Abstract
Increasing evidence suggests that capsaicin may play a role in modulating neuronal function and controlling motor behavior. However, the underlying mechanism is still unclear and the activation of transient receptor potential vanilloid 1 (TRPV1) might be involved in. This study investigated the potential neuroprotective role of capsaicin in a rat model of 6-hydroxydopamine (6-OHDA)-induced Parkinson’s disease (PD). Capsaicin was treated intraperitoneally for the 6-OHDA induced PD rats and the locomotor activity and abnormal involuntary movements were found alleviated. Besides, brain oxidative stress (lipid peroxidation, superoxide dismutase and catalase) was also assessed, and oxidative insults were investigated relieved. Both the expression of tyrosine hydroxylase and TRPV1 were increased in the striatal and substantia nigra areas of 6-OHDA induced rats after the treatment of capsaicin by the semi-quantitative analysis of Western Blot. And the immunostaining of substantia nigra further suggested that capsaicin might protect against dopaminergic neuronal loss. Our results showed that TRPV1 might be a novel therapeutic target for PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by the loss of dopaminergic (DA) neurons in the substantia nigra (SN) pars compacta [1]. One major goal of PD research is to explore potential disease-modifying drugs that slow or stop the underlying neurodegenerative process [2]. However, no effective neuroprotective drugs or therapies have been found [3]. Recently, the potential mechanisms of promising drugs have mainly focused on protein aggregation, neuroinflammation, endoplasmic reticulum stress, mitochondria dysfunction and oxidative stress [4].
Oxidative stress is one of the key biochemical abnormalities involved in the progression of PD [5], which can be caused by many environmental toxins, including MPTP, Rotenone and 6-OHDA [6]. So far, 6-OHDA has been widely used in experimental models of PD for in vivo and in vitro tests [7]. Apart from the direct toxicity of 6-OHDA on DA neurons, oxidative stress is thought to be the main mechanism involved in [8]. Insufficient antioxidants and excessive ROS, such as superoxide, hydrogen peroxide and hydroxyl radicals can lead to DA neuron degeneration and neuron network dysfunction [6, 9]. Furthermore, locomotor activity decreases significantly in a 6-OHDA-induced hemiparkinsonian rat model due to the oxidative stress [10]. Drugs that can reduce oxidative stress might be useful to rescue DA neuron and alleviate behavioral deficits effectively in a rat model of PD.
Transient receptor potential vanilloid subfamily member 1 (TRPV1) is a non-selective cation channel and a subfamily of TRP ion channels [11]. Although this channel can be activated by various types of endogenous and exogenous chemical ligands [12], the classical agonist is capsaicin [13], which is an alkaloid found primarily in the fruit of the Capsicum genus [14]. Traditionally, TRPV1 was considered to be involved in broad areas of disease, including pain (inflammatory, visceral, cancer and neuropathic), inflammatory bowel disease, interstitial cystitis, urinary incontinence, airway diseases, pancreatitis and migraine [15]. Recently, research has demonstrated that TRPV1 is not only highly expressed in sensory neurons but also present in various brain zones, which suggests that it might be susceptible to neurodegenerative insults and contribute to the cellular processes involved in neuronal death [16]. Moreover, experiments have shown that TRPV1 can significantly reduce markers of oxidative stress and brain infarction as well as reduce deficits in motor and cognitive functions [17]. However, another in vivo study showed neuroprotective effects against the excitotoxicity of TRPV1 activated by endogenous cannabinoid anandamides in rats [18]. The controversial effects of TRPV1 on cell survival might be dependent on the dosage of agonists used and the method of capsaicin administration [19], and further study are needed to confirm. Thus, TRPV1 might be an effective neuroprotective target for neurodegenerative diseases, especially for PD [20].
Accordingly, we determined whether TRPV1 could be activated and upregulated by capsaicin in the SN and striatum. Our experiments showed that oxidative insults involved in the progression of PD could be diminished and behavioral deficits could be improved in a 6-hydroxydopamine (6-OHDA) lesion rat model of PD. We further demonstrated that capsaicin was mainly involved in rescuing nigral neuron survival by inhibiting oxidative stress on the ipsilateral side, whereas the effect on antioxidant systems on the contralateral side was limited.
Materials and Methods
Animals
Albino Wistar rats have been widely used for the induction of PD symptoms by 6-OHDA [21]. Male Wistar rats weighing 220–250 g (Experimental Animal Center of Shandong University, Jinan, China) were used. The rats were maintained in an animal room with water and food supplied ad libitum under controlled conditions and a 12-h light/12-h dark cycle at an ambient temperature of 22 °C.
Ethics Statement
All animal care and experiments were performed in accordance with the European community Council Directive (2010/63/EU), and were approved by Animal Experimentation Ethics Committee of Shandong University.
Experimental Design
The animals were randomly allocated to four groups as follows: Control (n = 20), sham operated group with stereotaxic injection of saline with saline treatment for 7 days after the operation (SOG, n = 20), 6-OHDA-induced group without capsaicin treatment (6-OHDA, n = 40), and 6-OHDA-induced group with capsaicin treatment for 7 days after the operation (CAP, n = 40). On the 4th week, apomorphine-induced rotations were observed, and successful model rats were selected from the 6-OHDA and CAP groups for the subsequent tests.
Stereotaxic Surgery
Stereotaxic surgeries were conducted under chloral hydrate as previously described [22].The animals were anesthetized by intraperitoneal injection of chloral hydrate (1 ml/100 g). After the rats were deeply anaesthetized (loss of corneal and toe pad reflexes), they received a stereotaxic injection of the DA toxin 6-OHDA (8 μg; 2 μg/μl of 0.02% l-ascorbic acid, Sigma-Aldrich Co., St. Louis, MO, USA) in the right medial forebrain bundle (MFB) at two coordinates (from the bregma: right 2.5 mm; posterior 1.8 mm; depth 7.5 mm, and right 2.5 mm; posterior 1.8 mm; depth 8.0 mm). Sham animals were injected with 8 μl of saline (0.9% NaCl). Rats were injected intraperitoneally with capsaicin (1 mg/kg, a single injection/day for 7 days, Sigma) for 1 week after 6-OHDA injection.
Behavioral Tests
All behavioral tests were performed during the 4th week after the stereotaxic surgery. These tests were conducted in an isolated room between 8:00 and 11:00 am.
Rotational Behavior Test
Apomorphine (1 mg/kg, injected intraperitoneally) induced rotational behavior that could determine whether the creation of the 6-OHDA-induced lesion had been successful [22]. At 4 weeks post-lesion, rotations were counted for 30 min beginning at 5 min after intraperitoneal injection of apomorphine. Rats with at least 210 rotational turns in 30 min were regarded as successful models and selected for subsequent tests.
Open Field Test
The open field test was used to assess spontaneous locomotor activity as previously described [23]. Each rat was placed in the center of a square cage (100 × 100 × 40 cm). The floor was divided by black lines into 25 squares of 20 × 20 cm. After the 1-min test, rats were returned to their home cages, and the open field was cleaned with 75% ethyl alcohol and dried between tests. The total distance, amount of rearing and time spent in the center area were recorded.
Western Blot
Western blot analysis was performed as previously described [24]. Rat brain tissues from the SN and striatum were homogenized on ice in RIPA lysis buffer (Solarbio, Beijing, China) using a Diax 900 homogenizer (Sigma). The protein concentration was determined using a BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, USA). Homogenate samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis with antibodies. The following primary antibodies and dilutions were used: rabbit anti-tyrosine hydroxylase (TH, 1:200, Abcam, Cambridge, UK), rabbit anti-TRPV1 (1:1000, Abcam) and rabbit anti-GAPDH (1:5000, Abcam). Horseradish peroxidase-conjugated anti-rabbit IgG (1:5000, Abcam) was used as a secondary antibody.
Immunostaining
Rats were anesthetized with chloral hydrate and transcardially perfused with phosphate buffered saline (PBS) followed by treatment with 4% paraformaldehyde in PBS. Brains were carefully removed from the skull, post-fixed overnight in buffered 4% paraformaldehyde at 4 °C and stored in a 30% sucrose solution for 24 h until they sank. Coronal Sections (40 µm thick) of SN were collected using a microtome (Leica, Germany). Immunohistochemistry was performed as previously described [25]. The sections were incubated with the following primary antibodies: rabbit anti-TH (1:1000, Abcam) and horseradish peroxidase-conjugated anti-rabbit IgG (1:5000, Abcam). Slide digitization was used to estimate the TH+ cells in the SN with Pannoramic Scan (3DHISTECH, Budapest, Hungary). And images at ×1 and ×40 magnification were captured.
Biochemical Estimations
Determination of SN and Striatum MDA
The amount of MDA was used as an indirect measure of lipid peroxidation. MDA content was determined in the striatum and SN using a commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) that determined the thiobarbituric acid (TBA) reactivity. The assay was conducted according to the manufacturer’s instructions. The absorbance of samples was determined at 532 nm using a spectrophotometer.
Determination of SN and Striatum SOD and CAT Activity
SOD and CAT activity were determined by SOD and CAT kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocols. The absorbance of samples was determined at 550 nm for SOD and 405 nm for CAT at the end of the reaction on a spectrophotometer.
Statistical Analysis
All statistical analyses were carried out using GraphPad Prism 6.0 software. The data were presented as mean ± standard error of the mean (SEM). The statistical significant difference of rotational behavior test between groups was calculated by t-test (two groups). We used one-way analysis of variance (ANOVA) analysis followed by Sheffè post-hoc test to correct for multiple comparisons (≥3 groups). P values of less than 0.05 were considered statistically significant.
Results
Capsaicin Alleviated Behavioral Deficits and Enhanced Motor Ability
Rats that received apomorphine (1 mg/kg) intraperitoneally exhibited contralateral rotations. Unilateral MFB lesions in rats in the CAP group led to a significant decrease in the number of contralateral rotations compared to rats in the 6-OHDA group (n = 10, P < 0.05). In the open field test, the CAP rats spent a shorter time in the central area (n = 10, P < 0.05), displayed more rearing movements (n = 10, P < 0.05) and traveled over a longer total distance (n = 10, P < 0.05) than the 6-OHDA model group. No differences were found between the SOG and Control groups in the indexes mentioned above, (n = 10, P > 0.05). These results indicate that capsaicin treatment can ameliorate behavioral deficits and enhance motor ability in a rat model of PD (Fig. 1).
Capsaicin alleviated behavioral deficits and enhanced motor ability. a Apomorphine-induced circling in rats in the 6-OHDA and CAP groups, which was measured for 30 min. b–d The open field test results, duration of movement in the central area, number of rearing movements and total distance were measured and analyzed for rats in each group (n = 10). These three indexes showed significant differences between the CAP and 6-OHDA groups and between the 6-OHDA and SOG groups. No differences were observed between the SOG and Control groups. *P < 0.05, NS (no significance, P > 0.05)
TRPV1 Activation by Capsaicin Prevented Degeneration of DA Neurons
TRPV1 expression levels in the rat SN and striatum are shown in Fig. 2. Compared to 6-OHDA rats, a higher level of TRPV1 was observed in the SN (n = 6, P < 0.05) and striatum (n = 6, p < 0.05) of the CAP group. As shown in Fig. 2, the protein expression of TH in the SN and striatum of the CAP rats was higher than that in the 6-OHDA group (n = 6, P < 0.05). No differences in TH expression in the SN and striatum (n = 6, P > 0.05) were found between the SOG and Control groups. The immunostaining results showed that capsaicin increased the number of TH+ cells in the SN of the CAP group compared to the 6-OHDA rats (Fig. 3). These results indicated that capsaicin can alleviate degeneration of DA neurons by activating TRPV1 in vivo.
Capsaicin increases the protein expression of TH in the SN and striatum in the 6-OHDA group. a, c, e Protein levels of TH and TRPV1 in the SN of rats. b, d, f Protein levels of TH and TRPV1 in the striatum. a, b Western blot analyses of the immunoreactive bands of GAPDH, TH and TRPV1 are shown. c, d Decreased TH protein expression was observed in both the SN (c) and striatum (d) in rats from the 6-OHDA group compared to the SOG group, whereas expression of TH was increased in the CAP group compared to the 6-OHDA group. No significant differences were observed in the SN and striatum in the SOG and Control groups. e, f Increased TRPV1 protein expression was observed in both the SN (e) and striatum (f) in rats of the CAP group compared to the 6-OHDA group. No significant differences were observed in the SN and striatum in the SOG and Control groups or in the 6-OHDA and SOG groups. *P < 0.05, NS (no significance, P > 0.05)
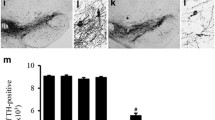
Capsaicin protects against the loss of DA neurons in the SN. a–d Tyrosine hydroxylase (TH) immunohistochemistry of SN pars compacta (SNpc) sections from rats in the Control (a), SOG (b), 6-OHDA (c) and CAP (d) groups. a–d TH+ neurons of SNpc at ×1 magnification. e–h Images were obtained at ×40 magnification from the pane areas shown in images of a, b, c and d respectively, and showed the ipsilateral TH+ neurons. The number of ipsilateral TH+ neurons was significantly higher in the CAP group (h) than in the 6-OHDA group (g), whereas a lower number of TH+ neurons was observed in the SOG (f) and control (e) groups. No significant difference was observed between the SOG (f) and control (e) groups
The Effects of Capsaicin on MDA, CAT and SOD Levels in 6-OHDA-Induced PD Rats
The results indicated that treatment with capsaicin produced a significant (n = 6, P < 0.05) decrease in MDA levels in the right SN and striatum compared to the 6-OHDA rats. Treatment with capsaicin significantly increased CAT (n = 6, P < 0.05) and SOD (n = 6, P < 0.05) activity in the right SN and striatum compared to 6-OHDA rats. In the left SN and striatum, these four experimental indexes exhibited no differences between model rats treated with capsaicin and 6-OHDA rats (n = 6, P > 0.05). Similarly, no differences in these four experiment indexes were observed between the SOG and Control groups in the right or left SN and striatum (Fig. 4).
Capsaicin increased the levels of MDA, CAT and SOD in 6-OHDA-induced PD rats. SOD (a, b), CAT (c, d) and MDA (e, f) were measured in both the ipsilateral (a, c, e) and contralateral hemispheres (b, d, f). In the ipsilateral hemisphere, lower SOD levels (a), lower CAT levels (c) and higher MDA (e) levels were observed in the 6-OHDA group than in the SOG group, whereas these three markers showed opposite trends in the CAP group to those in the 6-OHDA group. No significant differences in the CAP and 6-OHDA groups or in the 6-OHDA and SOG groups were observed in the contralateral hemispheres. No significant differences were found in the SOG and Control groups in the ipsilateral or contralateral hemispheres. *P < 0.05, NS (no significance, P > 0.05)
Discussion
The present work indicates that capsaicin might have potential neuroprotective effect on PD and rescues dopamine neurons in midbrain. Our study identified that the protective effects of capsaicin are mediated via activation of TRPV1 and reducing oxidative stress. In this study, we observed that in 6-OHDA-induced rat models of PD, rats presented motor deficits, including apomorphine-induced rotations and abnormal behaviors in the open-field test. Consistent with this result, the expression levels of TH in the SN and striatum decreased significantly. Furthermore, the antioxidant system was also involved in, which included the downregulation of SOD and CAT and a higher MDA level. Interestingly, if capsaicin, an agonist of the TRPV1 receptor, was applied immediately after injection with 6-OHDA, rats presented less severe behavioral deficits and higher levels of TH and TRPV1, accompanying by reducing of oxidative stress.
In 6-OHDA lesioned rat models of PD, rats exhibited contralateral rotations after administration of apomorphine (1 mg/kg), indicating that rat models of PD had been established successfully. The open field test was conducted to evaluate locomotor activity. We found that rats in 6-OHDA group showed longer time in the central area, displayed less rearing movements and traveled over a shorter total distance, which were in parallel with the current study [23]. In contrast, rats in CAP group (capsaicin treated group) showed a significant decrease in the number of contralateral rotations and better locomotor activities in the open field test. These results indicated that capsaicin significantly alleviated behavioral deficits and enhanced motor ability in a rat model of PD. Previous studies have revealed that TRPV1, a receptor of capsaicin, might be a therapeutic target to ameliorate levodopa-induced dyskinesia [26, 27], however, the underlying mechanism needs to be further elucidated.
We demonstrated that the expression of TH significantly decreases in both the SN and striatum in 6-OHDA lesioned rats. Furthermore, we observed that capsaicin administration increased the protein expression of TH. In addition, TRPV1, a receptor of capsaicin, was examined by Western blot analysis of the SN and striatum in capsaicin-treated 6-OHDA lesioned rats. We investigated a higher level of protein expression both in SN and striatum in consistent with that of TH and alleviation of behavioral deficits. Therefore, we hypothesized that TRPV1 played a vital role in protecting against DA cell death. Although accumulating evidence has suggested that TRPV1 involvement in the neuroprotection of PD is complex and remains unclear, in vitro and in vivo research all indicates that activation of TRPV1 mediates cell death of mesencephalic DA neurons [28,29,30]. Furthermore, another in vivo study showed neuroprotective effects against the excitotoxicity of TRPV1 activated by endogenous cannabinoid anandamides in rats [18]. The controversial effects of TRPV1 on cell survival might be dependent on the dosage of agonists used and the method of capsaicin administration [19]. We observed that capsaicin at the doses of 1 mg/kg for 7 days alleviated degeneration of DA neurons by activating TRPV1 in vivo.
The current study shows that oxidative stress is one of the key mechanism in the progression of PD [8]. In this study, rats in the 6-OHDA group presented abnormalities of antioxidants system, resulting in a significant rise in MDA levels and decrease in the activities of anti-oxidant enzymes, including SOD and CAT. On the other hand, we observed that capsaicin decreased the levels of MDA and increased the activities of SOD and CAT. Thus, the antioxidants system was effectively enhanced by capsaicin. An previous in vitro study [31] showed that capsaicin enhanced neuroprotection by upregulating antioxidant enzymes. Our in vivo experiments also demonstrated antioxidative properties of capsaicin. Interestingly, oxidative stress measurements showed no significant differences in the contralateral hemisphere in 6-OHDA lesioned rats regardless of whether capsaicin treatment was given. This result suggests that the reactive antioxidant system might be enhanced by the activation of TRPV1 in only certain situations, such as the toxicity induced by 6-OHDA.
Taken together, we investigated the neuroprotective role of capsaicin in a 6-OHDA-induced rat model of PD and found that capsaicin led to DA cell survival and behavioral recovery. In addition, greater expression of TRPV1 in both the SN and striatum in capsaicin-treated rats was observed, accompanied by activation of the reactive antioxidant system in the ipsilateral hemisphere. Although 6-OHDA is a relevant tool to mimic the symptoms of PD, it does not show the complete pathophysiology of PD. Thus, future research with capsaicin in genetic models is needed.
Conclusion
This study shows that TRPV1 activated by capsaicin could protect against DA neuron loss and enhance TH enzyme activity, which suggests that TRPV1 is a promising therapeutic target for alleviating the progression of PD. We demonstrated that TRPV1 activation by capsaicin protects against loss of DA neurons and promotes behavioral recovery in the 6-OHDA rat model of PD by inhibiting oxidative stress.
References
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912
Athauda D, Foltynie T (2015) The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol 11:25–40
Lindholm D, Makela J, Di Liberto V, Mudo G, Belluardo N, Eriksson O, Saarma M (2016) Current disease modifying approaches to treat Parkinson’s disease. Cell Mol Life Sci 73:1365–1379
Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol 7:97–109
Subramaniam SR, Chesselet MF (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 106–107:17–32
Bove J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2:484–494
Wachter B, Schurger S, Rolinger J, von Ameln-Mayerhofer A, Berg D, Wagner HJ, Kueppers E (2010) Effect of 6-hydroxydopamine (6-OHDA) on proliferation of glial cells in the rat cortex and striatum: evidence for de-differentiation of resident astrocytes. Cell Tissue Res 342:147–160
Lim JL, Wilhelmus MM, de Vries HE, Drukarch B, Hoozemans JJ, van Horssen J (2014) Antioxidative defense mechanisms controlled by Nrf2: state-of-the-art and clinical perspectives in neurodegenerative diseases. Arch Toxicol 88:1773–1786
Ozsoy O, Yildirim FB, Ogut E, Kaya Y, Tanriover G, Parlak H, Agar A, Aslan M (2015) Melatonin is protective against 6-hydroxydopamine-induced oxidative stress in a hemiparkinsonian rat model. Free Radic Res 49:1004–1014
Baker K, Raemdonck K, Dekkak B, Snelgrove RJ, Ford J, Shala F, Belvisi MG, Birrell MA (2016) Role of the ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), in allergic asthma. Respir Res 17:67
Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG (2014) Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol 171:2593–2607
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Reyes-Escogido Mde L, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E (2011) Chemical and pharmacological aspects of capsaicin. Molecules 16:1253–1270
Immke DC, Gavva NR (2006) The TRPV1 receptor and nociception. Semin Cell Dev Biol 17:582–591
Gunthorpe MJ, Szallasi A (2008) Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des 14:32–41
Maiese K (2014) “Tripping out” with the TRP superfamily and TRPV1 for novel neuroprotection. Curr Neurovasc Res 11:91–93
Veldhuis WB, van der Stelt M, Wadman MW, van Zadelhoff G, Maccarrone M, Fezza F, Veldink GA, Vliegenthart JF, Bar PR, Nicolay K, Di Marzo V (2003) Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J Neurosci 23:4127–4133
Razavinasab M, Shamsizadeh A, Shabani M, Nazeri M, Allahtavakoli M, Asadi-Shekaari M, Esmaeli-Mahani S, Sheibani V (2013) Pharmacological blockade of TRPV1 receptors modulates the effects of 6-OHDA on motor and cognitive functions in a rat model of Parkinson’s disease. Fundam Clin Pharmacol 27:632–640
Starowicz K, Cristino L, Di Marzo V (2008) TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr Pharm Des 14:42–54
Carvalho MM, Campos FL, Coimbra B, Pego JM, Rodrigues C, Lima R, Rodrigues AJ, Sousa N, Salgado AJ (2013) Behavioral characterization of the 6-hydroxidopamine model of Parkinson’s disease and pharmacological rescuing of non-motor deficits. Molecular Neurodegener 8:14
Qin X, Han W, Yu Z (2012) Neuronal-like differentiation of bone marrow-derived mesenchymal stem cells induced by striatal extracts from a rat model of Parkinson’s disease. Neural Regen Res 7:2673–2680
Zhang YM, Zhang L, Wang Y, Sun YN, Guo Y, Du CX, Zhang J, Yao L, Yu SQ, Liu J (2016) Activation and blockade of prelimbic 5-HT6 receptors produce different effects on depressive-like behaviors in unilateral 6-hydroxydopamine-induced Parkinson’s rats. Neuropharmacology 110:25–36
Choi SH, Joe EH, Kim SU, Jin BK (2003) Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J Neurosci 23:5877–5886
Park ES, Kim SR, Jin BK (2012) Transient receptor potential vanilloid subtype 1 contributes to mesencephalic dopaminergic neuronal survival by inhibiting microglia-originated oxidative stress. Brain Res Bull 89:92–96
Morgese MG, Cassano T, Cuomo V, Giuffrida A (2007) Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson’s disease: role of CB(1) and TRPV1 receptors. Exp Neurol 208:110–119
Gonzalez-Aparicio R, Moratalla R (2014) Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson’s disease. Neurobiol Dis 62:416–425
Kim SR, Lee DY, Chung ES, Oh UT, Kim SU, Jin BK (2005) Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J Neurosci 25:662–671
Di Marzo V, Bisogno T, De Petrocellis L (2001) Anandamide: some like it hot. Trends Pharmacol Sci 22:346–349
Shin CY, Shin J, Kim BM, Wang MH, Jang JH, Surh YJ, Oh U (2003) Essential role of mitochondrial permeability transition in vanilloid receptor 1-dependent cell death of sensory neurons. Mol Cell Neurosci 24:57–68
Lee JG, Yon JM, Lin C, Jung AY, Jung KY, Nam SY (2012) Combined treatment with capsaicin and resveratrol enhances neuroprotection against glutamate-induced toxicity in mouse cerebral cortical neurons. Food Chem Toxicol 50:3877–3885
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30970990) and Science and Technology Planning Project of Shandong Province (2014GSF118024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhao, Z., Wang, J., Wang, L. et al. Capsaicin Protects Against Oxidative Insults and Alleviates Behavioral Deficits in Rats with 6-OHDA-Induced Parkinson’s Disease via Activation of TRPV1. Neurochem Res 42, 3431–3438 (2017). https://doi.org/10.1007/s11064-017-2388-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2388-4