Abstract
Our previous investigations have demonstrated that microinjection of acetylcholine (ACh) or muscarinic ACh receptor activation in the cerebellar cortex induces a systemic blood pressure depressor response. This study aimed to determine the role of muscarinic ACh receptor-2 (M2 receptor) in the cerebellar cortex in cardiovascular function regulation in rats. A nonselective muscarinic receptor agonist (oxotremorine M, OXO; 30 mM), a selective M2 receptor agonist (arecaidine but-2-ynyl ester tosylate, ABET; 3, 10, and 30 mM), 30 mM OXO mixed with a selective M2 receptor antagonist (methoctramine hydrate, MCT; 0.3, 1, and 3 mM), and normal saline (0.9 % NaCl) were separately microinjected (0.5 µl/5 s) into the cerebellar cortex (lobule VI) of anaesthetized rats. We measured the mean arterial pressure (MAP), maximum change in MAP, and reactive time (RT; the duration required for the blood pressure to return to basal levels), heart rate (HR) and the maximum change in HR during the RT in response to drug activation. The results demonstrated that ABET dose-dependently decreased MAP and HR, increased the maximum change in MAP and the maximum change in HR, and prolonged the RT. Furthermore, MCT dose-dependently blocked the OXO-mediated cardiovascular depressor response. This study provides the first evidence that M2 receptors in the cerebellar cortex are involved in cardiovascular regulation, the activation of which evokes significant depressor and bradycardic responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to the classic afferents of climbing and mossy fibers, the cerebellum also receives many types of modulatory inputs from serotonergic, histaminergic, norepinephrinergic, dopaminergic, and cholinergic fibers, which are generalized as the third type of cerebellar afferent inputs [1, 2]. Cerebellar cholinergic projections predominantly originate from the lower brainstem, especially in the medial vestibular nucleus [3, 4]. These projections likely enter the cerebellum accompanied by mossy fibers through the inferior cerebellar peduncles, which subsequently innervate nearly all cerebellar regions and directly contact various types of cerebellar neurons [4, 5]. Moreover, radioligand binding, in situ hybridization, and immunohistochemical studies have demonstrated the existence of several subtypes of acetylcholine (ACh) receptors, such as α3, α4, α6, α7, β2, and β4 [6, 7], and potentially all subtypes of muscarinic (m)ACh receptors (M1–M5) [8, 9], in the cerebellum. These cholinergic receptors have been reported to have significant roles in cerebellar neuronal functions, such as the modulation of neuronal firing and synaptic plasticity [10, 11]. However, to date, cerebellar ACh receptor involvement in integral physiological functions has received minimal attention. Our previous reports demonstrated that muscarinic receptors in the cerebellar cortex exhibited an important influence in the systemic depressor response [12]. Nevertheless, the precise mechanism that underlies the cerebellar muscarinic receptor-mediated blood pressure down-regulation remains enigmatic.
The mACh receptor family comprises the M1–M5 subtypes. The M2 receptor has been the focus of substantial research interest because of its role in cardiovascular functions. For example, animals that lack functional M2 receptors or the use of autoantibodies against M2 receptors develop bradycardia and arrhythmia [13, 14]. In the present study, we focused on the role of the M2 receptor on cerebellar cortex-mediated cardiovascular modulation in anaesthetized rats using immunohistochemical and neuropharmacological techniques. The results demonstrate that M2 receptor activation in the cerebellar cortex dose-dependently decreases mean arterial pressure (MAP) and heart rate (HR), which may substantially contribute to the muscarinic receptor-mediated blood pressure depressor response.
Materials and Methods
Animals
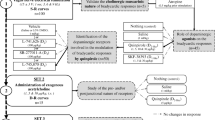
Young adult male Sprague-Dawley (SD) rats (2 months old, 230–260 g; n = 20) obtained from Shanghai Sippr BK Laboratory Animals Ltd. (Shanghai, China) were used in this study. The animals were individually housed in a temperature-controlled (23 ± 1 °C) environment with a 12/12 h light/dark-cycle with food and water ad libitum. All animal experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised in 1996) and were approved by our University’s Animal Care and Use Committee.
Surgical Procedures
The rats were anesthetized with urethane [1.4 g/kg, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China; intraperitoneal (i.p.) injection] and processed for cervical surgery with tracheal intubation. Rectal temperature was monitored, and body temperature was maintained at 37.0 ± 0.5 °C using an electric heating pad; supplementary doses of anesthesia (0.7 g/kg) were administered as required. A catheter (BL-2020; Taimeng Sci-Tec Co., Ltd, Chongqing, China) filled with normal saline and heparin (500 IU/ml) was inserted into the left carotid artery; it was connected to a signal collecting and processing instrument (BL-420F; Taimeng Sci-Tec Co., Ltd.) through a blood pressure transducer (PT-100, Taimeng Sci-Tech Co., Ltd.). The pulsatile blood pressure trace was consecutively collected through an analog-to-digital interface (sampling rate = 100 Hz) and subsequently processed using software (TM_WAV1; Taimeng Sci-Tec Co., Ltd.) that identifies inflection points on signals and generates beat-by-beat time series with systolic arterial pressure (SAP), diastolic arterial pressure (DAP), MAP, and HR values. The rats were then mounted on a stereotaxic instrument (51503 New Standard Stereotaxic; Stoelting Co., Wood Dale, IL, USA), and a craniotomy was performed under aseptic conditions. A Hamilton syringe needle (0.3 mm inner diameter, 0.5 mm outer diameter) was lowered into the granular layer of the cerebellar lobule VI (x: −10.8 to −11.8; y: 0.0–1.6; z: 1.8–2.0; according to a rat brain atlas [15] ) (Fig. 1), using a computer-controlled stepper motor (IVM-1000; Scientifica, UK). Repeated injections were administered at 30 min intervals, and the injections were separated by at least 1 mm to avoid interference between administrations. It has previously been demonstrated that a volume of 0.5 μl has an estimated diffusion range of 0.5–1.0 mm in brain tissue [16]; thus, this dose of cholinergic reagent should be restricted to the cerebellar cortex.
Schema of the cerebellar cortex microinjection sites. a Schematic diagram indicates the dorsal view of the rat cerebellum. The Latin numerals denote the lobules of the vermis according to Larsell’s descriptions [32]. The box in the dotted line (in lobule VI) illustrates the microinjection site region. b Histological reconstructions indicate the microinjection sites in the cerebellar granular layers (x: −10.8 to −11.8; y: 0.0–1.6; z: 1.8–2.0) according to a rat brain atlas [15] across 20 rats. The numbers in parentheses indicate the measurements performed. ML molecular layer, PCL Purkinje cell layer, GL granular layer, MS microinjection sites
Microinjections
The cholinergic reagents included the nonselective mACh receptor agonist oxotremorine M (OXO; 30 mM; Sigma-Aldrich, St. Louis, MO, USA), the selective M2 receptor agonist arecaidine but-2-ynyl ester tosylate (ABET; 3, 10, and 30 mM; Tocris Cookson Ltd., Bristol, UK), 30 mM of OXO mixed with the selective M2 receptor antagonist methoctramine hydrate (MCT; 0.3, 1, and 3 mM; Sigma-Aldrich), and normal saline (0.9 % NaCl).
In general, prior to drug injection, the blood pressure trace was observed for at least 10 min to ensure stability. Each rat received one saline injection (no blood pressure effect) and one 30 mM OXO injection (which induced a significant depressor response [12] ) for confirmation of blood pressure regulation. Each animal subsequently received 4–6 different drug injections based on the blood pressure status (blood pressure often appeared unstable after longer experiments), which were separately microinjected (0.5 µl/5 s) into the cerebellar cortex in at least 10 rats (2–3 injections/drug type/rat).
MAP and HR Measurements
The drug effects on MAP and HR regulation were considered substance-specific provided they were reversible and reproducible. The reactive time (RT; duration required for the MAP to return to baseline values), MAP during the RT, and maximum change in MAP in each ACh trial, as well as the HR (beats per minute; BPM) and the maximum change in HR during the RT in response to drug activation were calculated.
Immunohistochemistry
Three additional rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and subsequently perfused through the heart with 0.9 % NaCl until the liver became pale, followed immediately by 200 ml of a fixative solution that contained 4 % paraformaldehyde and 2.5 % glutaraldehyde in 0.1 M phosphate buffered saline (PBS, pH 7.4). The cerebellum was dissected and post-fixed overnight in the perfusion fixative. The blocks of tissue that contained lobule VI (approximately 5 mm × 5 mm × 3 mm) were subsequently washed in PBS, dehydrated in ethanol, cleared in xylene, and embedded in paraffin. 5 μm thick consecutive sagittal sections were cut using a microtome (Leica RM2245, Leica Microsystems, Wetzlar, Germany) prior to mounting on 3-aminopropyl-triethoxysilane (APES; Sigma-Aldrich, St. Louis, MO, USA; dilution: 1:50 in acetone) coated microscopic slides for histological staining.
Three sections of lobule VI [15], at intervals of approximately 50 μm, were obtained from each animal for immunohistochemical labeling, and the neighboring sections were processed as the negative controls; similar protocols have been described in our previous report [17]. Briefly, the sections were deparaffinized in xylene, hydrated using a graded ethanol series to distilled water, and then incubated with 3 % hydrogen peroxide (H2O2) to quench the endogenous peroxidase activity (10 min at room temperature). After washing in PBS (3 × 10 min at room temperature; the same washing procedure was performed between each step), the sections were incubated with 10 % goat serum to suppress the non-specific staining (10 min at room temperature). The sections were subsequently incubated for 24 h at 4 °C with anti-M2 receptor antibody (1:200, rabbit monoclonal; Abcam, Cambridge, UK), followed by incubation for 30 min at 37 °C with biotinylated anti-rabbit IgG (Boster Bioengineering Co., Ltd., Wuhan, China). The sections were then treated with preformed avidin–biotin–peroxidase complexes (Boster Bioengineering Co., Ltd.) for 30 min at 37 °C. Finally, the sections were incubated for 10 min in 0.05 % 3,3′-diaminobenzidine (DAB)/0.01 % H2O2 (Boster Bioengineering Co., Ltd.). The sections were counterstained with thionine (0.5 %, 37 °C, 10 min; Sigma-Aldrich, St. Louis, MO, USA), dehydrated in gradient alcohol, cleared in xylene, and cover slipped with permount. Immunohistochemical negative controls were performed on adjacent sections via the substitution of PBS for the primary antibody.
Statistical Analysis
All data are presented as the mean ± SE. A Student’s t test and one-way analysis of variance (ANOVA) followed by a Bonferroni correction post hoc test were conducted for the statistical analyses. P values <0.05 were considered significant.
Results
The M2 Agonist ABET Dose-Dependently Decreases MAP
The M2 receptors in the cerebellar cortex exhibited significant effects on somatic MAP and HR modulations, which typically recovered within 2.5 min after the microinjections (Fig. 2). Compared to the saline treatment (Fig. 2a), microinjection of the M2 receptor selective agonist ABET into the cerebellar cortex induced a significant down-regulation of systemic MAP (Fig. 2c). ABET administration (3, 10, and 30 mM) resulted in a significant dose-dependent MAP response. Compared with the saline administration, the MAP (F (3,84) = 30.524; P < 0.001; Fig. 3a) decreased by 7, 16, and 23 mmHg, respectively; the maximum change in MAP (F (2,66) = 26.716; P < 0.001; Fig. 3b) was 19 ± 4, 24 ± 4, and 27 ± 4 mmHg, respectively; and the RT (F (2,66) = 70.858; P < 0.001; Fig. 3c) lasted 50 ± 6, 63 ± 7, and 77 ± 10 s, respectively. These results demonstrate that ABET decreases MAP via M2 receptor activation in the cerebellar cortex, which indicates a homodromous characteristic of OXO (Fig. 2a) and ACh administration [18].
Representative recordings of the pulsatile arterial pressure and HR responses to cerebellar injections. a, b Compared to saline treatment, microinjection of 30 mM OXO (a nonselective mACh receptor agonist) into the cerebellar cortex induced a significant decrease in MAP and HR. c, d Dose-dependent effects of ABET (M2 receptor selective agonist) on MAP and HR reductions. e, f MCT (selective M2 receptor antagonist) dose-dependently blocked the 30 mM OXO mediated MAP and HR reductions. In the blood pressure recording, the gray line indicates the pulsatile arterial pressure, the black line indicates the MAP, and the arrows indicate the time of injection. ABET arecaidine but-2-ynyl ester tosylate, BP blood pressure, HR heart rate, MAP mean arterial pressure, MCT methoctramine hydrate, OXO oxotremorine M, MCMAP maximum change in MAP, RT reactive time
Histograms of cerebellar cholinergic microinjectionon blood pressure modulation. a MAP, b maximum change in MAP, and c RT (the duration required for the blood pressure to return to basal values) in response to ABET stimulation. d MAP, e maximum change in MAP, and f RT in response to 30 mM OXO mixed with different concentrations of MCT (0.3, 1, and 3 mM). There was no decrease in MAP with saline; therefore, this group was not included in the statistical analyses in b and c. The numbers presented in parentheses denote the number of animals measured per group. *P < 0.05; **P < 0.01. ABET arecaidine but-2-ynyl ester tosylate, BP blood pressure, MAP mean arterial pressure, MCT methoctramine hydrate, OXO oxotremorine M, RT reactive time
The M2 Receptor Antagonist MCT Dose-Dependently Blocks the OXO-Mediated MAP Decrease
Microinjection of 30 mM OXO into the cerebellar cortex induced a significant decrease in MAP compared to the saline treatment (21.57 %; P < 0.001 via t test; Fig. 2a), which was consistent with our previous report [12]. As shown in Fig. 2e, the selective M2 receptor antagonist MCT blocked the OXO-mediated MAP depressor response. One-way ANOVA demonstrated that MCT dose-dependently (0.3, 1, and 10 mM) attenuated the 30 mM OXO-mediated MAP decrease: MAP (F (3,89) = 34.546, P < 0.001; Fig. 3d) was blocked by 6, 13, and 19 mmHg, respectively; the maximum change in MAP (F (3,89) = 47.334, P < 0.001; Fig. 3e) was attenuated by 6, 11, and 15 mmHg, respectively, and the RT (F (3,89) = 79.081, P < 0.001, Fig. 3f) was shortened by 13, 23, and 32 s, respectively. These results indicate that M2 receptor inactivation in the cerebellar cortex via MCT effectively blocks the OXO-mediated MAP depressor response.
Cerebellar M2 Receptor-Mediated HR Decrease
The HR response to drug stimulation during the RT was also analyzed. The timing of HR change was almost synchronous with MAP changes (Fig. 2b, d, f). Compared to the saline treatment, ABET induced a reduction in HR activity (Fig. 2d). Statistical analysis indicated that ABET (3, 10, and 30 mM) induced a dose-dependent decrease in HR (F (3,85) = 28.026, P < 0.001; Fig. 4a; which was reduced by 22, 39, and 58 BPM, respectively) and an increase in the maximum change in HR (F (2,66) = 128.422, P < 0.001; Fig. 4b; which was 19 ± 4, 34 ± 6, and 47 ± 7 BPM, respectively). Moreover, MCT blocked the HR decrease in response to 30 mM OXO stimulation (Fig. 2f). One-way ANOVA indicated that MCT dose-dependently (0.3, 1, and 10 mM) attenuated the 30 mM OXO-mediated bradycardia: the HR decrease (F (3,89) = 28.388, P < 0.001; Fig. 4b) was blocked by 15, 32, and 47 BPM, respectively; and the maximum change in the HR (F (3,89) = 51.594, P < 0.001; Fig. 4b) was attenuated by 13, 22, and 29 BPM, respectively. These results indicate that cerebellar cortex M2 receptors are involved in cardiac regulation, and the activation of these receptors results in bradycardia.
Histograms of cerebellar cholinergic microinjectionon HR modulation. a HR and maximum change in HR alterations in response to different concentrations of ABET treatments. b HR and maximum change in HR alterations in response to 30 mM OXO mixed with different concentrations of MCT (0.3, 1, and 3 mM). The numbers presented in parentheses denote the number of animals measured per group. *P < 0.05; **P < 0.01. ABET arecaidine but-2-ynyl ester tosylate, HR heart rate, MCT methoctramine hydrate, OXO oxotremorine M
Confirmation of M2 Receptor Expression in the Cerebellar Cortex
The immunohistochemical results demonstrated that the M2 receptor was expressed in the cerebellar cortex (lobule VI) (Fig. 5a–c), whereas no positive staining was observed in the negative control (Fig. 5d–f). These findings confirm an unequivocal localization of M2 receptors in the cerebellar cortex.
Expression and distribution of mACh receptor-2 in the cerebellar cortex. a Immunohistochemical staining, counterstained with thionine, indicates that mACh receptor-2 was present in a sagittal section of the cerebellar cortex (lobule VI), b the molecular layer, and c the Purkinje cell and granular layers at a higher magnification. The arrowheads indicate immunoreaction. d Negative controls were performed on adjacent sections via substitution of the primary antibody for PBS and were counterstained by thionine. e The molecular layer and f the Purkinje cell and granular layers at a higher magnification. The dashed boxes in a and d indicate the origination of “b, c” and “e, f”, respectively. Scale bars a and d = 200 μm; b and e = 100 μm; c and f = 20 μm
Discussion
It has long been established that a cholinergic system is present in the cerebellum [12, 18], and this brain area is involved in central cardiovascular modulation [2]. Thus, it is plausible to suggest that cholinergic neurotransmission in the cerebellum may modulate cardiovascular activities. Notably, the cholinergic effects on cardiovascular regulation depend on the various brain sites and/or actions of different ACh receptors. For example, mACh receptor activation in the rostral ventrolateral medulla (RVLM) resulted in a significant increase in MAP and HR [19], whereas microinjection of ACh into the ventrolateral periaqueductal gray areas and cuneiform nucleus caused a significant MAP depressor response with no striking alterations in HR [20, 21]. Furthermore, nicotinic ACh (nACh) receptor activation in the caudal ventrolateral medulla results in significant depressor and bradycardic responses [22]. Our previous findings demonstrated that ACh microinjection or muscarinic receptor activation in the cerebellar cortex induced a marked down-regulation in systemic MAP [12, 18]; however, the detailed mechanism remains poorly understood.
The present study focused on the role of the muscarinic family M2 receptor in cerebellar cortex-mediated cardiovascular functions. The selective M2 receptor agonist ABET dose-dependently down-regulated systemic MAP (Fig. 3a), and the selective M2 receptor antagonist MCT dose-dependently blocked the nonselective muscarinic agonist OXO-mediated depressor response (Fig. 3d). These data indicate that the M2 receptor contributes homodromously to the muscarinic receptor-mediated MAP depressor response [12]. Moreover, the magnitude of the cerebellar-mediated MAP depressor by an M2 receptor agonist (30 mM, ABET) approached that of a non-selective mACh receptor agonist (30 mM, OXO) (Fig. 3a vs. d in MAP; Fig. 3b vs. e in maximum change in the MAP), which suggests that M2 receptors in the cerebellar cortex substantially contribute to the non-selective mACh receptor-induced MAP depressor response. These physiological effects were also in agreement with previous reports showing that the M2 receptor represents the predominant mACh receptor subtype localized in the cerebellum based on autoradiographic and immunohistochemical studies [8, 9, 23]. In addition, the current findings indicate that M2 receptor activation in the cerebellar cortex exerts a dose-dependent decrease in HR (Fig. 4a), and inactivation of the intrinsic M2 receptor with MCT effectively attenuates OXO-mediated bradycardia, which indicates that the cerebellar M2 receptor is involved in HR modulations. Furthermore, the immunohistochemical results also confirm that the M2 receptor is unequivocally expressed in the cerebellar cortex (Fig. 5).
In the current study and our previous research [12, 18], we demonstrated that the cerebellar cortex induced a significant MAP depressor response as a result of ACh stimulation, and mACh receptors (M2 specifically) substantially contributed to cerebellar ACh-mediated blood pressure modulation; however, the neurochemical circuitry that underlies this process remains largely unknown. The cerebellar cortex is innervated with cholinergic projections [24] and contains various mACh receptors [8, 9]. The output of this region (lobule VI) is principally projected to the fastigial nucleus (FN) [2], which is involved in the baroreceptor reflex [25]. More importantly, the FN (especially in the rostral portion) modulates the renal sympathetic nerve activity, which subsequently affects renal vasoconstriction and blood redistribution [25]. Thus, we speculate that cholinergic signaling in the cerebellar cortex likely influences cardiovascular control through the FN and baroreceptor reflex involvement.
Despite these novel findings, several study limitations should be considered in the interpretation of the results to improve future investigations. First, although MCT is an M2 receptor-selective competitive antagonist, it exhibits low-affinity binding to the M3 receptor at high concentrations [26], which raises the possibility of cross reactivity with other muscarinic receptor subtypes at higher doses. However, this cross reactivity effect, if present, is not expected to be severe because the M2 receptor is the predominant mACh receptor subtype in the cerebellum, based on mRNA expression and protein levels [8, 9]. Second, anesthesia has been reported to blunt effects on baroreflex activity [27, 28]. Although some reports have demonstrated that urethane anesthesia did not significantly affect the blood pressure response or baroreflexive HR activities [29], a similar experiment in conscious animals is worth consideration in the future. Third, mACh receptors comprise five subtypes, including M1, M3, and M5 receptors (which are preferentially coupled to Gq/11 and activate neuronal excitatory cascades) and M2 and M4 receptors (which link to Gi/o and initiate inhibitory procedures) [30, 31]. Therefore, a thorough investigation of the elaborate receptor mechanism for cerebellar mACh receptor-mediated MAP depression is needed in future investigations.
In summary, the present study provides novel evidence that M2 receptors in the cerebellar cortex are involved in MAP and HR regulation, which may represent an important contribution to the muscarinic receptor-mediated blood pressure depressor response. Additional investigations using electrophysiological techniques are needed to clarify the mechanisms that underlie these effects.
References
Schweighofer N, Doya K, Kuroda S (2004) Cerebellar aminergic neuromodulation: towards a functional understanding. Brain Res Rev 44:103–116
Ito M (2012) The cerebellum: brain for an implicit self. FT Press, Upper Saddle River
Zhang Y, Kaneko R, Yanagawa Y, Saito Y (2014) The vestibulo- and preposito-cerebellar cholinergic neurons of a ChAT-tdTomato transgenic rat exhibit heterogeneous firing properties and the expression of various neurotransmitter receptors. Eur J Neurosci 39:1294–1313
Lan CT, Wen CY, Tan CK, Ling EA, Shieh JY (1995) Multiple origins of cerebellar cholinergic afferents from the lower brainstem in the gerbil. J Anat 186(Pt 3):549–561
Ojima H, Kawajiri S, Yamasaki T (1989) Cholinergic innervation of the rat cerebellum: qualitative and quantitative analyses of elements immunoreactive to a monoclonal antibody against choline acetyltransferase. J Comp Neurol 290:41–52
Graham A, Court J, Martin-Ruiz C, Jaros E, Perry R, Volsen S, Bose S, Evans N, Ince P, Kuryatov A (2002) Immunohistochemical localisation of nicotinic acetylcholine receptor subunits in human cerebellum. Neuroscience 113:493–507
Gotti C, Zoli M, Clementi F (2006) Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27:482–491
Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR (1991) Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 11:3218–3226
Tayebati SK, Vitali D, Scordella S, Amenta F (2001) Muscarinic cholinergic receptors subtypes in rat cerebellar cortex: light microscope autoradiography of age-related changes. Brain Res 889:256–259
Prestori F, Bonardi C, Mapelli L, Lombardo P, Goselink R, De Stefano ME, Gandolfi D, Mapelli J, Bertrand D, Schonewille M, De Zeeuw C, D’Angelo E (2013) Gating of long-term potentiation by nicotinic acetylcholine receptors at the cerebellum input stage. PLoS ONE 8:e64828
Rinaldo L, Hansel C (2013) Muscarinic acetylcholine receptor activation blocks long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses via cannabinoid signaling. Proc Natl Acad Sci USA 110:11181–11186
Zhou P, Zhu Q, Liu M, Li J, Wang Y, Zhang C, Hua T (2015) Muscarinic acetylcholine receptor in cerebellar cortex participates in acetylcholine-mediated blood depressor response in rats. Neurosci Lett 593:129–133
Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J (1999) Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA 96:1692–1697
Hong CM, Zheng QS, Liu XT, Shang FJ, Wang HT, Jiang WR (2009) Effects of autoantibodies against M2 muscarinic acetylcholine receptors on rabbit atria in vivo. Cardiology 112:180–187
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Academic Press, Boston
Zhang WN, Bast T, Xu Y, Feldon J (2014) Temporary inhibition of dorsal or ventral hippocampus by muscimol: distinct effects on measures of innate anxiety on the elevated plus maze, but similar disruption of contextual fear conditioning. Behav Brain Res 262:47–56
Zhang C, Hua T, Zhu Z, Luo X (2006) Age-related changes of structures in cerebellar cortex of cat. J Biosci 31:55–60
Zhu Q, Zhou P, Wang S, Zhang C, Hua T (2015) A preliminary study on cerebellar acetylcholine-mediated blood pressure regulation in young and old rats. Exp Gerontol 63:76–80
Kumar NN, Ferguson J, Padley JR, Pilowsky PM, Goodchild AK (2009) Differential muscarinic receptor gene expression levels in the ventral medulla of spontaneously hypertensive and Wistar-Kyoto rats: role in sympathetic baroreflex function. J Hypertens 27:1001–1008
Deolindo MV, Pelosi GG, Busnardo C, Resstel LB, Correa FM (2011) Cardiovascular effects of acetylcholine microinjection into the ventrolateral and dorsal periaqueductal gray of rats. Brain Res 1371:74–81
Shafei MN, Niazmand S, Enayatfard L, Hosseini M, Daloee MH (2013) Pharmacological study of cholinergic system on cardiovascular regulation in the cuneiform nucleus of rat. Neurosci Lett 549:12–17
Aberger K, Chitravanshi VC, Sapru HN (2001) Cardiovascular responses to microinjections of nicotine into the caudal ventrolateral medulla of the rat. Brain Res 892:138–146
de Toro ED, Juíz JM, Smillie FI, Lindstrom J, Criado M (1997) Expression of α 7 neuronal nicotinic receptors during postnatal development of the rat cerebellum. Dev Brain Res 98:125–133
Jaarsma D, Ruigrok TJ, Caffe R, Cozzari C, Levey AI, Mugnaini E, Voogd J (1997) Cholinergic innervation and receptors in the cerebellum. Prog Brain Res 114:67–96
Nisimaru N (2004) Cardiovascular modules in the cerebellum. Jpn J Physiol 54:431–448
Jakubik J, Zimcik P, Randakova A, Fuksova K, El-Fakahany EE, Dolezal V (2014) Molecular mechanisms of methoctramine binding and selectivity at muscarinic acetylcholine receptors. Mol Pharmacol 86:180–192
Fluckiger JP, Sonnay M, Boillat N, Atkinson J (1985) Attenuation of the baroreceptor reflex by general anesthetic agents in the normotensive rat. Eur J Pharmacol 109:105–109
Shimokawa A, Kunitake T, Takasaki M, Kannan H (1998) Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J Auton Nerv Syst 72:46–54
Alves FH, Crestani CC, Resstel LB, Correa FM (2007) Cardiovascular effects of carbachol microinjected into the bed nucleus of the stria terminalis of the rat brain. Brain Res 1143:161–168
Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A, Wess J (2014) Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov 13:549–560
Haga T (2013) Molecular properties of muscarinic acetylcholine receptors. Proc Jpn Acad Ser B Phys Biol Sci 89:226–256
Larsell O (1952) The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol 97:281–356
Acknowledgments
This work was supported by Grants from Natural Science Foundation of Anhui Province (No. 1308085MH127), Natural Science Foundation of Anhui Provincial Education Bureau (No. KJ2013B124), and the Scientific Foundations of Anqing Normal University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors confirm that there are no conflicts.
Rights and permissions
About this article
Cite this article
Zhang, C., Sun, T., Zhou, P. et al. Role of Muscarinic Acetylcholine Receptor-2 in the Cerebellar Cortex in Cardiovascular Modulation in Anaesthetized Rats. Neurochem Res 41, 804–812 (2016). https://doi.org/10.1007/s11064-015-1755-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1755-2