Abstract
Justicidin A is a structurally defined arylnaphthalide lignan, which has been shown anti-cancer activity; however, the neuroprotective effect of justicidin A is still untested. In this study, we investigated the action of justicidin A on amyloid beta (Aβ)25–35-induced neuronal cell death via inhibition of the hyperphosphorylation of tau and induction of autophagy in SH-SY5Y cells. Pretreatment with justicidin A significantly elevated cell viability in cells treated with Aβ25–35. Western blot data demonstrated that justicidin A inhibited the Aβ25–35-induced up-regulation the levels of hyperphosphorylation of tau in SH-SY5Y cells. In addition, treatment with justicidin A significantly induced autophagy as measured by the increasing LC3 II/I ratio, an important autophagy marker. These studies showed that justicidin A inhibited activity of glycogen synthase kinase-3beta (GSK-3β), which is an important kinase in up-stream signaling pathways; inhibited hyperphosphorylation of tau in AD; and enhanced activity of AMP-activated protein kinase (AMPK), which is the key molecule for both hyperphosphorylation of tau and induction of autophagy. These data provide the first evidence that justicidin A protects SH-SY5Y cells from Aβ25–35-induced neuronal cell death through inhibition of hyperphosphorylation of tau and induction of autophagy via regulation the activity of GSK-3β and AMPK, and they also provide some insights into the relationship between tau protein hyperphosphorylation and autophagy. Thus, we conclude that justicidin A may have a potential role for neuroprotection and, therefore, may be used as a therapeutic agent for AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the progressive loss of neurons and production of amyloid beta (Aβ). There are two major markers for AD: extracellular plaques consisting of Aβ fibrils and intracellular deposits of neurofibrillary tangles (NFTs) composed of the hyperphosphorylated form of tau, a microtubule-binding protein [1, 2]. Specifically, hyperphosphorylation of tau protein is proposed to be an early event in the evolution of AD, and it may play an important role in Aβ-induced neurodegeneration. In normal physiological processes, tau protein binds to microtubules, thereby stimulating polymerization and promoting stabilization; however, accumulation of Aβ can cause tau hyperphosphorylation, which reduces its ability to bind to microtubules and leads to aggregation of tau protein. Therefore, reduction of tau hyperphosphorylation or removal of aggregated tau protein will be useful treatments for curing AD.
Justicidin A is an arylnaphthalide lignan (Fig. 1a) and has been reported to suppress in vitro growth of several tumor cell lines [3, 4]. Nevertheless, no information is available regarding the neuroprotective effects of justicidin A against the pathogenesis of AD, although the bioactivities of justicidin A are known. However, it is noteworthy that justicidin A has shown anti-cancer effects mainly through inducing autophagic flux, thus enhancing apoptosis, in cancer cells [4]. Autophagy has been reported to have dual roles, both cell-destructive and cell-protective. The beneficial roles of autophagy in the nervous system are mainly associated with maintaining the normal balance between the formation and degradation of cellular proteins, as inactivation of the autophagy-lysosomal system could lead to the accumulation of tau [5]. Therefore, it is necessary to ascertain whether justicidin A exerts neuroprotective effects by inducing autophagy.
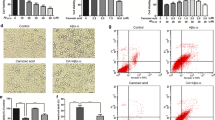
a Chemical structure of justicidin A. Protective effect of justicidin A on Aβ25–35-induced cytotoxicity in SH-SY5Y cells. b Cells were treated with the indicated concentrations of justicidin A alone for 24 h, and cell viability was then detected using the MTT assay. c Cells were pre-treated with the indicated concentrations of justicidin A for 30 min and then treated with 30 μM Aβ25–35 for 24 h. d SH-SY5Y cells were pre-treated with 30 μM Aβ25–35 for 2 h and then treated with justicidin A (62.5, 125, and 250 nM) for 24 h. Data are presented as the means ± SEM (n = 3). ***p < 0.001 compared with the control group. ## p < 0.01 and ### p < 0.001 compared with the Aβ25–35-treated group
Moreover, in neurons, the products of degradation are transported from the axon to the cell body through retrograde transport [6]. Thus, it seems likely that the nature and regulation of retrograde axonal organelle transport are central to the regulation of the autophagic-lysosomal pathway of protein degradation in neurons. In addition, microtubules which are stabilized by tau protein, provide platforms for intracellular transport. Thus, there may be a potential relationship between tau hyperphosphorylation and the process of autophagy. We therefore examined whether justicidin A inhibits the hyperphosphorylation of tau in Aβ25–35-induced neuronal cell death in SH-SY5Y cells.
Materials and Methods
Chemicals and Reagents
Justicidin A was synthesized at AK Scientific Inc. (Union, CA, USA). Aβ25–35, okadaic acid (OA), lithium chloride (LiCl, a potent GSK-3β inhibitor), 3-Methyladenine (3-MA) and Rabbit anti-phospho-tau (pSer199/202) antibody were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Hyclone (Logan, UT, USA). Penicillin/streptomycin mixture and 0.25 % trypsin–EDTA were obtained from GIBCO–BRL (Grand Island, NY, USA). Rabbit anti-phospho-tau (Ser396) and Rabbit anti-phospho-tau (Ser404) antibodies were purchased from Abcam (Cambridge, MA, USA). Rabbit anti-phospho-AMPK, rabbit anti-AMPK, rabbit anti-SQSTM1/p62, rabbit anti-LC3B, rabbit anti-phospho-GSK-3β (Ser9), rabbit anti-GSK-3β, GAPDH, rabbit anti-phospho-tau (Ser202), and anti-rabbit horseradish peroxidase (HRP)-linked IgG antibodies were purchased from Cell Signaling Technology Inc. (Boston, MA, USA). Rabbit anti-tau antibody was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). All other chemicals were of analytical grade.
Cell Culture and Treatment
SH-SY5Y cells were grown in DMEM supplemented with 10 % heat-inactivated FBS and 1 % penicillin/streptomycin at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. Aβ25–35 was dissolved in deionized distilled water at 1 mM as a stock solution and stored at -80 °C. The stocks were diluted to the desired final concentrations in treatment medium prior to use.
Measurement of Cell Viability
Cell viability was measured using an EZ-Cytox kit (DAEILLAB Co, Ltd, Seoul, Republic of Korea) according to the manufacturer’s instructions. SH-SY5Y cells were incubated at 37 °C with 30 μM of Aβ25–35 for the indicated time with or without justicidin A pre-treatment and then incubated with EZ-Cytox solution for 1 h at 37 °C. The absorbance at 450 nm was measured with a microplate reader (BIO-TEK® Dower Wave XS, Winooski, VT, USA). The results are expressed as the percentage of MTT reduction relative to the absorbance of control cells.
Western Blot Analysis
Treatment of SH-SY5Y cells and preparation of total cell lysates were performed as described previously [7]. Proteins (20 μg) were separated on 8–15 % SDS–polyacrylamide gels, transferred onto polyvinylidene difluoride (PVDF) transfer membranes and blocked with 3 % BSA or 5 % skim milk. Membranes were incubated with the primary antibody, followed by HRP-linked secondary antibody, and detected with enhanced chemiluminescence ECL reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Immunofluorescence Assay
To detect expression of phosphorylated tau and total tau, SH-SY5Y cells were seeded on sterile coverslips placed on 24-well culture plates. After treatment with the indicated drugs for 24 h, cells were washed with PBS and fixed with 3.8 % paraformaldehyde for 20 min, permeabilized with 0.2 % Triton X-100 for 30 min, blocked with 5 % BSA in PBS for 1 h, incubated with primary antibodies for 2 h, and incubated with secondary antibodies labeled with Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature in the dark place. This was followed by incubation with of 4′,6-diamidino-2-phenylindole (DAPI) at 37 °C for 30 min in the dark. The coverslips were then prepared with one drop of Pro-Long Gold Antifade Reagent (Invitrogen) and sealed to slides. Images were obtained using a Leica TCS SP5 confocal microscope (Leica, Mannheim, Germany).
Statistical Analysis
Data were analyzed with Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, USA) and are expressed as the mean ± SEM Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by the Newman–Keuls test. Statistical significance was set at p < 0.05.
Results
Effect of Justicidin A Against Aβ25–35-Induced Cytotoxicity in SH-SY5Y Cells
To detect the effect of justicidin A on Aβ25–35-induced cytotoxicity, cells were treated with justicidin A at various concentrations (62.5, 125, 250, and 500 nM). Justicidin A did not show any significant cytotoxic effect (Fig. 1b). To test the neuroprotective effect of justicidin A, SH-SY5Y cells were pre-treated with the indicated concentrations of justicidin A for 30 min and then treated with 30 μM Aβ25–35 for 24 h. Only 74 ± 1.15 % of the cells survived after treatment with 30 μM Aβ25–35 for 24 h, and cell viability in justicidin A-treated groups increased to 87 ± 3.13, 89 ± 2.49, and 98 ± 0.83 % at concentrations of 62.5, 125, and 250 nM, respectively (Fig. 1c). In addition, when SH-SY5Y cells were pre-treated with 30 μM Aβ25–35 for 2 h and then treated with justicidin A (62.5, 125, and 250 nM) for 24 h, 77 ± 0.58 % of SH-SY5Y cells survived after treatment with 30 μM Aβ25–35 for 24 h, and in the justicidin A post-treatment groups, the cell viability was significantly recovered to 89 ± 0.89, 97 ± 1.33 and 103 ± 2.08 % of control values (Fig. 1d).
Effect of Justicidin A on Aβ25–35-Induced Hyperphosphorylation of Tau in SH-SY5Y Cells
To evaluate the possible involvement of justicidin A in Aβ25–35-induced hyperphosphorylation of tau, cells were treated with Aβ25–35 in the presence or absence of justicidin A. Treatment with 30 μM Aβ25–35 significantly increased the levels of hyperphosphorylation of tau at the sites Ser202, Ser396 and Ser404 (Fig. 2a). However, this increased hyperphosphorylation of tau was significantly and dose-dependently inhibited by pre-treatment with justicidin A at 62.5, 125, and 250 nM. We also monitored the alteration of tau hyperphosphorylation via cellular immunofluorescence staining. As shown in Fig. 2b, increased staining for phosphorylated tau at Ser202, Ser396 and Ser404 were detected upon Aβ25–35 treatment, with no change in total tau immunoreactivity. This staining pattern for hyperphosphorylated tau was also reversed after pretreatment with justicidin A at 62.5, 125, and 250 nM, consistent with the western blotting results.
Justicidin A attenuated Aβ25–35-induced hyperphosphorylation of tau in SH-SY5Y cells. a Cells were pre-treated with the indicated concentrations of justicidin A for 30 min and then treated with 30 μM Aβ25–35 for 24 h. Levels of phosphorylation of tau (at Ser202, Ser396 and Ser404) and levels of GAPDH were evaluated using western blot analysis. b Immunofluorescence staining of phosphorylated tau protein (at Ser202, Ser396 and Ser404) and total tau (bar 20 μm). Data are presented as the means ± SEM (n = 3). **p < 0.01 compared with the control group. ## p < 0.01 compared with the Aβ25–35-treated group
Effect of justicidin A on OA-induced hyperphosphorylation of tau in SH-SY5Y cells
Okadaic acid, a widely used reagent in studies of tau protein hyperphosphorylation, was used as a positive control in this study [8, 9]. Cells were treated with OA in the presence or absence of justicidin A, and tau hyperphosphorylation was measured using western blot analysis. Treatment with 15 nM OA significantly increased the levels of hyperphosphorylation of tau at Ser202, Ser396 and Ser404 (Fig. 3). However, the increased hyperphosphorylation of tau was significantly and dose-dependently inhibited by pre-treatment with justicidin A at 62.5, 125, and 250 nM.
Justicidin A attenuated OA-induced hyperphosphorylation of tau in SH-SY5Y cells. Cells were pre-treated with the indicated concentrations of justicidin A for 30 min and then treated with 15 nM OA for 6 h. Levels of tau phosphorylation (at Ser202, Ser396 and Ser404) and levels of GAPDH were evaluated using western blot analysis. Data are presented as the means ± SEM (n = 3). **p < 0.01 and ***p < 0.001 compared with the control group. # p < 0.05, ### p < 0.001 compared with the OA-treated group
Effect of Justicidin A on Aβ25–35-Induced Phosphorylation Levels of GSK-3β and AMPK in SH-SY5Y Cells
We examined the levels of phosphorylation of GSK-3β and AMPK, which are possible up-stream components in the signaling pathway involved in Aβ25–35-induced tau hyperphosphorylation [10, 11]. Treatment with 30 μM Aβ25–35 significantly decreased the level of GSK-3β phosphorylation at Ser9 compared to the control group (Fig. 4a). Conversely, the Aβ25–35-induced decrease in phosphorylation of GSK-3β was significantly inhibited by pre-treatment with justicidin A at 62.5, 125, and 250 nM. In contrast, treatment with 30 μM Aβ25–35 significantly increased the level of AMPK phosphorylation compared to the control group. Furthermore, pre-treatment with justicidin A at 62.5, 125, or 250 nM significantly stimulated the phosphorylation of AMPK.
Justicidin A attenuated hyperphosphorylation of tau by inhibiting GSK-3β activity and enhancing AMPK activity. a Cells were pre-treated with the indicated concentrations of justicidin A for 30 min and then treated with 30 μM Aβ25–35 for 24 h. Levels of phosphorylation of GSK-3β and AMPK were evaluated using western blot analysis. b Cells were pre-treated with 5 mM LiCl or justicidin A for 30 min and then treated with 30 μM Aβ25–35 for 24 h. Levels of phosphorylation of tau (at Ser202, Ser396 and Ser404) and levels of GAPDH were evaluated using western blot analysis. c Cells were pre-treated with 5 mM LiCl or justicidin A for 30 min, treated with 30 μM Aβ25–35 for 24 h, and then evaluated using an MTT assay. Data are presented as the means ± SEM (n = 3). *p < 0.05, **p < 0.01 and ***p < 0.001 compared with the control group. # p < 0.05, ## p < 0.01 and ### p < 0.001 compared with the Aβ25–35-treated group
LiCl, a potent GSK-3β inhibitor, was used as a positive control. As shown in Fig. 4b, LiCl also significantly inhibited tau phosphorylation at Ser202, Ser396, and Ser404. In addition, LiCl significantly increased cell viability in Aβ-treated SH-SY5Y cells, but co-treatment with LiCl and justicidin A did not further increase the cell viability compared with LiCl treatment alone (Fig. 4c).
TDZD-8, another specific GSK-3β inhibitor, was used in this study as a positive control. TDZD-8 significantly increased the level of GSK-3β phosphorylation at Ser9 (Fig. 5a), with an effect as strong as that of justicidin A. Further, the MTT assay showed that TDZD-8 significantly increased cell viability in Aβ-treated SH-SY5Y cells, but co-treatment with TDZD-8 and justicidin A did not further increase the cell viability compared with TDZD-8 treatment alone (Fig. 5b). Immunofluorescence staining revealed increased staining for phosphorylated tau (at Ser202, Ser396 and Ser404), with no change in total tau immunoreactivity, upon Aβ25–35 treatment, and this hyperphosphorylated tau staining pattern was also reversed after pretreatment with justicidin A or TDZD-8 (Fig. 5c).
Justicidin A attenuated the hyperphosphorylation of tau by inhibiting GSK-3β activity. a Cells were pre-treated with 5 μM TDZD-8 or justicidin A for 30 min and then treated with 30 μM Aβ25–35 for 24 h. Levels of phosphorylation and total GSK-3β were evaluated using western blot analysis. b Cells were pre-treated with 5 μM TDZD-8 or justicidin A for 30 min and then treated with 30 μM Aβ25–35 for 24 h, and cell viability was evaluated using an MTT assay. c Immunofluorescence staining of phosphorylated tau protein (at Ser202, Ser396 and Ser404) and total tau (bar 20 μm). Data are presented as the means ± SEM (n = 3). ***p < 0.001 compared with the control group. ## p < 0.01 and ### p < 0.001 compared with the Aβ25–35-treated group
Effect of Justicidin A on Autophagy-Mediated Signaling Protein Levels in SH-SY5Y Cells
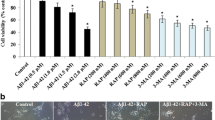
To evaluate the autophagic effects of justicidin A on SH-SY5Y cells under basal conditions, cells were treated with justicidin A at 62.5, 125, and 250 nM. The results revealed that justicidin A significantly increased the value of the LC3 II/I ratio in a dose-dependent manner, indicating that autophagy was induced by justicidin A (Fig. 6a). Treatment with justicidin A at concentrations of 62.5, 125, and 250 nM also significantly and dose-dependently increased the phosphorylation of AMPK, which is upstream of autophagy (Fig. 6a). In addition to LC3, p62/SQSTM1 is also possible to use as a marker for autophagy, since P62 itself is removed from the cytoplasm mainly by autophagy, it recognize toxic cellular waste, becomes incorporated into the completed autophagosome and is degraded in autolysosomes, its amount is generally considered to inversely correlate with autophagic activity [12]. As expected, justicidin A down-regulated p62 expression significantly and dose-dependently in Fig. 6a. Autophagy is a mechanism of cell homeostasis that becomes activated under stress conditions (nutrient deprivation, infection, toxins), but it is a short-term process because it is self-perpetuated within the cell. Therefore, continuous autophagy should be induced to protect cells from damage. We next evaluated the autophagic effects of justicidin A on SH-SY5Y cells under pathological conditions. As expected, co-treatment with justicidin A in both pre- and post-treatment with Aβ25–35 significantly increased the expression levels of LC3 II and P-AMPK compared with Aβ25–35 treatment alone, and there were no significant differences between the pre-treatment and post-treatment groups (Fig. 6b). These data indicate that the autophagy-inducing effect of justicidin A is a continuous and independent process. 3-Methyladenine (3-MA), a potent autophagy inhibitor, inhibits autophagy by blocking autophagosome formation via the inhibition of class Ш PI3 K [13]. 3-MA and justicidin A did not show any significant cytotoxic in SH-SY5Y cells, respectively. Justicidin A significantly increased cell viability in Aβ-treated SH-SY5Y cells, but this increase was reversed by co-treatment with 3-MA.These data indicate that justicidin A protects the cell death induced by Aβ25–35 through induction of autophagy (Fig. 6c).
Justicidin A induced autophagy by enhancing AMPK and reducing p62. a Cells were treated with the indicated concentrations of justicidin A for 24 h; levels of LC3, AMPK and p62 were evaluated using western blot analysis. b Cells were pre-treated with 250 nM justicidin A for 30 min, treated with 30 μM Aβ25–35 for 24 h, pre-treated with 30 μM Aβ25–35 for 2 h, and then treated with 250 nM justicidin A for 24 h, or only treated with justicidin A for 24 h. LC3 and AMPK levels were evaluated using western blot analysis. c Cells were pre-treated with justicidin A or co-treated with 5 mM 3-MA for 30 min, treated with 30 μM Aβ25–35 for 24 h, and then evaluated using an MTT assay. Data are presented as the means ± SEM (n = 3). *p < 0.05, **p < 0.01 and ***p < 0.001 compared with the control group. ## p < 0.01 and ### p < 0.001 compared with the Aβ25–35-treated group. $$ p < 0.01 compared with the Aβ25–35 and justicidin A treated group
Discussion
In the present study, we demonstrated for the first time that justicidin A exerts protective effects in a cellular model of AD induced by Aβ25–35. Neuroprotection by justicidin A has not previously been investigated in any models of neurodegenerative disease. Therefore, we investigated the possible molecular mechanism underlying the neuroprotective effect of justicidin A against Aβ25–35-induced cell death in SH-SY5Y cells.
Aβ is one of the main markers of AD, and it can induce axonopathy and neuronal apoptosis both in brain tissue and in neuronal cells [14]. The axonal disruption induced by Aβ is strongly related to the hyperphosphorylation and dissociation of the microtubule-associated protein tau. Previous studies demonstrated that axonopathy, not apoptosis, is an early marker of Aβ neurotoxicity [15], and cells may undergo apoptosis due to the removal of a trophic factor. In this study, we treated SH-SY5Y cells with 30 μM Aβ25–35 to induce cell death, and this treatment also successfully induced tau protein hyperphosphorylation. Justicidin A significantly prevented and/or ameliorated neuronal cell death both as a pre-treatment and as a post-treatment (Fig. 1).
The serine (S) 202 residue is located in the proline-rich region, and it is phosphorylated in fetal human brain, becomes dephosphorylated around birth, and remains unphosphorylated throughout life under normal circumstances. The abnormal phosphorylation of tau at Ser202 in AD thus recapitulates the normal phosphorylation during development [16]. In addition, the formation of intracellular deposits of NFTs probably occurs in several steps: pre-NFT, intra-neuronal NFT, and extra-neuronal NFT. One study showed that at all of these stages, tau was phosphorylated at Ser396 [17]. Therefore, to confirm the effect of justicidin A in attenuating the hyperphosphorylation of tau, it was necessary to detect tau phosphorylation at Ser202, Ser396, and Ser404. As expected, in our study, justicidin A significantly attenuated Aβ25–35-induced hyperphosphorylation of tau at Ser202, Ser396, and Ser404 in a dose-dependent manner (Fig. 2).
Recent studies have suggested that synthetic Aβ peptides and Aβ peptides isolated from the brains of AD patients promote tau phosphorylation [18–20]. Aβ peptides may interact directly or indirectly with neurons to induce tau hyperphosphorylation. In the direct process, Aβ peptides bind to the receptors, thus disturbing the balance of tau kinase and phosphatase activity in the affected neurons [21]. Although these observations suggest pathophysiological roles for Aβ peptides in AD pathogenesis, it remains unclear how Aβ induces the hyperphosphorylation of tau in AD brains [22]. It is difficult to anticipate whether justicidin A regulates tau phosphorylation through a non-specific pathway or through a specific molecular interaction, and this will be tested in future studies. OA, an inhibitor of protein phosphatases 1 (PP1) and 2A (PP2A) that directly increases the level of tau phosphorylation in cultured cells [23] and in situ [24], was used as a positive control to induce hyperphosphorylation of tau. Justicidin A significantly decreased the level of OA-induced hyperphosphorylation of tau, as expected (Fig. 3).
One protein kinase that has garnered significant attention as a “tau kinase” is GSK-3β [22], which is a constitutively active serine/threonine kinase and has been implicated in AD pathobiology. GSK-3β phosphorylates tau at select epitopes, increasing the propensity for the formation of oligomeric tau [25]. Phosphorylation of GSK-3β at Ser9 leads to inactivation of the enzyme, thereby reducing tau phosphorylation. Both LiCl and TDZD-8 inhibit hyperphosphorylation of tau by inhibiting GSK-3β activity [10], and they were used as positive controls for GSK-3β. As expected, justicidin A significantly inhibited the GSK-3β activity by increasing the phosphorylation of GSK-3β at Ser9 (Fig. 4a). LiCl significantly attenuates the Aβ25–35-induced hyperphosphorylation of tau to the same degree as justicidin A (Fig. 4b). Meanwhile, both justicidin A and TDZD-8 significantly inhibited GSK-3β activity in Aβ25–35-treated SH-SY5Y cells (Fig. 5a), and immunofluorescence showed that justicidin A and TDZD-8 attenuated the Aβ25–35-induced hyperphosphorylation of tau (Fig. 5c). Additionally, like justicidin A, both LiCl and TDZD-8 significantly protected cells from Aβ25–35-induced neuronal cell death, but co-treatment with justicidin A did not show additional protection (Fig. 4c, 5b). Notably, GSK-3β is the main molecule involved in Aβ- and OA-induced tau hyperphosphorylation [26, 27]. These findings indicated that justicidin A may decrease the hyperphosphorylation of tau by inhibiting GSK-3β activity.
AMPK may be activated by exposure of cells to Aβ, and our results were consistent with this hypothesis (Fig. 4a). It is unclear how AMPK regulates the phosphorylation of tau. AMPK is a tau kinase that can phosphorylate tau on a number of sites, altering the binding between tau and microtubules [28]. However, there are also many reports that AMPK can inhibit the hyperphosphorylation of tau. In a subsequent study, the same authors revealed that AMPK was able to inhibit GSK-3β activity and thus prevent the phosphorylation of tau protein [29]. Our results are consistent with the latter mechanism; justicidin A significantly increased the level of AMPK phosphorylation in a dose-dependent manner (Fig. 4a). It seems that the effect of AMPK on phosphorylation of tau is context-dependent; that is, under certain conditions, AMPK can directly phosphorylate tau, but in other contexts, AMPK can inhibit tau phosphorylation and aggregation through the activation of sirtuin 1 (SIRT1) and the inhibition of GSK-3β [30]. In additional, AMPK and GSK-3β kinases signaling are also the major regulators capable of activating autophagy [31–33]. LiCl, a potent GSK-3β inhibitor, which can induce autophagy to enhance clearance of mutant proteins [34, 35]. Meanwhile, AMPK promotes autophagy by directly activating Ulk1 through phosphorylation of Ser317 and Ser777 [36]. Since Justicidin A inhibited GSK-3β and increased the level of AMPK phosphorylation. Therefore, we investigated the effect of justicidin A on the induction of autophagy: SH-SY5Y cells were treated with various concentrations of justicidin A and incubated for 24 h. As expected, justicidin A increased the protein expression of LC3 II, a marker of autophagy, as well as the phosphorylation of AMPK, in a dose-dependent manner (Fig. 6a). Because autophagy is known to be activated by many types of stimulation, treating cells with Aβ25–35 before or after justicidin A treatment may influence the ability of justicidin A to induce autophagy. In our further experiment, justicidin A was applied before or after Aβ25–35 treatment, and cells were collected after 24 h of incubation. As shown in Fig. 6b, both pre- and post-treatment justicidin A with Aβ25–35 significantly increased the expression levels of LC3 II and the phosphorylation of AMPK, and there were no significant differences between this two groups. These results show that autophagy induced by justicidin A is not related to Aβ25–35. In addition, justicidn A co-treatment with 3MA attenuated its protective effect in Aβ-treated SH-SY5Y cells. Interestingly, our results show no induction of autophagy upon Aβ25–35 treatment, which has been reported to induce autophagy in other circumstances. However, it should be noted that amyloid-induced autophagy is a short-term process because it is self-perpetuated within the cell. In such experiments, amyloid-induced autophagy was most abundant after 4 h of incubation, but after that, the increase in autophagy faded with increased incubation time [37]. In this study, we detected the induction of autophagy after 24 h of incubation with Aβ25–35. It is therefore more relevant that justicidin A induced autophagy after a long incubation period.
Conclusion
In conclusion, justicidin A protected SH-SY5Y cells from neuronal cell death produced by Aβ25–35 by inducing autophagy, and it significantly attenuated the Aβ25–35-induced hyperphosphorylation of tau at Ser202, Ser396 and Ser404 through the phosphorylation of GSK-3β and AMPK. These results indicate that justicidin A inhibited the tau hyperphosphorylation induced by Aβ25–35. Taken together, the results presented here increase our knowledge about the neuroprotective effect of justicidin A, and suggest this compound as a candidate for the development of therapeutic agents for Alzheimer’s disease and other tau pathology-related neuronal degenerative diseases.
References
Holscher C (2005) Development of beta-amyloid-induced neurodegeneration in Alzheimer’s disease and novel neuroprotective strategies. Rev Neurosci 16(3):181–212
Yoon S-S, AhnJo S-M (2012) Mechanisms of amyloid-β peptide clearance: potential therapeutic targets for Alzheimer’s disease. Biomol Ther 20(3):245–255. doi:10.4062/biomolther.2012.20.3.245
Su CL, Huang LL, Huang LM, Lee JC, Lin CN, Won SJ (2006) Caspase-8 acts as a key upstream executor of mitochondria during justicidin A-induced apoptosis in human hepatoma cells. FEBS Lett 580(13):3185–3191. doi:10.1016/j.febslet.2006.04.085
Won SJ, Yen CH, Liu HS, Wu SY, Lan SH, Jiang-Shieh YF, Lin CN, Su CL (2015) Justicidin A-induced autophagy flux enhances apoptosis of human colorectal cancer cells via class III PI3K and Atg5 pathway. J Cell Physiol 230(4):930–946. doi:10.1002/jcp.24825
Funderburk SF, Marcellino BK, Yue Z (2010) Cell “self-eating” (autophagy) mechanism in Alzheimer’s disease. Mt Sinai J Med 77(1):59–68. doi:10.1002/msj.20161
Hollenbeck PJ (1993) Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol 121(2):305–315
Zeng KW, Ko H, Yang HO, Wang XM (2010) Icariin attenuates beta-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology 59(6):542–550. doi:10.1016/j.neuropharm.2010.07.020
Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I (2001) Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett 507(1):81–87
Harris KA, Oyler GA, Doolittle GM, Vincent I, Lehman RA, Kincaid RL, Billingsley ML (1993) Okadaic acid induces hyperphosphorylated forms of tau protein in human brain slices. Ann Neurol 33(1):77–87. doi:10.1002/ana.410330113
Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N (2009) Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett 455(3):191–194. doi:10.1016/j.neulet.2009.03.066
Lage R, Diéguez C, Vidal-Puig A, López M (2008) AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med 14(12):539–549. doi:10.1016/j.molmed.2008.09.007
Lippai M, Lőw P (2014) The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. BioMed Res Int 2014:11. doi:10.1155/2014/832704
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM (2010) Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285(14):10850–10861. doi:10.1074/jbc.M109.080796
Li YP, Bushnell AF, Lee CM, Perlmutter LS, Wong SK (1996) Beta-amyloid induces apoptosis in human-derived neurotypic SH-SY5Y cells. Brain Res 738(2):196–204
Datki Z, Papp R, Zadori D, Soos K, Fulop L, Juhasz A, Laskay G, Hetenyi C, Mihalik E, Zarandi M, Penke B (2004) In vitro model of neurotoxicity of Abeta 1–42 and neuroprotection by a pentapeptide: irreversible events during the first hour. Neurobiol Dis 17(3):507–515. doi:10.1016/j.nbd.2004.08.007
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3(4):519–526
Kimura T, Ono T, Takamatsu J, Yamamoto H, Ikegami K, Kondo A, Hasegawa M, Ihara Y, Miyamoto E, Miyakawa T (1996) Sequential changes of tau-site-specific phosphorylation during development of paired helical filaments. Dement Geriatr Cogn Disord 7(4):177–181
Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM (2009) Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci 29(28):9078–9089. doi:10.1523/jneurosci.1071-09.2009
Zempel H, Thies E, Mandelkow E, Mandelkow EM (2010) Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci 30(36):11938–11950. doi:10.1523/jneurosci.2357-10.2010
Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ (2011) Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA 108(14):5819–5824. doi:10.1073/pnas.1017033108
Stancu I-C, Vasconcelos B, Terwel D, Dewachter I (2014) Models of β-amyloid induced Tau-pathology: the long and “folded” road to understand the mechanism. Mol Neurodegener 9:51. doi:10.1186/1750-1326-9-51
Tokutake T, Kasuga K, Yajima R, Sekine Y, Tezuka T, Nishizawa M, Ikeuchi T (2012) Hyperphosphorylation of Tau induced by naturally secreted amyloid-beta at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3beta signaling pathway. J Biol Chem 287(42):35222–35233. doi:10.1074/jbc.M112.348300
Zhang Z, Simpkins JW (2010) Okadaic acid induces tau phosphorylation in SH-SY5Y cells in an estrogen-preventable manner. Brain Res 1345:176–181. doi:10.1016/j.brainres.2010.04.074
Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K (1995) Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem 65(2):732–738
Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX (2007) Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci 26(12):3429–3436. doi:10.1111/j.1460-9568.2007.05955.x
Hernandez F, Gomez de Barreda E, Fuster-Matanzo A, Lucas JJ, Avila J (2010) GSK3: a possible link between beta amyloid peptide and tau protein. Exp Neurol 223(2):322–325. doi:10.1016/j.expneurol.2009.09.011
Qian W, Shi J, Yin X, Iqbal K, Grundke-Iqbal I, Gong CX, Liu F (2010) PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3beta. J Alzheimer’s Dis JAD 19(4):1221–1229. doi:10.3233/jad-2010-1317
Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D (2011) AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem J 434(3):503–512. doi:10.1042/bj20101485
Greco SJ, Sarkar S, Johnston JM, Tezapsidis N (2009) Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biophys Res Commun 380(1):98–104. doi:10.1016/j.bbrc.2009.01.041
Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M (2011) AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem 118(4):460–474. doi:10.1111/j.1471-4159.2011.07331.x
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90(4):1383–1435. doi:10.1152/physrev.00030.2009
Parr C, Carzaniga R, Gentleman SM, Van Leuven F, Walter J, Sastre M (2012) Glycogen synthase kinase 3 inhibition promotes lysosomal biogenesis and autophagic degradation of the amyloid-beta precursor protein. Mol Cell Biol 32(21):4410–4418. doi:10.1128/mcb.00930-12
Marchand B, Arsenault D, Raymond-Fleury A, Boisvert FM, Boucher MJ (2015) Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem 290(9):5592–5605. doi:10.1074/jbc.M114.616714
Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170(7):1101–1111. doi:10.1083/jcb.200504035
Heiseke A, Aguib Y, Riemer C, Baier M, Schatzl HM (2009) Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J Neurochem 109(1):25–34. doi:10.1111/j.1471-4159.2009.05906.x
Kim J, Kundu M, Viollet B, Guan K-L (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141. doi:http://www.nature.com/ncb/journal/v13/n2/abs/ncb2152.html#supplementary-information
Hung SY, Huang WP, Liou HC, Fu WM (2009) Autophagy protects neuron from Abeta-induced cytotoxicity. Autophagy 5(4):502–510
Acknowledgments
This research was supported by the Institutional Program of the Korea Institute of Science and Technology (2Z04690), the Bio-Synergy Research Project (NRF-2012M3A9C4048793) and the Bio and Medical Technology Development Program (NRF-2015M3A9A5030735) of the Ministry of Science, ICT, and Future Planning through the National Research Foundation, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gu, MY., Kim, J. & Yang, H.O. The Neuroprotective Effects of Justicidin A on Amyloid Beta25–35-Induced Neuronal Cell Death Through Inhibition of Tau Hyperphosphorylation and Induction of Autophagy in SH-SY5Y Cells. Neurochem Res 41, 1458–1467 (2016). https://doi.org/10.1007/s11064-016-1857-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1857-5