–21 days following injury, but the packaging function is restored to the normal level thereafter.
Susceptibility of GAT-1 to Facial Nerve Transection
GAT-1, another GABA transporter for reuptaking GABA in the synaptic cleft, was examined in the axotomized facial nucleus. The levels of GAT-1 protein in the injured facial nucleus were downregulated over 5–14 days post-insult (Fig. 6a). The levels relative to those of the control nucleus were 95.5 ± 5.3%, 71.7 ± 20.2%, 39.5 ± 10.3%, 42.6 ± 2.5% and 31.3 ± 7.0% at 1, 3, 5, 7 and 14 days post-insult, respectively (Fig. 6b). Over 3–5 weeks post-insult, the GAT-1 protein levels in the injured nucleus seemed to recover (Fig. 6c). In fact, quantified data indicated that the values in the injured nucleus were 34.5 ± 13.4%, 74.6 ± 21.2% and 98.6 ± 2.7% at 3, 4 and 5 weeks, respectively, compared to those in the control nucleus (Fig. 6d). Similar to the case of the GAD and VGAT proteins, the GAT-1 protein was found to recover to the control level at 4–5 weeks post-insult.
Response of GAT-1 to motoneuron insult. a Changes in GAT-1 levels over the 1–14 days after insult. Sets of contralateral (L) and ipsilateral (R) facial nuclei recovered at 1, 3, 5, 7 and 14 days after transection were immunoblotted for GABA transporter 1 (GAT-1) and actin (Actin). The result shown is representative of experiments performed in triplicate using five rats. b Quantification of GAT-1 levels. The intensities of the GAT-1 bands in panel (a) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). Data shown are means ± SDs from three independent experiments (ns not significant, **P < 0.01). c Changes in GAT-1 levels over the 3–5 weeks after insult. Sets of contralateral (left side: L) and ipsilateral (right side: R) facial nuclei recovered at 3, 4 and 5 weeks after axotomy were immunoblotted for GAT-1 and actin (Actin). The result shown is representative of experiments performed in triplicate using three rats. d Quantification of GAT-1 levels. The intensities of the bands in panel (c) were determined by a densitometer, and the value for the transected facial nucleus (R) was expressed relative to that for the control nucleus (L) (defined as 100%). The data presented are means ± SDs from three independent experiments (ns not significant; *P < 0.05). e GAT-1 levels in the uninjured nucleus and control nucleus. Sets of left (L) and right (R) facial nuclei of 8 week-old rats (8w-0d), and sets of control (L) and injured (R) facial nuclei of 8 week-old rats recovered at 5 days post-insult (8w-5d) were immunoblotted for GAT-1 and actin (Actin). The result shown is representative of experiments performed in triplicate using two rats. f Quantification of GAT-1 levels in (e). The intensities of the GAT-1 bands in (e) were determined by a densitometer, and the values for R of 8w-0d and L of 8w-5d were expressed relative to that for L of 8w-0d (defined as 100%). The value for R of 8w-5d was expressed relative to that for L of 8w-5d (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant, **P < 0.01). g GAT-1 levels in uninjured nuclei of 8 and 13 week-old rats. Sets of left (L) and right (R) facial nuclei of 8 week-old (8w-0d) and 13 week-old (13w-0d) rats were immunoblotted for GAT-1 and actin (Actin). The result shown is representative of experiments performed in triplicate using two rats. h Quantification of GAT-1 levels in panel (g). The intensities of the GAT-1 bands in (g) were determined by a densitometer, and the values for R of 8w-0d and L and R of 13w-0d were expressed relative to that for L of 8w-0d (defined as 100%). The data shown are means ± SDs from three independent experiments (ns not significant). i Immunohistochemistry for GAT-1. The brainstem sections recovered at 5 days post-insult were stained with anti-GAT-1 antibody according to fluorescence methods. GAT-1—expressing cells on the control nucleus (ct) and injured nucleus (op) were visualized by Alexa Fluor-568 (red). The scale bar represents 50 µm. The images are representative of experiments performed using three rats, with three sections per animal. (Color figure online)
To determine the basal levels of GAT-1 protein in the uninjured facial nucleus, we compared the levels between the uninjured facial nucleus (L and R) of 8 week-old rats (8w-0d) and left control facial nucleus (L) of 8 week-old rats whose right facial nerve was transected 5 days previously (8w-5d) (Fig. 6e). When the level of GAT-1 protein in L of 8w-0d was defined as 100%, the levels in R of 8w-0d and L of 8w-5d were 101.1 ± 6.9% and 104.2 ± 3.7%, respectively (Fig. 6f). The level in R of 8w-5d was significantly lower (32.7 ± 3.8%) than that in L of 8w-5d (Fig. 6f). These quantified results suggested that the levels of GAT-1 protein in the control nucleus were not essentially modified by motoneuronal injury on the operated side.
We further compared the levels of GAT-1 protein between the uninjured nuclei of 8w-0d and 13w-0d rats (Fig. 6g). When the level in L of 8w-0d was defined as 100%, the levels in R of 8w-0d, and L and R of 13w-0d were 98.5 ± 4.5%, 103.7 ± 6.7% and 102.3 ± 6.9%, respectively (Fig. 6h), indicating that there was no significant difference in GAT-1 protein levels between nuclei in 8 week-old rats and 13 week-old rats. These results suggest that the levels of GAT-1 protein in the facial nucleus are not essentially changed over 8–13 weeks of age.
The GAT-1—expressing cells were immunohistochemically examined in cryosections of the injured facial nucleus taken at 5 days post-insult. In the control nucleus, many small cells were stained with anti-GAT-1 antibody (Fig. 6i, ct). However, fewer cells were stained in the ipsilateral nucleus (Fig. 6i, op). These results suggested that GABAergic neurons transiently reduce GABA-reuptake activity at 5 days to 3 weeks following transection, but then return to the control levels at 4–5 weeks post-injury.
Effects of GDNF Administration on the Recovery of GABAAR Levels
Our results indicated that GABAARα1 protein levels in the transected nucleus did not return to the control levels at 5 weeks post-insult (Fig. 1c, d). A possible reason for this is that motoneurons could not obtain sufficient amounts of target-derived neurotrophic factor by axotomy, which rendered them unable to recover the GABAARα1 protein level. To examine this possibility, we tested whether or not GDNF maintains GABAARα1 protein levels in the injured nucleus. GDNF was selected as a main candidate because the factor is known as a strong survival factor for motoneurons [25].
In the experiment, we divided six rat littermates into two groups. One group underwent transection of a facial nerve, and after 5 days the levels of GABAARα1 protein in the injured nucleus were compared to those in the control nucleus. The results clearly indicated that the amounts of GABAARα1 protein in the injured nucleus at 5 days post-insult were significantly reduced (Fig. 7a), consistent with the results in Fig. 1a. The levels in the injured nucleus of three rats were quantitatively estimated at 19.1 ± 12.6% (P < 0.01) (Fig. 7b, cut) versus 100% for the control nucleus (Fig. 7b, ct). The level of actin protein in the injured nucleus was 103.1 ± 6.1% (Fig. 7c, cut) of that in the control nucleus (Fig. 7c, ct) (defined as 100%), indicating that there was no significant difference in the actin protein level between the nuclei.
Effects of GDNF on levels of GABAARα1 in injured nucleus. a GABAARα1 levels in the transected facial nucleus. The right facial nerves of three rats (1–3) were transected, as described in “Materials and Methods”. Five days later, the rats were decapitated and their control nucleus (ct) and transected nucleus (cut) were immunoblotted for GABAARα1 and actin (Actin). b, c Quantification of GABAARα1 (b) and actin (c). The intensities of the GABAARα1 and actin protein bands in panel (a) were determined by densitometer and expressed as the value for the transected facial nucleus (cut) relative to that for the control nucleus (ct) (defined as 100%). Data shown are means ± SDs from an experiment using three rats (n = 3). (ns not significant, **P < 0.01). d GABAARα1 levels in transected and GDNF-administered facial nuclei. Both the right and left facial nerves from each of three rats (rats 1–3) were transected. At the left transected facial nerve, a piece of Gelfoam soaked in PBS (vehicle) was applied (cut + P), while at the right transected facial nerve a piece of Gelfoam soaked in GDNF/PBS was applied (cut + G) as described in “Materials and Methods”. Five days later, the rats were decapitated and their facial nuclei were immunoblotted for GABAARα1 and actin (Actin). e, f Quantification of GABAARα1 (e) and actin (f). The intensities of the GABAARα1 and actin protein bands in panel (d) were determined by a densitometer and expressed as the value for the transected and GDNF-applied facial nucleus (cut + G) relative to that for the transected nucleus (cut + P) (defined as 100%). Data shown are means ± SDs from an experiment using three rats (n = 3) (ns not significant, *P < 0.05). g GDNF contents in the uninjured nucleus and injured nucleus. Six rats were divided into two groups; one group (three rats) was not treated (group 1), and the other group (three rats) was subjected to axotomy of the right facial nerve (group 2). Five days later, the uninjured right facial nucleus (un) in group 1 and injured right facial nucleus (cut) in group 2 were taken from each rat, and their tissue extracts were immunoblotted for GDNF protein (GDNF) and actin protein (Actin). h, i Quantification of GDNF protein (h) and actin protein (i). The intensities of the GDNF and actin protein bands in panel (g) were determined by a densitometer and expressed as the value for the transected facial nucleus (cut) relative to that for the uninjured nucleus (un) (defined as 100%). Data shown are the means ± SDs from an experiment using three rats (n = 3) (ns not significant, *P < 0.05)
On the other hand, the other group was subjected to the transection of a facial nerve and the administration of GDNF, as described in the “Materials and Methods”. In the injured and GDNF-administered nucleus (Fig. 7d, cut + G), GABAARα1 protein levels were higher than those in the injured nucleus (Fig. 7d, cut + P). The quantified results indicated that the levels of GABAARα1 protein in the injured/GDNF-administered nucleus (cut + G) were 162.8 ± 18.1% (P < 0.05) of those in the injured nucleus (cut + P), which were defined as 100% (Fig. 7e). The level of actin protein in the injured/GDNF-administered nucleus was 101.7 ± 3.7% (Fig. 7f, cut + G) of that in the injured nucleus (Fig. 7f, cut + P) (defined as 100%), indicating that there was no significant difference in the actin protein level between the nuclei. These results suggested that GDNF administration ameliorated the reduction of GABAARα1 protein levels in the lesioned nucleus.
Additionally, we compared the contents of GDNF protein between the uninjured right nucleus (un) of 8 week-old rats and injured nucleus (cut) of 8 week-old rats recovered at 5 days post-insult. Immunoblotting indicated that GDNF protein contents in the injured facial nucleus (cut) were lower than those in the uninjured nucleus (un) (Fig. 7g). Quantified results indicated that the levels of GDNF protein in the injured nucleus (cut) were 34.1 ± 13.5% of those in the uninjured nucleus (un) (defined as 100%) (Fig. 7h), indicating that the amounts of GDNF protein in the injured nucleus were significantly decreased. Actin protein levels in the injured nucleus (cut) were 100.4 ± 3.3% of those in the uninjured nucleus (un) (Fig. 7i). This result pointed to a correlation between the levels of GABAARα1 protein and GDNF protein contents in the facial nucleus.
Discussion
Motoneuron activity in the cranial nervous system is regulated by excitatory and inhibitory synapses [12]. However, it has not been sufficiently determined how motoneuron insult affects the inhibitory system and how the system is restored after insult. In this study, we focused on the GABAergic system of the rat facial nerve, and analyzed GABAAR and functional components of GABAergic neurons in a facial nerve axotomy model.
GABAAR, an ionotropic receptor, is known to influx Cl− from the extracellular space in response to GABA, and to inhibit the formation of action potential [26]. GABAAR was previously recognized in the rat facial motonucleus, and the levels of GABAAR subunits have been observed to decrease in the axotomized facial nucleus based on in situ hybridization and immunohistochemical studies [21]. Here, we also found that the levels of GABAARα1 protein in the injured facial nucleus decreased from day 5 post-injury, and that the decreased levels did not recover over the 5 weeks post-injury (Fig. 1). These findings are essentially consistent with those of Vassias et al. [21].
The cells expressing GABAARα1 protein in the facial nucleus were identified as motoneurons by an immunohistochemical study (Fig. 2). In previous reports, excitatory glutamate receptors (GluRs) were also detected in facial motoneurons [27, 28]. Accordingly, we considered that facial motoneuron activity is regulated by a suitable balance of excitatory and inhibitory inputs. How is the balance regulated when motoneurons are injured? Levels of the metabotropic GluR mGluR1a have been shown to be decreased in axotomized motoneurons [29], and GluR2/3 and GluR4 levels were also decreased in injured hypoglossal motoneurons [30]. On the other hand, the mRNA levels of GABAAR subunits (α1, β2, and γ2) and the protein levels of α1/γ2 subunits were shown to be decreased in the axotomized facial nucleus [21]. The mRNA levels of GABABR subunits (B1B and B2) and the protein level of the B2 subunit were also found to be reduced in the transected facial nucleus [21]. In addition, we showed in the present study that the GABAARα1 protein was decreased in the axotomized facial nucleus. Thus, injured motoneurons tend to downregulate both excitatory and inhibitory transmissions by reducing expression of the respective receptors, reflecting a state in which they reject neurotransmission.
GABAAR expression levels have been reported to be relevant to pathological states of the central nervous system and peripheral nervous system. In the dentate gyrus of a kainic acid-epilepsy model and temporal lobe epilepsy, the levels of GABAAR subunits showed complicated patterns of alteration [31], suggesting that GABAAR-mediated inhibitory transmission is disturbed in epilepsy. In the central nucleus of the rat amygdala, an agonist and antagonist for GABAAR were shown to change the degree of pruritus, suggesting that GABAAR-mediated inhibitory transmission is involved in itch/pain modulation [32]. In rats whose GABAAR subunit Gabrα6 was reduced by infusing Gabrα6 siRNA into the trigerminal ganglia, the nociceptive response was increased, indicating that the GABAAR level in the trigerminal ganglion regulates myofascial nociception [33].
The responses of GAD, VGAT and GAT-1 proteins to motoneuron injury have not been fully analyzed. In this study, we quantitatively demonstrated that the levels of the GAD, VGAT and GAT-1 proteins in the injured nucleus were transiently decreased at 5–14 days post-insult, but recovered at 4–5 weeks post-injury. This means that GABAergic neurons decreased the synthesis of GABA, the packaging of GABA into the vesicles, and the reuptake of GABA at 5–14 days post-insult, but that at 4–5 weeks post-insult their functions were restored to the control levels (Figs. 4, 5, 6). Although these transition profiles of the GAD/VGAT/GAT-1 proteins were not fully elucidated, we found that they essentially resembled those of ChAT and VAchT, whose levels declined at 3–14 days but recovered at 4–5 weeks post-insult [10]. Immunohistochemical analysis suggested that the GAD/VGAT/GAT-1—expressing cells in the facial nucleus are GABAergic neurons (Figs. 4i, 5i, 6i). We thus found that the GABAergic neurons were influenced by neighboring injured motoneurons in the facial nucleus.
The finding that the levels of functional proteins in the GABAergic neurons were decreased in response to motoneuron injury suggested that a certain stimulus released from lesioned motoneurons was delivered to GABAergic neurons. Humoral substances such as neuropeptides, nucleotides, neurotransmitters and ions (Na+ and K+) have been predicted to act as motoneuron-derived stimuli [34], and electric distortion and/or physical stresses are also possible candidates for motoneuron-derived stimuli. Presumably, these molecules or other stimuli are released from the injured motoneurons and trigger the stimulation of GABAergic neurons in a manner that downregulates the GAD, VGAT and GAT-1 proteins. It is plausible that the release of these stimuli is regulated depending on the health of the motoneuron. Motoneurons would release a large amounts of stimuli just after injury, then reduce the amount during recovery. Much effort has been expended in exploring the potential stimulus molecules produced and released from injured motoneurons.
As shown above, the levels of GABAARα1 protein were depressed over the 5 weeks post-insult and did not return to the control level (Fig. 1). We attributed this to a disturbance in the supply of neurotrophic factor due to the nerve transection; the shortage would have depressed the GABAARα1 protein levels. To examine this possibility, we tested whether or not a neurotrophic factor could prevent the reduction of GABAARα1 protein levels in injured motoneurons. Among the neurotrophic factors for motoneurons, which include neurotrophins and transforming growth factor beta superfamily proteins, we selected GDNF because it is known to exhibit a strong survival effect on motoneurons [25]. Interestingly, administration of GDNF at the cut nerve significantly decreased the reduction in GABAARα1 protein levels (Fig. 7d, e). This result strongly suggested that the injured motoneurons downregulate the levels of GABAARα1 protein, due to the shortage of neurotrophic factors such as GDNF.
The present study revealed an important phenomenon related to the intercellular interaction between injured motoneurons and GABAergic neurons in the lesioned facial nucleus.
Conclusion
Transection of a rat facial nerve led to the downregulation of GABAARα1 protein levels in injured motoneurons from 5 days to 5 weeks post-insult. The long-term reduction was thought to be caused by a shortage of target-derived neurotrophic factor(s). Accompanying the motoneuronal change, the GAD, VGAT and GAT-1 protein levels in GABAergic neurons were decreased at 5–21 days post-insult, but subsequently returned to the control levels at 4–5 weeks post-insult. These results suggested that some specific information derived from injured motoneurons is transmitted to nearby GABAergic neurons, which temporarily downregulate specific functional components of the GABAergic system.
References
Martin MR, Mason CA (1977) The seventh cranial nerve of the rat. Visualization of efferent and afferent pathways by cobalt precipitation. Brain Res 121:21–41
Kreutzberg GW (1996) Principles of neuronal regeneration. Acta Neurochir (Suppl 66):103–106
Moran LB, Graeber MB (2004) The facial nerve axotomy model. Brain Res Brain Res Rev 44:154–178
Hoover DB, Hancock JC (1985) Effect of facial nerve transection on acetylcholinesterase, choline acetyltransferase and [3H]quinuclidinyl benzilate binding in rat facial nuclei. Neuroscience 15:481–487
Che YH, Yamashita T, Tohyama M (2002) Changes in mRNA for VAMPs following facial nerve transection. J Chem Neuroanat 24:147–152
Eleore L, Vassias I, Vidal PP, de Waele C (2005) Modulation of the glutamatergic receptors (AMPA and NMDA) and of glutamate vesicular transporter 2 in the rat facial nucleus after axotomy. Neuroscience 136:147–160
Haas CA, Streit WJ, Kreutzberg GW (1990) Rat facial motoneurons express increased levels of calcitonin gene-related peptide mRNA in response to axotomy. J Neurosci Res 27:270–275
Saika T, Senba E, Noguchi K, Sato M, Kubo T, Matsunaga T, Tohyama M (1991) Changes in expression of peptides in rat facial motoneurons after facial nerve crushing and resection. Brain Res Mol Brain Res 11:187–196
Akazawa C, Nakamura Y, Sango K, Horie H, Kohsaka S (2004) Distribution of the galectin-1 mRNA in the rat nervous system: its transient upregulation in rat facial motor neurons after facial nerve axotomy. Neuroscience 125:171–178
Ichimiya T, Yamamoto S, Honda Y, Kikuchi R, Kohsaka S, Nakajima K (2013) Functional down-regulation of axotomized rat facial motoneurons. Brain Res 1507:35–44
Petroff OA (2002) GABA and glutamate in the human brain. Neuroscientist 8:562–573
Roth FC, Draguhn A (2012) GABA metabolism and transport: effects on synaptic efficacy. Neural Plast 805830:12. doi:10.1155/2012/805830
Buddhala C, Hsu CC, Wu Y (2009) A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem Int 55:9–12
McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM (1997) Identification and characterization of the vesicular GABA transporter. Nature 389:870–876
Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J (1998) The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci 18:9733–9750
Sperk G, Furtinger S, Schwarzer C, Pirker S (2004) GABA and its receptors in epilepsy. Adv Exp Med Biol 548:92–103
Kudo Y, Abe N, Goto S, Fukuda H (1975) The chloride-dependent depression by GABA in the frog spinal cord. Eur J Pharmacol 32:251–259
Bowery NG, Brown DA (1974) Depolarizing actions of gamma-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br J Pharmacol 50:205–218
Matsumoto RR (1989) GABA receptors: are cellular differences reflected in function? Brain Res Brain Res Rev 14:203–225
Chebib M, Johnston GA (1999) The ‘ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol 26:937–940
Vassias I, Lecolle S, Vidal PP, de Waele C (2005) Modulation of GABA receptor subunits in rat facial motoneurons after axotomy. Brain Res Mol Brain Res 135:260–275
Graeber MB, López-Redondo F, Ikoma E, Ishikawa M, Imai Y, Nakajima K, Kreutzberg GW, Kohsaka S (1998) The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Res 813:241–253
Lowry OJ, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Konigsmark BW (1970) Methods for counting of neurons. In: Nauta WJH, Ebbesson SOE (eds) Contemporary research methods in neuroanatomy. Springer, Berlin Heidelberg, pp 315–340
Yan Q, Matheson C, Lopez OT (1995) In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature 373:341–344
Ito S (2016) GABA and glycine in the developing brain. J Physiol Sci 66:279–375
Xing GG, Wang R, Yang B, Zhang D (2006) Postnatal switching of NMDA receptor subunits from NR2B to NR2A in rat facial motor neurons. Eur J Neurosci 24:2987–2992
Chen P, Song J, Luo L, Cheng Q, Xiao H, Gong S (2016) Gene expression of NMDA and AMPA receptors in different facial motor neurons. Laryngoscope 126:E6–E11
Alvarez FJ, Dewey DE, Carr PA, Cope TC, Fyffe RE (1997) Downregulation of metabotropic glutamate receptor 1a in motoneurons after axotomy. Neuroreport 8:1711–1716
García del Caño G, Gerrikagoitia I, Sarasa M, Matute C, Martínez-Millán L (2000) Ionotropic glutamate receptor subunits are differentially regulated in the motoneuronal pools of the rat hypoglossal nucleus in response to axotomy. J Neurocytol 29:509–523
Sperk G, Drexel M, Pirker S (2009) Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia 50(Suppl 12):29–31
Chen L, Wang W, Tan T, Han H, Dong Z (2016) GABA(A) Receptors in the central nucleus of the amygdala are involved in pain- and itch-related responses. J Pain 17:181–189
Kramer PR, Bellinger LL (2013) Reduced GABAA receptor α6 expression in the trigeminal ganglion enhanced myofascial nociceptive response. Neuroscience 245:1–11
Nakajima K, Kohsaka S (2005) Response of microglia to brain injury. In: Kettenmann H, Ransom BR (eds) Neuroglia second edition. Oxford University Press, New York, pp 443–453
Acknowledgements
We thank Yoko Tohyama for her very good and careful care of the animals.
Funding
This research was not supported by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RK performed the operation on the animals and was primarily responsible for analyzing the GABAARα1, GAD, VGAT and GAT-1 proteins by immunoblotting and immunohistochemistry. MH examined the effects of GDNF on the levels of GABAARα1. MK carried out Nissl staining. SK and KN participated in designing the study and performed some supporting experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Kikuchi, R., Hamanoue, M., Koshimoto, M. et al. Response of the GABAergic System to Axotomy of the Rat Facial Nerve. Neurochem Res 43, 324–339 (2018). https://doi.org/10.1007/s11064-017-2427-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2427-1