Abstract
Purinergic P2X3 receptors (P2X3Rs) play extensive roles in nerve cells in the central nervous system, particularly in hyperexcitability and calcium (Ca2+) influx. However, the role of P2X3Rs in epilepsy has not been previously investigated. To determine the relationship between P2X3Rs and epilepsy, the expression and cellular location of P2X3Rs in patients with intractable temporal lobe epilepsy (TLE) and in a lithium chloride-pilocarpine-induced chronic rat model of epilepsy were assessed. Furthermore, the function of P2X3Rs was assessed in vitro. Real-time quantitative polymerase chain reaction (RT-qPCR) and Western blot analysis were used to evaluate the expression levels of P2X3Rs in brain tissues from TLE patients and an epileptic rat model, whereas immunofluorescence labeling was applied to determine the distribution of target proteins. Whole-cell recording was subsequently performed to identify the influence of P2X3Rs on seizure-like discharges. P2X3Rs were located at the cell bodies and dendrites of neurons with significantly increased expression in the TLE patients and epileptic rat model. In vitro, P2X3R activation accelerated sustained repetitive firing, whereas P2X3R inhibition led to relatively low-frequency discharges. To the best of our knowledge, this is the first study provide evidence that upregulated P2X3R expression exists in both epileptic humans and rats and may aggravate the epileptic state in vitro. Thus, P2X3Rs may represent a novel therapeutic target for antiepileptic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a severe and chronic neurological disease that exhibits a substantial influence on the health and quality of life of patients, particularly individuals who live in low-income regions [1]. To date, 65 million individuals suffer from epilepsy and epilepsy-related disabilities worldwide [2]. Furthermore, individuals with epilepsy suffer from psychological damage because of the societal discrimination and misunderstanding associated with this disease [3]. Although an increasing number of antiepileptic drugs (AEDs) have been investigated, which has led to increased treatment efficacy, the mortality of intractable epilepsy remains high [4]. Therefore, the development of novel therapeutic targets for epilepsy is critical.
Purinergic P2X receptors comprise a family of ligand-gated ion channels activated by adenosine triphosphate (ATP) and upregulated by pain, oxidative stress, inflammation, and other stimuli [5–7]. The P2X3 receptor (P2X3R) is a subtype of the P2X receptor family, which includes seven types (P2X1-P2X7), and it increases the membrane permeability to potassium (K+) and calcium (Ca2+). The P2X3R is a tripolymer that is broadly distributed in the central and peripheral nervous systems, such as the hippocampus, the main brain region involved in epilepsy, the dorsal root ganglion, and other regions [8]. Furthermore, these purinergic receptors produce a positive current and play key roles in neuropathic pain, inflammatory mediation, and increased neuronal excitability [9–11].
P2X receptors are closely related to neuronal excitability, and the P2X7 subtype has been reported to reduce status epilepticus (SE) in immature rats [12]. However, the potential effect of P2X3Rs on epilepsy has not previously been investigated. To further demonstrate the influence of P2X3Rs on temporal lobe epilepsy (TLE), real-time quantitative polymerase chain reaction (RT-qPCR), Western blot analysis, and immunofluorescence were employed to investigate P2X3R expression in patients with TLE and in a lithium chloride-pilocarpine-induced chronic rat model of epilepsy. Whole-cell recording was used to evaluate the effects of the P2X3R via antagonist (AF-353) and agonist (α,β-methylene-ATP) treatment on epileptic activity induced in vitro.
Materials and Methods
Patient and Control Selection
The human subject procedures in this study complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki), as well as the National Institutes of Health in China and the Ethics Committee of Chongqing Medical University. All participants and their relatives were informed and provided written informed consent prior to surgery. Fifteen samples of patients with TLE were randomly selected from our epilepsy brain bank, which comprises 300 patients with medically intractable TLE (Table 1). All patients presented with typical ictal clinical features of TLE, characteristic interictal and ictal electroencephalogram (EEG) manifestations, and significant imaging alterations assessed via computed tomography (CT) or magnetic resonance imaging (MRI). Furthermore, these patients had not previously responded to three or more AEDs, such as valproic acid, carbamazepine, or phenytoin. Video-EEG and intraoperative electrocorticography were employed during surgery to determine the focal location. No other nervous system diseases were present in these patients based on their medical histories and clinically relevant examinations. Following resection, part of the epileptic focus underwent pathological examination. The pathological examination results indicated that neuronal loss and gliosis were common. For comparison, ten control temporal tissues were obtained from patients with brain trauma who underwent surgery (Table 2). The pathological examination results were normal. These control individuals were definitively diagnosed with brain trauma and had no history of seizure, AEDs, or other nervous system diseases.
Chronic Rat Model of Epilepsy
All animal procedures were approved by the Animal Care Committee and Ethical Commission of Chongqing Medical University. Twelve eight-week-old male Sprague–Dawley rats (180–220 g) were obtained from the animal experimental center of Chongqing Medical University. All rats were randomly divided into two groups, which included the experimental and control groups (n = 6 per group). For the experimental group, the rats were initially intraperitoneally (i.p.) injected with lithium-chloride (127 mg/kg, Sigma Aldrich, St Louis, MO, USA). After 20 h, pilocarpine (50 mg/kg, i.p., Sigma Aldrich) was administered, followed by atropine methyl nitrate (1 mg/kg, i.p.) after 30 min [13]. In the absence of an epileptic seizure, pilocarpine (10 mg/kg, i.p.) was repeatedly injected every 10 min until the rats in the experimental group reached stage 4 seizures, according to a refractory TLE model [14]. Diazepam (10 mg/kg, i.p.) was injected into the enterocoelia of the rats to terminate convulsions when the SE would last for 1 h. The rats with stage 4 or 5 seizures were selected for further experimentation. For the control group, the rats were injected i.p. with an equal volume of saline. A standard video recorder was used to observe spontaneous seizures in the rat model for 2 months. Rats with spontaneous seizures in the experimental group were euthanized 2 months after the onset of SE following anesthetization with 10 % chloral hydrate (3 ml/kg, i.p.), whereas the rats in the control group underwent the same experimental procedures, including anesthetization following saline injection [13]. The cortex and hippocampus were subsequently dissected for further study.
Tissue Processing
The tissues obtained from human and rat subjects were divided into two sections. One section of the brain tissue was immediately placed in liquid nitrogen at −80 °C for RNA and protein extraction. The remaining tissue section was fixed in 4 % phosphate-buffered paraformaldehyde for 24 h, followed by sucrose for 48 h. The tissue was subsequently sliced into 10-μm-thick sections at an optimal cutting temperature and mounted on polylysine-coated slides. The specimens were stored at −80 °C for trinal-labeled immunofluorescence analysis.
Western-Blot Analysis
According to the manufacturer’s instructions, total proteins were extracted from the brain using a whole protein extraction kit (KeyGen Biotech, Nanjing, China) and homogenized in radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology, China). A bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, China) was used to determine the protein concentrations. A sample of the extractive (50 μg/lane) was separated by 10 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes at 350 mA for 80 min. The samples were immersed in blocking buffer at room temperature for one hour, and the membranes were incubated with primary polyclonal rabbit anti-P2X3 (1:600; Abcam, Cambridge, UK) or monoclonal rabbit anti-β-actin antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C overnight. The membranes were subsequently washed with Tween-Tris-buffered saline three times (10 min per wash). Then, the membranes were incubated in a goat anti-rabbit secondary antibody (HRP-labeled, 1:4000; Zhongshan Golden Bridge, China) at room temperature for 2 h. Immunoreactive bands were displayed via an enhanced chemiluminescence (ECL) kit in a darkroom. The images were then scanned and analyzed using Quantity One software (Bio-Rad Laboratories). The optical density (OD) values were normalized to the β-actin value.
RT-qPCR Analysis
Total RNA was obtained using RNAiso Plus (Takara, China) according to the manufacturer’s instructions. The concentration and purity of the RNA were evaluated at a wavelength of 260/280 nm. Complementary DNA was synthesized using the PrimeScript™ RT reagent kit with gDNA Eraser. A Bio-Rad CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA) was used. The housekeeping gene β-actin was used to normalize mRNA levels. The RT-qPCR cycling conditions were 30 s at 95 °C, 40 cycles for five seconds at 95 °C, 30 s at 60 °C, and 65–95 °C for 5 s. The forward and reverse sequences of the primers were as follows: P2X3 (Homo sapiens), 5′-ATCTCGTCTCCCTGTCCTGT-3′ and 5′-TCTTCACAACCACCGACTTG-3′; P2X3 (Rattus norvegicus), 5′-TAGGAGGACCACAGGAGTGC-3′ and 5′-CAAGCAGAGGGAAGAAAGGA-3′; β-actin (Homo sapiens), 5′- CCTGGCACCCAGCACAAT-3′ and 5′-GGGCCGGACTCGTCATAC-3′; β-actin (Rattus norvegicus), 5′-TGTCACCAACTGGGACGATA-3′ and 5′-GGGGTGTTGAAGGTCTCAAA-3′. Data collection and analyses were performed using Bio-Rad CFX96 Manager (Bio-Rad, Hercules, CA, USA).
Immunofluorescence Labeling
The frozen sections were immersed in acetone for 30 min and washed using PBS three times (5 min per wash). Each section was subsequently permeabilized with 0.4 % Triton X-100 for 20 min at 37 °C. After washing in PBS for 5 min, antigen retrieval was performed using microwave heating with 10 mmol/L sodium citrate buffer. Antigen retrieval buffers were used for 20 min and washed. The sections were subsequently blocked in 5 % goat serum (Zhongshan Golden Bridge Inc., China) for 30 min at 37 °C. The sections were then incubated in a mixture of polyclonal rabbit P2X3 antibody (1:120; Abcam, Cambridge, UK), mouse anti-MAP2 antibody (1:50; Abcam, Cambridge, UK) at 4 °C overnight. On the following day, the sections were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:100; Zhongshan Golden Bridge, Inc.) and DyLight 549-Affinipure goat anti-mouse IgG (1:100, EarthOx, Millbrae, CA, USA) secondary goat anti-rabbit for two hours at 37 °C in a darkroom. The sections were then incubated in DAPI at room temperature for 8 min. After washing with PBS, the sections were mounted in 50 % glycerol. Laser scanning confocal microscopy (Leica Microsystems, Wetzlar, Germany) was used to detect the fluorescence in each section.
Slice Preparation and Whole-Cell Recording
Brain slices were obtained from two-week-old Sprague–Dawley rats. The rats were euthanized via decapitation within 1 min, and the brain tissues were immediately placed in a cold cutting solution with standard gas (95 % O2 and 5 % CO2 at 0 °C). The 1 L cutting solution contained 2.5 mM KCl, 1.6 mM NaH2PO4, 2.0 mM Cacl2, 1.0 mM Mgcl2.6H2O, 119.0 mM NaCl, 26.0 mM NaHCO3, and 25.0 mM d-glucose (pH 7.4, osmolarity of 295–305 mosmol/L). Following immersion in cold cutting solution for 2 min, the brain tissues were trimmed and placed in a vibratome to obtain a slice thickness of 350–400 μm. The slices were then rapidly moved to a vessel filled with normal artificial cerebrospinal fluid (ACSF) that contained the same constituents as the cutting solution. The solution was mixed with 95 % O2 and 5 % CO2 and incubated at 26 °C for 60 min prior to use. Twenty-four slices from ten rats were examined.
Whole cell patch-clamp recordings were performed using a MultiClamp 700B amplifier (Molecular Devices, Silicon Valley, CA, USA), a microscope, and a 3–5 MΩ glass electrode (Sutter Instrument, Novato, USA). The pipette solution contained 60 mM K2SO4, 60 mM NMG, 40 mM HEPES, 4 mM Mgcl2, 0.5 mM BAPTA, 12 mM phosphocreatine, 2 mM NA2ATP, 0.2 mM NA3ATP with a pH of 7.2 and an osmolarity of 265-270 mosmol/L. To measure cell excitability, pyramidal cells in the CA1 region of the hippocampus were recorded. Seizure-like discharges were induced via magnesium-free ACSF, which contained the same constituents as normal ACSF with the exception of Mg2+. A nonselective agonist, α,β-MeATP, was diluted in Mg-free ACSF at an appropriate concentration of 10 μM. Then, AF-353, an effective selective antagonist of P2X3, was dissolved in DMSO and diluted in Mg-free ACSF at a concentration of 10 μM. The concentrations of α,β-MeATP and AF-353 have previously been reported to be effective [15, 16]. Additional concentrations were also performed. To exclude the influence of DMSO on seizure-like discharges, equivalent DMSO was diluted in Mg-free ACSF for contrast. Whole-cell current-clamp techniques were used to identify the effects of α,β-MeATP and AF-353 on seizure-like activity evoked by Mg-free ACSF. The final data were analyzed using pCLAMP 10.3 Software (Molecular Devices, Silicon Valley, CA, USA).
Statistical Analysis
The data are expressed as the means ± standard deviations and were analyzed using SPSS Statistics for Windows, Version 17.0 (SPSS, Chicago, IL, USA). Student’s t-tests and Chi square tests were used to identify significant differences between the experimental and control groups, and comparisons of more than two groups were conducted by Kruskal–Wallis analysis. P < 0.05 was considered statistically significant.
Results
Clinical Characteristics
The patients with intractable TLE included nine male and six female patients with a mean age of 30.60 ± 10.32 years. The patients in the control group included six male and four female patients with a mean age of 26.50 ± 9.71 years. There was no significant difference in age or gender between the intractable TLE and control groups (P > 0.05).
Upregulated Expression of P2X3 Protein and mRNA in Patients with Intractable TLE and a Rat Model of Epilepsy
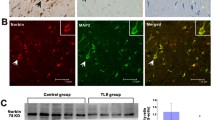
Western-blot analysis and RT-qPCR were used to determine the P2X3 protein level in the temporal neocortex of the patients with intractable TLE and in the hippocampus and adjacent cortex of the epileptic rats. The Western-blot and RT-qPCR analyses were repeated three times. The bands of the P2X3 protein were located at the 49 KD position (Fig. 1). As the target protein was normalized to β-actin, the P2X3 immunoreactivity in the patient group (n = 15) was significantly increased compared with the control group (n = 10) (t = 14.58, df = 23, P < 0.05; Fig. 2a, b). In the rat experiments, the same tendency was identified in which the P2X3 protein levels in the epileptic rats (n = 6) were significantly increased (t = 4.73, df = 10, P < 0.05; Fig. 2c, d). RT-qPCR was performed to further investigate the expression of P2X3R in epilepsy. The relative P2X3 mRNA expression level in the patients with TLE increased greatly compared with the controls (Fig. 3a). Furthermore, there was also an apparent increase in the hippocampus and adjacent cortex of the epileptic rats (Fig. 3b).
P2X3 protein expression in the temporal neocortex of patients with intractable TLE and in the hippocampus and adjacent cortex of the rat model was analyzed via Western blot assay. a Representative bands of the P2X3 receptor (P2X3R) in patients with intractable TLE and controls. The P2X3 expression in the patients with TLE was strong compared with the controls. b The intensity ratio of P2X3 protein in the patients with intractable TLE (n = 15) was significantly increased compared with the controls (n = 10, *P < 0.05). c Representative P2X3 expression in the rat model. d Comparison of the intensity ratios indicates increased P2X3 expression in the rat model compared with control (n = 6; *P < 0.05)
P2X3 mRNA measured via RT-qPCR analysis of the temporal neocortex in patients with intractable TLE and in the hippocampus and adjacent cortex of the rat model. a The P2X3 receptor (P2X3R) mRNA expression was significantly increased in the patients with intractable TLE (n = 15) compared with the controls (n = 10; *P < 0.05). b Compared with the control group, the P2X3R mRNA expression was increased in the epileptic rats (n = 6; *P < 0.05)
P2X3 Localization in the Cortex of Patients with TLE and in the Hippocampus and Adjacent Cortex of Epileptic Rats
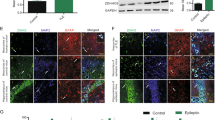
P2X3 protein expression was located at the dendrites instead of the nucleus of neurons in the hippocampal CA1 region and temporal neocortex (Fig. 4a, b) For the patients with intractable TLE (Fig. 4c), the P2X3R location also demonstrated the same conclusion in the temporal cortex. P2X3 (green signal) co-localized with microtubule-associated protein 2 (red signal), which is a dendrite-specific marker in neurons in both the hippocampus and cortex of humans and rats. However, P2X3 (green signal) was not co-expressed with the nucleus (blue signal) in all subjects.
Immunofluorescence staining indicates that there were P2X3-positive cells in the hippocampal CA1 region and adjacent cortex of the epileptic rats and in the temporal neocortex of the patients with intractable TLE. a In the CA1 region, P2X3 (green) was co-expressed with MAP2 (red), but not DAPI (blue), in the epileptic rats. These findings indicate that P2X3 receptors (P2X3Rs) were located at the dendrite and not at the nucleus of CA1 region neurons. b The same dendrite location was identified in the adjacent cortex of the epileptic rats. c In the temporal neocortex of the patients with intractable TLE, P2X3 (green) was co-expressed with MAP2 (red), but not DAPI (blue). Scale bar = 100 μm (a, b); scale bar = 50 μm (c) (Color figure online)
Effects of P2X3R Inhibition and Agitation on Epileptic Activity
Our findings indicated that the P2X3R was located at neuronal cells and was increased in epilepsy. However, no evidence indicates whether P2X3 influenced epilepsy or seizures. Therefore, the patch-clamp system was used to determine whether a causal relation between the target protein and epilepsy exists. For hippocampal CA1 cells, Mg-free ACSF induced sustained repetitive firing (SRF) and resulted in high-frequency seizure-like discharges. The self-contrast method was used to determine the effects of the P2X3R (n = 6 per group). The cells were recorded if the initial resting membrane potential was depolarized at −50 to −60 mV. The frequency induced by Mg-free ACSF was significantly increased (3.15 ± 0.32 Hz) compared with the frequency of action potentials (APs) with normal ACSF (1.18 ± 0.36 Hz, t = −8.43, df = 5, P < 0.05; Fig. 5a). DMSO was dissolved in Mg-free ACSF, and the frequency of APs did not significantly change (t = −0.18, df = 5, P > 0.05; Fig. 5b). 3 min after AF-353 was mixed in Mg-free ACSF, the frequency of APs was reduced to 2.49 ± 1.01 Hz (t = 3.92, df = 5, P < 0.05, Fig. 5c). When the slices were treated with α,β-MeATP for 3 min, seizure-like discharges significantly increased (4.93 ± 1.06 Hz, t = -5.12, df = 5, P < 0.05; Fig. 5d). We subsequently performed a comparison of the AP frequencies at different concentrations of AF-353 and α,β-MeATP (n = 6 per group). The mean AP frequency induced by Mg-free ACSF was 3.24 ± 0.53 Hz. When slices were treated with 1 μM, 10 μM, or 100 μM Af-353, the mean AP frequencies were 2.19 ± 0.60, 2.21 ± 0.68, and 1.97 ± 0.33 Hz, respectively (H = 9.69, df = 3, P < 0.05, Fig. 6). For α,β-MeATP, 1 μM of agonist did not significantly affect the AP frequencies. However, 10 μM and 100 μM of α,β-MeATP facilitated seizure-like discharges (H = 17.39, df = 3, P < 0.05, Fig. 7). These findings indicate that P2X3 activation facilitated epileptic activity, and the inhibition of P2X3R provided a protective effect on neurons regarding SRF.
Action potential (AP) frequency measured via whole-cell recording. A self-contrast method was adopted (n = 6 per group), and the membrane potential was maintained at −50 to −60 mV. a Continuous high-frequency seizure-like discharges were induced in pyramidal cells of the hippocampal CA1 region of the slices perfused using Mg-free ACSF. b No apparent change in AP frequency was observed following DMSO treatment. c The selective antagonist AF-353 (10 μM) reduced the continuous seizure-like discharges. d The agonist α,β-meATP (10 μM) rapidly increased the AP frequency. e The quantitative analysis results from the different groups are shown (*P < 0.05)
Whole-cell recordings from hippocampal pyramidal cells of brain slices exposed to different concentrations of AF-353 (n = 6 per group). a Continuous high-frequency discharges in the hippocampal pyramidal cells of brain slices are induced after Mg-free ACSF. b AP frequency recorded after 1 μM AF-353. c AP frequency recorded after 10 μM AF-353. d AP frequency recorded after 100 μM AF-353. e The quantitative analysis results from the different groups are shown (*P < 0.05)
Whole-cell recordings at different concentrations of α,β-meATP (n = 6 per group). a Continuous high-frequency discharges in the hippocampal pyramidal cells of brain slices were induced after Mg-free ACSF. b AP frequency recorded after 1 μM α,β-meATP. c AP frequency recorded after 10 μM α,β-meATP. d AP frequency recorded after 100 μM α,β-meATP. e The quantitative analysis results from the different groups are shown (*P < 0.05)
Discussion
It is well established that changes in neuronal excitability are important factors in the pathogenesis of central nervous system diseases. Accumulating evidence has suggested that hyperexcitability exists in the pathophysiological process of epileptogenesis and plays a key role in it [17–19]. Many studies have demonstrated that P2X3R plays a role in the formation of neuronal excitability [20, 21]. However, to date, no studies have directly investigated whether P2X3R is involved in hyperexcitability-induced epilepsy.
Relevant studies have suggested that P2X3Rs comprise ligand-gated positive ion channels and exhibit properties that generate calcium influx [22]. For excitable cells, inward and outward currents are in dynamic equilibrium at the level of the resting membrane potential. Calcium current induced by P2X3Rs leads to an inward positive ion current. The inward current subsequently leads to membrane depolarization and decreases the threshold intensity of excitable cells. Consequently, the excitability of cells increases. It is widely accepted that the hippocampus and adjacent tissue are the main brain regions involved in epilepsy. In our study, we speculated that upregulated P2X3Rs in the hippocampus and adjacent cortex may play an important role in epilepsy by depolarizing the resting membrane potential and increasing neuronal excitability.
Furthermore, abnormal synaptic transmission has also been identified as a vital risk factor for epilepsy [23, 24]. In physiological conditions, glutamic acid, the major excitatory neurotransmitter, maintains a dynamic balance with the GABAergic inhibitory transmission. Both neurotransmitters act to maintain the normal function and activity of the neural network. Thus, an abnormal regulation of glutamic or aminobutyric acid (GABA) may result in alterations in neuronal excitability and subsequently induce epilepsy [25]. It has been reported that P2X3Rs are also located at glutamatergic nerve terminals and lead to glutamate release [26]. An increase in the excitatory postsynaptic current (EPSC) has also been identified in neurons via P2X3R activation [27]. Therefore, P2X3Rs play a critical role in the regulation of neuronal excitability via another mechanism that affects synaptic transmission, which is closely related to the occurrence and development of epilepsy.
Our findings indicate that P2X3Rs were located at neurons in the hippocampus and adjacent cortex and were significantly increased in the patients with intractable TLE and in epileptic rats. According to the function of the P2X3R, the results indicated that this protein may potentially be associated with the pathogenesis of epilepsy via the regulation of excitability and generation of abnormal synaptic transmission.
In summary, our findings demonstrate that P2X3R activation may aggravate the hyperexcitability of neurons in the hippocampus, and P2X3R inhibition may have a remission effect. Despite these promising findings, there are several limitations that should be considered in the interpretation of these results. First, because of ethical constraints, live brain tissue could not be obtained from normal human controls. Thus, we compared brain tissues from patients with TLE to brain tissues obtained from patients with brain trauma. Although the control tissues were not obtained from healthy controls, a comprehensive examination indicated the pathology of these tissues was normal. Second, anesthesia often has effects on post-mortem measurements in rat models. However, all animals received the same anesthetic compound. Finally, this study comprised a descriptive study; therefore, the specific molecular mechanisms must be further investigated to address these issues. Nevertheless, these novel findings provide a theoretical basis for the treatment of epilepsy and indicate that P2X3Rs may represent a therapeutic target for AEDs.
References
Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR (2010) Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 51:883–890
Thurman DJ, Beghi E, Begley CE et al (2011) Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 52(suppl 7):2–26
Quintas R, Raggi A, Giovannetti AM et al (2012) Psychosocial difficulties in people with epilepsy: a systematic review of literature from 2005 until 2010. Epilepsy Behav 25:60–67
Neligan A, Bell GS, Shorvon SD, Sander JW (2010) Temporal trends in the mortality of people with epilepsy: a review. Epilepsia 51(11):2241–2246
Wu JX, Xu MY, Miao XR, Lu ZJ, Yuan XM, Li XQ, Yu WF (2012) Functional up-regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur J Pain 16(10):1378–1388
Zhang Q, Siroky M, Yang JH, Zhao Z, Azadzoi K (2014) Effects of ischemia and oxidative stress on bladder purinoceptors expression. Urology 84(5):1249.e1-7
Chen Y, Zhang L, Yang J, Zhang L, Chen Z (2014) LPS-induced dental pulp inflammation increases expression of ionotropic purinergic receptors in rat trigeminal ganglion. NeuroReport 25(13):991–997
Burnstock G (2015) Physiopathological roles of P2X receptors in the central nervous system. Curr Med Chem 22(7):819–844
Bele T, Fabbretti E (2015) P2X receptors, sensory neurons and pain. Curr Med Chem 22(7):845–850
Wang S, Dai Y, Kobayashi K, Zhu W, Kogure Y, Yamanaka H, Wan Y, Zhang W, Noguchi K (2012) Potentiation of the P2X3 ATP receptor by PAR-2 in rat dorsal root ganglia neurons, through protein kinase-dependent mechanisms, contributes to inflammatory pain. Eur J Neurosci 36(3):2293–2301
Xing J, Lu J, Li J (2008) Purinergic P2X receptors presynaptically increase glutamatergic synaptic transmission in dorsolateral periaqueductal gray. Brain Res 1208:46–55
Henshall DC, Engel T (2015) P2X purinoceptors as a link between hyperexcitability and neuroinflammation in status epilepticus. Epilepsy Behav 49:8–12
Cao Q, Wang W, Gu J, Jiang G, Wang K, Xu Z, Li J, Chen G, Wang X (2014) Elevated expression of acid-sensing ion channel 3 inhibits epilepsy via activation of interneurons. Mol Neurobiol. doi:10.1007/s12035-014-9014-0
Racine RJ (1972) Modification of seizure activity by electrical stimulation II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Martínez-Cutillas M, Gil V, Gallego D, Mañé N, Clavé P, Martín MT, Jiménez M (2014) α, β-meATP mimics the effects of the purinergic neurotransmitter in the human and rat colon. Eur J Pharmacol 740:442–454
Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE, Kinnamon SC (2015) Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol 593(5):1113–1125
Brigo F, Bongiovanni LG, Nardone R, Trinka E, Tezzon F, Fiaschi A, Manganotti P (2013) Visual cortex hyperexcitability in idiopathic generalized epilepsies with photosensitivity: a TMS pilot study. Epilepsy Behav 27(2):301–306
Iori V, Frigerio F, Vezzani A (2015) Modulation of neuronal excitability by immune mediators in epilepsy. Curr Opin Pharmacol 26:118–123
Carletti F, Gambino G, Rizzo V, Ferraro G, Sardo P (2015) Cannabinoid and nitric oxide signaling interplay in the modulation of hippocampal hyperexcitability: study on electrophysiological and behavioral models of temporal lobe epilepsy in the rat. Neuroscience 303:149–159
Liu M, Yang H, Fang D, Yang JJ, Cai J, Wan Y, Chui DH, Han JS, Xing GG (2013) Upregulation of P2X3 receptors by neuronal calcium sensor protein VILIP-1 in dorsal root ganglions contributes to the bone cancer pain in rats. Pain 154(9):1551–1568
Chen XQ, Wang B, Wu C, Pan J, Yuan B, Su YY, Jiang XY, Zhang X, Bao L (2012) Endosome-mediated retrograde axonal transport of P2X3 receptor signals in primary sensory neurons. Cell Res 22(4):677–696
Elsa Fabbretti and Andrea Nistri (2012) Regulation of P2X3 receptor structure and function. CNS Neurol Disord Drug Targets 11(6):687–698
Saffarzadeh F, Eslamizade MJ, Mousavi SM, Abraki SB, Hadjighassem MR, Gorji A (2016) TRPV1 receptors augment basal synaptic transmission in CA1 and CA3 pyramidal neurons in epilepsy. Neuroscience 314:170–178
Häussler U, Rinas K, Kilias A, Egert U, Haas CA (2015) Mossy fiber sprouting and pyramidal cell dispersion in the hippocampal CA2 region in a mouse model of temporal lobe epilepsy. Hippocampus. doi:10.1002/hipo.22543
Salazar P, Tapia R (2015) Epilepsy and hippocampal neurodegeneration induced by glutamate decarboxylase inhibitors in awake rats. Epilepsy Res 116:27–33
Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA (2005) Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci 25(27):6286–6295
Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC (2004) Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24(20):4709–4717
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81171225). The authors express their sincere gratitude to the patients with TLE and brain trauma, as well as their families. The authors also thank Xinqiao Hospital for supplying the brain tissues, as well as the National Institutes of Health of China and the local Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no conflicts of interest in this work.
Additional information
Xin Zhou and Li-Min Ma have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, X., Ma, LM., Xiong, Y. et al. Upregulated P2X3 Receptor Expression in Patients with Intractable Temporal Lobe Epilepsy and in a Rat Model of Epilepsy. Neurochem Res 41, 1263–1273 (2016). https://doi.org/10.1007/s11064-015-1820-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1820-x