Abstract
Phenylketonuria and hyperphenylalanemia are inborn errors in metabolism of phenylalanine arising from defects in steps to convert phenylalanine to tyrosine. Phe accumulation causes severe mental retardation that can be prevented by timely identification of affected individuals and their placement on a Phe-restricted diet. In spite of many studies in patients and animal models, the basis for acquisition of mental retardation during the critical period of brain development is not adequately understood. All animal models for human disease have advantages and limitations, and characteristics common to different models are most likely to correspond to the disorder. This study established similar levels of Phe exposure in developing rats between 3 and 16 days of age using three models to produce chronic hyperphenylalanemia, and identified changes in brain amino acid levels common to all models that persist for ~16 h of each day. In a representative model, local rates of glucose utilization (CMRglc) were determined at 25–27 days of age, and only selective changes that appeared to depend on Phe exposure were observed. CMRglc was reduced in frontal cortex and thalamus and increased in hippocampus and globus pallidus. Behavioral testing to evaluate neuromuscular competence revealed poor performance in chronically-hyperphenylalanemic rats that persisted for at least 3 weeks after cessation of Phe injections and did not occur with mild or acute hyperphenylalanemia. Thus, the abnormal amino acid environment, including hyperglycinemia, in developing rat brain is associated with selective regional changes in glucose utilization and behavioral abnormalities that are not readily reversed after they are acquired.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenylketonuria (PKU) and hyperphenylalanemia comprise a group of inborn errors of metabolism of phenylalanine arising from defects in phenylalanine hydroxylase, dihydropteridine reductase (the co-enzyme required for regeneration of the oxidized cofactor, tetrahydrobiopterin), or in the enzymatic steps to synthesize the cofactor [1, 2]. Classical PKU is caused by the absence of phenylalanine hydroxylase, and is characterized by increased plasma and tissue phenylalanine levels, severe mental retardation, epilepsy, neurotransmission disorders, myelination deficits, and other biochemical and behavioral abnormalities in untreated children [3, 4]. Hyperphenylalanemia arises from incomplete inhibition of phenylalanine hydroxylase or other deficits in metabolic pathways required for conversion of phenylalanine to tyrosine, and the affected patients also acquire serious neurological deficits [3, 4]. The most effective treatment for PKU is detection of high phenylalanine levels in blood in the newborn and placement of the subject on a phenylalanine-restricted diet. Close control and monitoring of hyperphenylalanemia during the period of brain development prevents or minimizes acquisition of mental retardation, but dietary control is difficult to sustain and the patients do not have normal metabolic homeostasis. A number of additional treatment paradigms (e.g., dietary supplementation with tetrahydrobiopterin, synthetic forms of the cofactor, or large neutral amino acids) have, therefore, been undertaken to improve clinical care and reduce the deleterious effects of PKU and hyperphenylalanemia [5].
The pathogenesis and basis for acquisition of cognitive defects associated with PKU and hyperphenylalanemia has been intensively studied in patients and animal models during the past 50 years, but the critical mechanisms that lead to disruption of brain development and cause mental retardation are still not adequately understood. Blockade of Phe hydroxylation to generate tyrosine leads to its metabolism by alternative pathways to generate increased levels of minor metabolites, including phenylethylamine, phenylacetate, 2-hydroxyphenylacetate, phenylpyruvate, and phenyllactate. High levels of Phe and its metabolites can have concentration- and exposure duration-dependent effects on transport systems, metabolic pathways, and developmental processes in vitro or in vivo, ranging from inhibition or modulation of amino acid transport across the blood–brain barrier and cellular membranes, glycolytic and oxidative metabolism of glucose, fatty acid metabolism, neurotransmitter turnover, myelination, dendritic arborization, and synaptic spines [6–8]. However, the pathophysiological relevance of any one or combination of these effects to neurological deficits of PKU remains to be established.
To better understand the biochemical, developmental, physiological, and behavioral changes associated with hyperphenylalanemia, a number of animal models have been developed by treatments with their onset at various postnatal ages for different durations using specific metabolites of Phe given alone (e.g., phenylacetate [9, 10]), or different doses of Phe either alone [11] or in combination with an inhibitor of Phe hydroxylase (p-chlorophenylalanine (pCPhe) or α-methylphenylalanine (αMePhe) [12]). A PKU mouse model (BTBR background-Pahenu2) was subsequently developed by producing germline mutagenesis with ethylnitrosourea (enu) and identification of Phe hydroxylase deficiency by Phe clearance screening [13]. These models reproduce many of the biochemical, developmental, and behavioral characteristics of PKU patients, but the use of enzyme inhibitors involves side effects of the drugs and differential toxicity, with pCPhe having greater deleterious effects than αMePhe or high doses of Phe alone, and the residual Phe hydroxylase activity in the rat models causes high levels of tyrosine (e.g., [12, 14–16]). The Phe hydroxylase-deficient mouse model is generally considered advantageous due to lack of inhibitor side effects, but lack of sufficient demand led Jackson Laboratory to maintain the mutant lines by cryopreservation, increasing the cost and time to obtain the animals unless they are available from active PKU researchers [17]. All animal models for human disease have advantages and limitations, and characteristics common to different models are most likely to represent those of the human disorder. In addition, specific models may be useful to evaluate the contributions of different characteristics to pathophysiology, as for example differential effects on levels of neurotransmitters due to elevated levels of tyrosine. If similar biochemical characteristics are established in non-genetic PKU animal models, various studies could be readily carried out in many laboratories and subsequently compared with the Pahenu2 mouse model. The present study, therefore, established similar levels of Phe exposure in three models for hyperphenylalanemia in developing rats between 3 and 16 days of age, and identified changes in brain amino acid content common to all models. In one model, changes in local rates of glucose utilization (CMRglc) were observed in specific brain structures, and behavioral testing to evaluate neuromuscular competence revealed poor performance in the chronic hyperphenylalanemic rats that persisted for at least 3 weeks after cessation of Phe injections.
Materials and Methods
Materials
l-Phenylalanine (Phe) and α-methyl-l-phenylalanine (αMePhe) were obtained from ICN Life Sciences Group (Cleveland, OH, USA). p-Chlorophenylalanine (pCPhe) and other reagents were obtained from Sigma-Aldrich (St. Louis, MO) and were of the highest purity available. Amino acid standards and norleucine were purchased from Pierce Chemical Co. (Rockford IL, USA). 2-Deoxy-D-[U-14C]glucose ([14C]DG, 343 mCi/mmol) and calibrated [14C]methylmethacrylate standards were obtained from DuPont/NEN (Boston, MA, USA).
Experimental Procedures
Hyperphenylalanemia
Procedures were similar to those previously described [12, 18, 19] and used either αMePhe or pCPhe to inhibit liver phenylalanine hydroxylase along with large doses of Phe in the presence or absence of enzyme inhibition. Stock solutions (all were adjusted to pH 7.2) for injection of αMePhe (70 μmol/mL), αMePhe (70 μmol/mL) plus Phe (152 μmol/mL), or Phe (152 μmol/mL) were prepared in 0.9 % NaCl (w/v) with warming. Suspensions of pCPhe (26 μmol/mL) were prepared in 0.9 % NaCl or in Phe (152 μmol/mL), sonicated, and aliquots were taken during rapid stirring and injected immediately. For chronic treatments of animals used for brain amino acid analyses at age 10 or 16 days, Fisher CDF rats were injected subcutaneously once-daily with αMePhe (2.4 μmol/g body weight), αMePhe (2.4 μmol/g) + Phe (5.2 μmol/g), or with a similar volume of 0.9 % NaCl from age 3 to 10 days, and the daily Phe dose was doubled from 11 to 16 days (i.e., 2.4 μmol/g αMePhe + 10.4 μmol/g Phe) to maintain plasma and brain Phe concentrations at elevated levels for a longer duration. In addition, two other procedures also were used to produce chronic hyperphenylalanemia from age 3 to 10 or 16 days, (1) pCPhe (2.6 μmol/g given on alternate days starting on day 3) + Phe (5.2 μmol/g given daily) [12], and (2) two daily injections of Phe, each consisting of 6.1 μmol/g given 2 h apart, from age 3 to 10 days [11]. For acute treatments, animals were given single or short-term injections as described in the text. For glucose utilization and behavioral assays, separate groups of Wistar rats from a commercial supplier were used because these studies were carried out at a different institution and Fisher rats from the ‘in house’ colony were not available. The Wistar rats were injected daily with either (1) αMePhe (2.4 μmol/g) + Phe (5.2 μmol/g) from 3 to 27 days, or (2) a daily supplemental dose of Phe (2.6 μmol/g) was injected about 10–12 h after the daily injection of αMePhe (2.4 μmol/g) + Phe (5.2 μmol/g) from 3 to 25 or 27 days; these animals were also used for the string test behavioral assays. Animals were weaned at age 21 days and given free access to water and Purina Chow. All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Amino Acid Analysis
Fisher CDF rats were killed by decapitation, brains were quickly excised and grossly dissected on an ice-cold platform to obtain tissue samples comprised of cerebral cortex, hippocampus, and caudate (hereafter referred to as brain). Brains from 4 to 6 animals were pooled for each sample and homogenized in ice-cold deionized water, followed by addition of ice-cold trichloroacetic acid (final concentration 0.3 mol/L) containing norleucine as an internal standard. After mixing and standing for 20 min, the samples were centrifuged at 12,000g for 20 min, and the supernatant fractions were stored at −20 °C until analyzed. Amino acids in the acid-soluble fractions were separated on a Beckman 120C automatic amino acid analyzer using a program for physiological fluids [20], ninhydrin detection, and a Beckman system AA computing integrator that was calibrated with amino acid standards (Pierce Chemical Cl., Rockford IL, USA) [19]. Recovery of norleucine ranged from 91 to 99 %. Arginine and glutamine were not resolved by the separation procedure, but brain contains very low amounts of arginine [21], and results are tabulated as glutamine. Postmortem increases in the concentrations of γ-aminobutyric acid (GABA) that is derived from glutamate, which decreases proportionally, and alanine occur with a lag of about 1 min after death, increasing thereafter [22, 23]. Since the time between death and protein precipitation was about 2–2.5 min, the reported concentrations of these amino acids are not their true values. Brain amino acid concentrations in the NaCl-treated rats showed little variation with respect to time of day when tissue was harvested, and effects of any daily rhythms to changes in amino acid levels in the hyperphenylalaninemic animals is considered negligible; there were no differences in amino acid levels in animals given chronic or acute NaCl injections. Values for all NaCl-injected animals were, therefore, averaged to obtain mean (±SD) control values.
Local Rates of Cerebral Glucose Utilization (CMRglc)
CMRglc was assayed in awake, non-fasted 25–27-day-old control and acute and chronic hyperphenylalaninemic Wistar rats (Charles River, Wilmington, MA, USA) that were given treatments described in the text and weaned at 21 days. Assays were carried out according to the procedures of Sokoloff et al. [24], adapting the methods previously described for adult rats [25] for use in weanlings. In brief, tail arterial (PE10 Intramedic polyethylene tubing, Clay Adams division of Becton–Dickinson, Parsippany, NY, USA) and external jugular venous catheters (Silastic, Dow Corning medical-grade tubing; Midland, MI, USA) were inserted under halothane anesthesia (1–1.5 % in air with dose adjustments as needed to maintain the unconscious state), the animals were restrained by the hind limbs on plastic boards, and they were allowed about 4 h recovery until the experiment. Rectal temperature was maintained at 37 °C with a heat lamp connected to a thermistor throughout the recovery and experimental intervals. Baseline blood pressure, rectal temperature, and arterial plasma glucose levels were determined immediately prior to the pulse intravenous injection of [14C]DG (10 μCi in 0.1 mL 0.9 % NaCl). Timed samples of arterial blood (10 μL) were drawn for analysis of plasma [14C]DG and glucose concentrations by liquid scintillation counting with external standardization and Beckman glucose analyzer, respectively. At the end of the 45 min experimental period the rats were anesthetized with thiopental (50 mg/kg, iv), decapitated, and their brains were removed and frozen in isopentane at about −45 °C. Frozen 20 μm-thick coronal sections were cut in a cyrostat at −20 °C, dried, and exposed to Kodak SB-5 medical X-ray film along with calibrated 14C-standards. Local 14C concentrations in brain regions of interest were determined by densitometry, and CMRglc was calculated with the operational equation of the method using values for the lumped constant and rate constants for normal adult rats [24, 25].
String Test to Evaluate Coordination
Behavioral testing using a tightrope to evaluate neuromuscular competence [26] was carried out essentially as described by Barclay et al. [27] except that a different scoring procedure was used. The testing apparatus consisted of a nylon cord (3 mm thick, 50 cm long) tightly tied between two vertical poles 60 cm above a 2.5 cm-thick foam landing pad; ring stand platforms were located at each end of the string. The test was initiated by holding the rat by the tail and allowing it to grab the string with both forepaws near the midpoint between the platforms. Then the animal was gently released and scored by one or two observers blind to the treatment of the animal; the duration of the test was 1 min unless terminated sooner if the rat fell off the string or traversed to a platform. Because hyperphenylalaninemic rats were less active than controls discrimination between groups was increased by awarding points for contact of paws and tail with the string and speed of traversing the string to the platform and assigning penalty (negative) points for less contact with the string, inactivity, and falling. Rats were tested at twice daily, at 4–6 h after the last phenylalanine injection when brain phenylalanine levels were highest, in the range of 2–4 μmol/g, and at about 12 h after the last injection when the brain phenylalanine level had fallen below from peak levels (See “Results” section, Fig. 1). If a rat received a score of ≤2 it was re-tested about 2–3 min later and both scores averaged. Details of the testing and scoring systems are presented in the legend to Fig. 4.
Chronic hyperphenylalanemia produced by three procedures. Brain phenylalanine (a) and tyrosine (b) levels were determined at intervals after the last Phe injection and are reported as % control (see Table 1 for absolute values). Rats were injected once daily from age 3 to 5, 7, 10 or 16 days with α-methylphenylalanine (αMePhe, 2.4 μmol/g body weight) + phenylalanine (Phe, 5.2 μmol/g from age 3 to 10 days and 10.4 μmol/g from age 11 to 16 days), p-chlorophenylalanine (pCPhe, 0.9 μmol/g on alternate days) + Phe (5.2 μmol/g daily), or two daily doses of Phe (6.1 μmol/g) given 2 h apart. Each sample is derived from a pool comprised of 4–6 brains. Lines illustrate the temporal trends
Statistics
Statistically-significant differences between control and experimental values were identified with the Student’s t test or one- or two-way analysis of variance (ANOVA) with Dunnett’s test for multiple comparisons against a single control. P < 0.05 was considered significant.
Results
Optimization of Phenylalanine Dosing to Produce Sustained Elevations of Phe in Plasma and Brain
The study establishing the use of αMePhe in a model for chronic hyperphenylalanemia showed that a dose of 2.4 μmol/g αMePhe reduced liver Phe hydroxylase activity by about 65–75 % and injections of αMePhe + Phe caused 20–30-fold increases in plasma Phe concentration and about twofold increases in plasma tyrosine level; age-related gains in body and brain weight, brain protein and DNA content, and developmental profiles of 6 enzymes were similar to those of controls whereas phosphoserine phosphatase was elevated in hyperphenylalanemic rats [12]. In contrast, rats treated with pCPhe had a 30–60 % mortality rate and grew more slowly than those given αMePhe [12].
Initial experiments in the present study (not shown) evaluated the time courses of Phe clearance from plasma and brain at 10 and 16 days of age after a single injection of Phe and after chronic αMePhe + Phe injections, and demonstrated that the rate constant for Phe clearance was linearly correlated with liver phenylalanine hydroxylase activity. In brief, in 10-day-old rats the plasma Phe concentration peaked at about 6 μmol/mL within 30 min after a dose of αMePhe + 5.2 μmol Phe/g body wt., fell by about 30 % at 2 h, remained above 1 μmol/g for 16 h, and returned to near-normal between 16 and 20 h. Because clearance of Phe from plasma and brain was faster in older rats due to higher phenylalanine hydroxylase activity that is not prevented by αMePhe, the daily Phe doses were doubled starting at day 11. In 16 day-old rats that were given once-daily αMePhe + 5.2 μmol/g Phe doses from 3 to 10 days and αMePhe + 10.4 μmol/g Phe from 10 to 16 days, plasma Phe peaked at about 9 μmol/mL at 0.5–2 h, and declined by about 30 and 80 % at 2 and 8 h, respectively, becoming near normal by 14–16 h. At both ages, maximal brain Phe level lagged that in plasma, consistent with transport limitation, and it had a lower peak of 2.5–3 μmol/g at about 6 h after the injections (Fig. 1a). In contrast, inhibition of phenylalanine hydroxylase by pCPhe was more effective at 10 and 16 days than with αMePhe, and brain Phe was in the range of 0.7–2.1 μmol/g during 6–20 h after the 5.2 μmol/g Phe injection, i.e., the 16-day-old pCPhe-treated rats had elevated brain Phe levels for a longer period with lower Phe doses than with α-MePhe treatments (Fig. 1a). Double injections of Phe alone raised the brain Phe level in 10-day-old rats to 3.7 μmol/g at 6 h and it normalized by 20 h (Fig. 1a). Brain tyrosine levels were highest in rats injected with only Phe and no inhibitor, peaking at 12–15-fold above normal, whereas the rats treated with αMePhe or pCPhe had 2–4-fold elevations in brain tyrosine content due to incomplete inhibition of the enzyme (Fig. 1b). The temporal profiles of brain Phe concentrations were generally similar in the different models for hyperphenylalanemia, except that its levels were highest in rats given the highest Phe doses (i.e., double injections of Phe alone and higher doses of Phe in the αMePhe-treated rats from 11 to 16 days) (Fig. 1a).
The brain concentrations of the phenylalanine hydroxylase inhibitors were also estimated. The amino acid analysis procedure did not separate αMePhe from tyrosine, and the results for tyrosine in Fig. 1b are reported as the sum of the two amino acids. However, the ninhydrin color coefficient (area/μmol) for tyrosine was 6.3 times that of αMePhe, and overestimation of tyrosine level would be <15 % if both amino acids were present in equimolar amounts. In separate assays for tyrosine using a fluorometric procedure [28, 29], concentrations of standard tyrosine agreed within 5 % of those obtained by amino acid analysis and αMePhe did not contribute fluorescence or interfere with the assay. Brain αMePhe concentrations were, therefore, estimated by difference, from tyrosine levels determined by the two methods. Approximate αMePhe concentration ranges in brain of 10-day-old rats were 0.3–0.6 μmol/g at 6 and 12 h after the last injection and 0.9–1.2 at 20 h; in 16-day-old rats αMePhe was 0.2–0.3 μmol/g at 6 and 12 h after the injection. Brain tyrosine levels ranged from 0.2 to 0.4 μmol/g in the same animals indicating that any contributions of αMePhe to tyrosine levels were generally small (<15 %). Phe, Tyr, and pCPhe were resolved by the analytical procedure, and brain pCPhe levels ranged from about 0.2–0.4 and 0.2–0.3 μmol/g in 10- and 16-day old rats, respectively, at 6–20 h after the last pCPhe injection.
To summarize, the rate constant for clearance of Phe from plasma correlated with liver Phe hydroxylase activity in αMePhe-treated rats, but different relationships were obtained with pCPhe- and Phe-treated rats (not shown), indicating that brain and plasma Phe concentrations cannot be predicted simply from knowledge of Phe hydroxylase activity and Phe dose; other factors, perhaps cofactor availability, are also involved. An important consideration is that there is little benefit of greatly increasing the Phe dose to maintain elevated brain Phe levels because the plasma levels appear to be sufficiently high to saturate brain Phe uptake (Kt of the large neutral amino acid transporter ≈ 0.45–0.9 mmol/L in the presence of other amino acids [30, 31]). More frequent injections using lower Phe doses are a better approach to sustain its level in brain. The doses of Phe used in the above three models to produce chronic hyperphenylalaninemia cause large, dynamic changes in plasma and brain levels that vary with Phe dose and time after injection (Fig. 1a). These levels approximate or exceed the range of values in plasma (~1.8–3.3 μmol/mL) of fasting human phenylketonuric children [3], in brain of adult phenylketonuric patients determined with magnetic resonance spectroscopy (~0.2–0.9 mmol/L or per kg wet wt.) [32–40], or in adult phenylketonuric brain at autopsy (~0.8–1.4 μmol/g) [41, 42]. Blood and tissue Phe levels in PKU patients are governed by clinical and dietary intervention treatments, diet composition, and time after eating, and Phe levels would be higher in untreated patients. All three rat models have the disadvantage that they cause elevated levels of tyrosine and the enzyme inhibitors have side effects. Nevertheless, biochemical changes common to all three experimental animal models are likely to be most relevant to the pathophysiology of phenylketonuria.
Brain Amino Acid Levels in Chronic Hyperphenylalanemic Rats
Daily injections of αMePhe alone increased brain Phe concentration about fourfold at 10 and 16 days of age without increasing brain Tyr levels (Table 1). Ile and phosphoethanolamine levels were depressed and glycine increased at 10 days. Inclusion of single daily injections of Phe (5.2 μmol/g from 3 to 10 days and 10.4 μmol/g from 11 to 16 days) with αMePhe greatly increased brain Phe and Tyr levels, depressed concentrations of neutral amino acids by 30–50 %, and increased Gly level to a greater extent than inhibitor alone, as previously reported [19]. At 10 days the concentrations of phosphoserine and Glu were reduced by about 20 %, whereas at 16 days the levels of Tau and Asp were elevated by 50 and 84 %, respectively. Thus, there were age-related and Phe concentration-dependent effects on specific brain amino acid levels in the αMePhe + Phe-treated rats (Table 1).
Temporal profiles of changes in concentrations of brain amino acids at intervals after the last Phe injection and comparisons with effects of treatment with αMePhe + Phe, pCPhe + Phe, and double daily doses of Phe are illustrated in Fig. 2. Most but not all of the trends observed for the 10-day-old αMePhe + Phe-treated rats (solid lines) were evident at 5, 7, and 16 days of age, and were also observed for the two other treatments to produce chronic hyperphenylalanemia. Elevated levels of Gly were sustained in the range of about 1.5–3.5-fold above normal throughout the day, with highest concentrations in the rats given the largest dose of Phe (double injections with no inhibitor), intermediate in pCPhe-treated rats, lower but still 1.6–3-fold above normal in the αMePhe + Phe-treated rats (Fig. 2a), and lowest in those given only αMePhe (Table 1); these findings suggest Phe-concentration–time dependence of hyperglycinemia. This conclusion is supported by findings in the 16-day-old rats in which the brain glycine concentrations determined at 6 and 12 h after the last Phe injection were 2–3-fold higher in rats injected with (1) αMePhe + 10.4 μmol/g Phe from age 11 to 16 days compared with those given 5.2 μmol/g Phe during the same interval, or (2) pCPhe-treated rats given Phe doses 5.2 μmol/g compared with 2.6 μmol/g from age 3 to 16 days (data with lower Phe doses not shown). The brain concentrations of other neutral amino acids (Fig. 2b–f) were lowest, in the range of 30–75 % of control, when Phe was highest (i.e., about 6–12 h, Fig. 1a), and they tended to normalize when Phe was cleared from brain. Brain Phe concentration fell more rapidly (Fig. 1a) in the 16-day-old αMePhe + Phe-treated rats where Phe hydroxylase activity (not shown) increased in spite of the inhibitor and cleared Phe from plasma and brain more rapidly. Of interest, the mean levels of Glu (Fig. 2g) and phosphoserine (Fig. 2j) between 2.5 and 15 h after the last injection were depressed by about 20–40 % (P < 0.05) in all three animal models in 10-day-old rats, but not for Glu in the 16-day-old αMePhe + Phe-treated rats or for P-Ser in the pCPhe + Phe-treated 16-day-old rats, indicating age- and treatment-dependent effects of hyperphenylalanemia (Table 1; Fig. 2). Curiously, Arg levels were significantly reduced at 10 days in the pCPhe + Phe- and double dose-phenylalanine-treated rats and tended to be below normal in αMePhe + Phe-treated rats but rose above normal as Phe was cleared from brain (Fig. 2i). Disruption of brain amino acid levels was most severe in the pCPhe-treated rats, perhaps due to side effects of the drug, and these animals had significantly elevated levels of Lys that was not observed in the two other models (Fig. 2h). Both pCPhe + Phe-treated and Phe-injected 10-day-old rats also had significantly reduced mean levels (range 23–39 %, P < 0.05) over the 2.5–15 h interval for Gln (Fig. 2k), Asp (Fig. 2l), and Ser (not shown) that were not evident in the 10 day-old αMePhe + Phe-treated rats (Table 1), perhaps reflecting differences in brain Phe exposure (i.e., area under curve in plots of concentration vs. time that was greatest in these two groups). The effects of higher daily Phe dosage are also evident when pCPhe-treated rats were given daily Phe doses of 2.6 (data not shown) or 5.2 μmol/g. With the higher dose, the mean levels of Asp, Ser, Gln, Glu, Ala, Met, GABA, Orn, and Arg were depressed to a greater extent during the 6, 12, and 20 h interval after the last Phe injection. To sum up, at 10 days all three animal models had elevated Gly content throughout the day and reduced levels of phosphoserine, Glu, and neutral amino acids, most of which varied with brain Phe content, and had some age- and treatment-dependence of the magnitude of change. Because the αMePhe + Phe-treated rats exhibited most of the characteristics of the two other animal models but were healthier and had tyrosine levels in the lower range (2–3-fold increases), these animals were used for assays of CMRglc and behavior.
Temporal and age-dependent changes in brain amino acid concentrations evoked by chronic hyperphenylalanemia produced by three procedures. Treatments are described in the legend to Fig. 1. Brains were sampled at the indicated times after the last Phe injection, and each value represents a single pool comprised of 4–6 brains or the mean of two independent pooled samples for that time point. For the αMePhe + Phe-treated rats there were two pooled samples at 6, 12, and 20 h in 10-day-old rats, 2 pooled samples at 6 and 12 h at 16 days, single pooled samples at 2.5, 9, and 15 h at 10 days, and at 12 h at 5 and 7 days. The pCPhe + Phe-treated rats had 2 pooled samples each at 6, 12, and 20 h at 10 days, and 2 each at 6 and 12 h at 16 days. The 10 day-old Phe-treated rats had 2 pooled samples at 6 and 12 h, and 1 at 20 h. Lines connect the points in the 10-day-old rats to illustrate the temporal trends for brain amino acid levels in the three models at this age
Local Rates of Cerebral Glucose Utilization in Chronic Hyperphenylalanemic Rats
Phenylalanine or its metabolites, e.g., phenylpyruvate, can interfere with whole-brain glucose utilization in brain in vivo after a single Phe injection [43] and with pyruvate and ketone body metabolism in vitro (e.g., [44, 45]). Rates of glucose utilization (CMRglc) were, therefore, determined in brain of weanling rats that were given once-daily injections from age 3 to 25 days of either NaCl (controls), αMePhe + Phe (5.2 μmol/g), or only αMePhe. CMRglc was also assayed after a single injection of αMePhe + Phe (5.2 μmol/g). CMRglc was measured at 4 and 24 h after the last Phe injection when brain Phe concentrations would be highest (2–4 μmol/g) or near-normal (0.05 μmol/g), respectively (Fig. 1a). Glucose utilization rates throughout the brain were largely unaffected by chronic hyperphenylalaninemia, with selective decreases (some time-dependent) in CMRglc in the mamillary body and two structures in the auditory pathway, the cochlear nucleus and inferior colliculus (Table 2). Chronic treatment with αMePhe alone that causes modest chronic hyperphenylalanemia (Table 1) had no effect on CMRglc, whereas a single injection of αMePhe + Phe caused increases in CMRglc at 4 h in a number of gray and white matter regions that were unaffected by chronic treatment (Table 2), suggesting adaption to chronic treatment. Baseline values for physiological variables, arterial plasma glucose concentration, hematocrit, and blood pressure were similar in all experimental groups (Table 2).
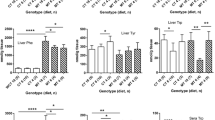
Because single daily injections of αMePhe + Phe (5.2 or 10.4 μmol/g, Fig. 1) do not maintain elevated brain Phe concentrations for the entire day after age 10 days, CMRglc was also determined in a second group of rats that was given supplementary daily injections of 0.9 % NaCl (n = 8) or Phe (2.6 μmol/g, n = 8) at 10–12 h after the αMePhe + Phe (5.2 μmol/g) injection from age 3 to 27 days. There were selective statistically-significant changes in four structures, frontal cortex (82 % control), radiatum layer of hippocampus (115 % control), thalamus (85 % control), and globus pallidus (113 % control) (Fig. 3), that differed from those of the lower duration of brain Phe exposure (Table 2). Baseline physiological variables were similar to those of animals given single daily injections (see legend to Table 2).
Selective changes in glucose utilization in rats given two daily injections of phenylalanine. Rats were given daily injections from age 3 to 27 days of 0.9 % NaCl or α-methylphenylalanine (αMePhe, 2.4 μmol/g) + phenylalanine (Phe, 5.2 μmol/g) plus a second daily injection 10–12 h later of only Phe (2.6 μmol/g) for the hyperphenylalanemic rats (n = 8) or of NaCl for the controls (n = 8). Statistically significant (*P < 0.05, t test) differences were observed in four structures, with no changes in CMRglc in the other brain regions of interest that are presented in Table 2 in which the rats did not receive second daily Phe injections. CMRglc was measured in the same rats used for the behavioral testing in Fig. 4a
Behavioral Changes in Chronic Hyperphenylalanemic Rats
Prior to determination of CMRglc in the rats given supplementary daily Phe injections (Fig. 3), their muscular coordination was evaluated with the string test. The chronic hyperphenylalanemic rats performed worse than the age-matched controls when assayed for several consecutive days at 4 or 24 h after the last injection (Fig. 4a, left panel). Because the Phe-treated rats tended to be less mobile, hang onto the string with forepaws without traveling towards the rest platform, and then to drop to the landing pad, the initial scoring system (A) was changed to assign penalties for these behaviors and award points for faster travel to the platform using more limbs and tail (scoring system B, see legend Fig. 4); scoring system B amplified difference between the control and chronically hyperphenylalanemic rats (Fig. 4a, right panel). Poor performance on the string test persisted for at least 3 weeks after the twice-daily Phe injections were terminated at age 22 days (Fig. 4b), and poor performance was not observed during mild hyperphenylalanemia (treatment with only αMePhe, Fig. 4c), or after a single injection of Phe or after 36 h treatment with αMePhe + Phe (Fig. 4d). Thus, persistent behavioral abnormalities depend on chronic hyperphenylalanemia and are associated with selective changes in brain amino acid levels and CMRglc during the resting state, not global CMRglc deficits.
Behavioral testing of hyperphenylalanemic rats. a Rats were given daily injections of 0.9 % NaCl or α-methylphenylalanine (αMePhe, 2.4 μmol/g) + phenylalanine (Phe, 5.2 μmol/g) plus a second injection 10–12 h later of only Phe (2.6 μmol/g) or NaCl from age 3 to 27 days. Rats were weaned at age 21 days. Twice-daily string tests were initiated at age 23 days; the odd-numbered trials were carried out at 6 h after the NaCl or α-MePhe + Phe injection when brain Phe concentrations would be highest (see Fig. 1), and subsequent even numbered trials at ages 24–26 days were carried out at 12 h after the NaCl or α-MePhe + Phe injection (just prior to the second Phe injection) when brain Phe level would be lower. Scoring system A (see below) was used for trials 1–5 and scoring system B was used for trials 6–8 (n = 13 controls and n = 6–13 hyperphenylalanemic rats). These rats were then used to determine CMRglc (Fig. 3). b Rats were given daily injections as described above from age 3 to 22 days, then the injections were terminated. Once-daily string tests using scoring system B were carried out from age 19 to 54 days (n = 5 controls and 4–5 hyperphenylalanemic rats). c Rats were given single daily injections from age 3 to 35 days of 0.9 % NaCl (n = 5) or αMePhe (2.4 μmol/g; n = 7) and string tests scored with system B were carried out at intervals beginning on day 25. d Rats were given a single injection of 0.9 % NaCl (n = 6) or phenylalanine (Phe, 5.2 μmol/g; n = 6) at age 31 days and string tests scored with system B were carried out at timed intervals. At 12 and 24 h after the last string test, the rats were given αMePhe (2.4 μmol/g) + Phe (5.2 μmol/g), followed by Phe (5.2 μmol/g) at 36 h, and αMePhe (2.4 μmol/g) + Phe (5.2 μmol/g) at 48 h; string tests carried out at 3 h and 7 h after the last injection. The major difference between the scoring systems A and B is that system B gives more points for rapid movement along the string to the platform and penalizes hanging in place; these changes increase behavioral discrimination between the control and experimental rats. Scoring systems A and B, respectively, allocated points as follows, where dashes indicate a characteristic that was not included in the scoring system: each paw kept on string for ≥5 s (1 point, 1 point); tail kept on string for ≥5 s (2, 2); continuous travel along string for each 5 s of travel (3,3); reaching one of vertical poles (2, –) within 0–5 s (–, 16), 5–15 s (–, 7), 16–30 s (–, 4), or 31–60 s (–, 2); falling off string within 0–15 s (−3, −3), 16–30 s (−2, −2), or 31–60 s (−1, −1); penalties for each occurrence of hanging motionless for 5 s (−3, −3) or hanging in place without moving paws from their position on the string (–, −2). Values are mean ± SEM. Rats in all experimental groups were weaned at age 21 days. The string test scores for the hyperphenylalanemic rats were significantly lower than controls in panels A and B (P < 0.001, 2-way ANOVA), whereas there were no significant differences among the groups in panels C and D. Body weights at 23, 25, and 27 days, respectively, were 48.7 ± 1.1, 57.8 ± 1.3, and 69.4 ± 1.4 g for controls and 40.6 ± 1.8, 46.9 ± 2.0, and 51.6 ± 0.5 g for hyperphenylalanemic rats; weights of the Phe-treated rats were significantly less than controls at all ages (P < 0.001, 2-way ANOVA and Bonferroni multiple comparisons test)
Discussion
Chronic Hyperphenylalanemia-Evoked Hyperglycinemia is Common to Different PKU Models
The major findings of the present study demonstrate that three procedures to produce chronic hyperphenylalanemia in the developing rat cause large, sustained increases in brain phenylalanine content, smaller increases in brain tyrosine level, and time-, age-, and phenylalanine-concentration dependent disruption of brain amino acid levels, most of which are common to all three models. Fluctuations in the brain amino acid levels after the last Phe injection were generally related to changes in brain Phe concentration due to the Phe injection and its appearance in and clearance from blood. The main exception was glycine, which was elevated at 12 h in 5- and 7-day-old rats and stayed high throughout the day in 10, 16, and 30-day-old rats (Table 1; Fig. 2, [14, 19]). Glycine levels were not increased in spinal cord or liver of hyperphenylalanemic rats [19]. Significant increases in brain glycine content are also evoked in 6-week-old rats fed a high Phe diet for 2 weeks [46] and a 2-day-old monkey fed a high Phe diet for 20 weeks [47]. Notably, elevated brain glycine levels are reported in 10-day-old and 4-week-old Phe hydroxylase-deficient mice in conjunction with abnormal levels of other amino acids at 4 weeks, including reduced levels of glutamate, aspartate, and glutamine but no change in GABA [48, 49]. Elevated glycine evoked by chronic hyperphenylalanemia in 3 different rat models, a monkey, and the genetic mouse PKU model is particularly important because it demonstrates that the cause(s) of hyperglycinemia are not related to side effects of the Phe hydroxylase inhibitors or of elevated blood and brain tyrosine concentrations. These data are relevant to high brain glycine levels that are considered to be neurotoxic in patients with nonketotic hyperglycinemia [50–52]. Glycine is detectable in human brain by 1H-MRS [53], but, to our knowledge, brain tissue glycine level has not been carefully examined in PKU patients. MRS technology appears to mainly have been used in PKU patients to determine brain Phe levels and relationships between blood and brain Phe levels under various conditions and amino acid supplementation therapy (e.g., [36, 54]). Metabolic profiling of PKU patients by 1H-MRS may be a useful approach for future studies.
The mechanisms responsible for chronic hyperphenylalaninemia-evoked hyperglycinemia are not known, and some possibilities have been previously discussed in detail [19]. In brief, brain glycine levels fall from peak levels shortly after birth to lowest levels at about 15 days, then slowly rise to adult levels, and chronic hyperphenylalaninemia prevents this fall [19]. A threshold and tissue specificity for Phe exposure to increase brain glycine content is suggested by the finding that 3–4-fold increases in brain Phe level caused by chronic treatment with αMePhe (Table 1) or pCPhe alone cause smaller increases brain glycine level [19], with no changes in spinal cord and liver even though these tissues have elevated Phe levels [19]. Furthermore, chronic treatment with serine, leucine, tyrosine or phenylacetate did not raise brain glycine levels, indicating that disruption of neutral amino acid transport is not a likely cause, nor is accumulation of a potentially-toxic Phe metabolite [19]. Disruption of 1-carbon metabolic pathways was considered as a possibility to alter serine-glycine interconversion, but supplementation of the αMePhe + Phe treatments from age 3–10 days with injections (given on day 9 at intervals after the last Phe injection) of pyridoxamine, pyridoxol, glutamate, serine, methionine, B12, folate or a combination of folate + B12 + pyridoxol + methionine did not reduce glycine level [19]. Deficits in glycine cleavage system cause non-ketotic hyperglycinemia, severe mental retardation, seizures, and other neurological problems [50–52]. Valproic acid (2-propylpentanoic acid or dipropylacetic acid) is an anti-epileptic agent that inhibits the glycine cleavage system and elevates glycine levels in brain, liver, and blood [55–57]. Valproate-evoked increases in glycine levels worsen seizures in patients with epilepsy [58] and non-ketotic hyperglycinemia [59, 60]. Various α-keto acids inhibit glycine oxidation [61], raising the possibility that metabolic disruption in brain by phenylalanine and its metabolites may generate compounds that interfere with glycine metabolism. This possibility needs to be taken into account when assaying glycine cleavage system activity in vitro because dilution of metabolite levels in tissue homogenates and use of optimal assay conditions may prevent inhibition by compounds that are present at higher levels in brain in vivo. Understanding the mechanisms that elevate brain glycine level in PKU may lead to improved therapeutic approaches, and reducing brain glycine concentration in PKU animals and patients may improve outcome.
The αMePhe Rat Model for PKU
The rats treated with αMePhe to inhibit Phe hydroxylase were healthiest and had lower brain tyrosine levels compared to the other treatment groups, but use of this inhibitor requires an additional daily dose of Phe to maintain elevated Phe levels for most of the day due to residual liver phenylalanine hydroxylase activity. Conceivably, increasing the dose of the inhibitor at older ages may be useful to further inhibit the enzyme. On the other hand, variable Phe and amino acid levels associated with the injections may, in part, be a reasonable representation of fluctuations in PKU patients in whom the daily Phe levels would vary with meals and dietary control of Phe intake. As a cautionary note, most but not all amino acid changes are observed in different studies using the same rat model (compare Table 2; Fig. 2 with [14]), but the most robust changes are similar in rat and mouse models, taking into account the likelihood of age-dependent differences [14, 48, 49, 62]. The αMePhe-treated rats that received double daily Phe injections exhibited post-weaning behavioral abnormalities that persisted for at least 3 weeks after phenylalanine treatments were halted. These animals also had regionally-selective changes in rates of glucose utilization (Table 2; Fig. 3), including forebrain which was also affected in the genetic mouse PKU model [63]. Normal energetic status [64] and selective, as opposed to global, changes in CMRglc suggest that in vitro metabolic studies [65] and global in vivo assays of CMRglc during acute hyperphenylalanemia [43] need not represent actual pathway fluxes in chronically-hyperphenylalanemic animals. For technical reasons related to serial blood sampling to measure the specific activity of [14C]deoxyglucose, the CMRglc assays were carried out in the resting state in the present study. However, it is likely that assays of CMRglc during functional activation when energy demand is increased would be more sensitive to detect local metabolic abnormalities or deficiencies. For example, adult (but not 5–7-week-old) PKU mice are sensitive to audiogenic seizures, with seizure onset dependence on blood Phe concentration [66]. Involvement of auditory structures (cochlear nucleus and inferior colliculus) in some of the hyperphenylalanemic rats (Table 2) suggests that CMRglc assays in hyperphenylalanemic animals during auditory (or other physiological) stimulation may generate useful information to understand audiogenic sensitivity (or other pathway disturbances). CMRglc studies in human PKU patients are sparse and difficult to compare with those in animals due to a heterogeneous patient population with differences in age, diet, and clinical intervention. Global energy metabolism is, however, intact in PKU patients, as in the rats, and there are selective changes in relatively few brain structures but affected regions differ among the studies [38, 67–69].
Many other laboratories have shown that αMePhe + Phe-treated rats exhibit characteristics common to other PKU models and relevant to the pathophysiology of PKU, including the presence of phenylalanine metabolites (phenylpyruvate, phenyllactate), lower brain weight and serotonin levels, decreased synaptic density, reduced levels of myelin, polysome disaggregation, persistent behavioral or cognitive abnormalities (e.g., [14, 64, 70–76]). These studies also showed that αMePhe has much less toxicity than pCPhe. There are, however, potential side effects of αMePhe that need to be taken into account when evaluating relevance of the animal model to PKU, including inhibition of tyrosine hydroxylase and catecholamine depletion in brain and other tissues that depend on dosage [16, 77, 78]. Estimates of brain αMePhe levels in the present study are within the range that may have effects on catecholamines, but dopamine and norepinephrine levels were normal in brain of αMePhe- and pCPhe-treated rats at 10 and 30 days [14], although homovanillic acid levels (a metabolite of dopamine) and 5-hydroxyindolacetic acid (metabolite of serotonin) were significantly reduced [76]. Notably, chronic treatment with αMePhe alone caused only a few changes in brain amino acid levels, and the rise in glycine level was amplified by Phe, suggesting Phe concentration-dependence and tyrosine independence (Table 1; Fig. 2a). Also, αMePhe treatment alone did not alter local rates of brain glucose utilization (Table 2) or behavioral activity in the string test (Fig. 4). Taken together, these findings indicate that the αMePhe + Phe model may be a useful, low-cost, readily-available alternative model to the phenylalanine hydroxylase-deficient mouse models and it would be a good model to investigate the basis for elevated glycine levels. The αMePhe + Phe model can complement the genetic mouse models as long as appropriate control studies are carried out for all models; rat strain and the background mouse strain used to generate the PKU mutation and the Phe content of the mouse diet may also influence outcome. Thus, the novel treatment paradigms that successfully prevented decreases in dendritic spine density in αMePhe + Phe-treated rats [79] should also be tested in Phe hydroxylase-deficient mice. Finally, some caution must be exercised with interpretation of results from mouse and rat PKU models because rodent diets differ from those of omnivorous humans, and compensatory metabolic changes in response to enzyme deletion or inhibition may differ among species and give rise to different pathophysiological consequences. Compensatory gene expression changes after gene knockdown or knockout are, in fact, extensive and involve both up- and down-regulation of many genes related to a wide range of cellular functions [80, 81].
PKU is a very complex disease and the basis for acquired mental retardation and other neurological deficiencies is not yet understood due to the broad biochemical and physiological impact of chronic hyperphenylalanemia during brain development and in the adult. Recent reviews of findings in PKU patients and animal models [82–84] tend to emphasize the genetic mouse model [85] that shares many of the pathophysiological phenotypes previously reported in earlier animal studies with or without inhibition of phenylalanine hydroxylase, as well as the concepts derived from these data. Interpretation of relevance of the earlier findings is obviously complicated by use of inhibitors and elevated tyrosine levels but useful information has been obtained from them and characteristics common to all models are likely to be most relevant to PKU. In fact, elevated tyrosine levels and any of its downstream effects on brain catecholamine levels or turnover can be ruled out contributing to hyperglycinemia. New treatment paradigms for PKU patients, in addition to Phe-restricted diet, are designed to target enzyme and cofactor deficiencies with gene, enzyme, or cofactor therapies, and with dietary supplementation with large neutral amino acids to compete with phenylalanine for uptake into brain and reduce brain Phe level while increasing the brain levels of neutral amino acids [84, 86, 87]. These approaches use modern techniques and are derived, in large part, from knowledge generated by many studies in various PKU animal models.
Consequences of Elevated Brain Glycine Levels
High glycine levels are linked to brain dysfunction, damage, and mental retardation in nonketotic hyperglycinemia, and glycine may be involved in brain dysfunction and abnormal glutamatergic neurotransmission in experimental PKU. Discussion of PKU and animal models [85, 87] tended to discount findings in the early rat models and chronic hyperphenylalanemia-evoked hyperglycinemia [19] that is now shown to also occur in the Phe hydroxylase-deficient mouse [48, 49]. Several recent studies have implicated high brain glycine levels in oxidative stress and disruption of brain energy metabolism, and showed that these effects were reduced or prevented by the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 [88–91], linking high glycine levels to excitatory glutamatergic activity. A second interesting aspect of the above work was that glycine, at levels observed in hyperphenylalanemic rat brain, had a number of inhibitory effects on mitochondrial energy metabolism, and it impaired 14CO2 production from 14C-labeled acetate but not from glucose or citrate in homogenates of cerebral cortex. Use of homogenates eliminates the astrocyte-specific oxidation of acetate that arises from monocarboxylic acid transporter substrate specificity [92], but may implicate astrocytes in aspects of metabolic dysfunction in PKU rodents. The levels of glutamate, aspartate, and glutamine tended to be reduced in the young rats in some but not all studies and ages (Table 2; Fig. 2, [48, 49]) raising the question of whether CO2 fixation is impaired. Pyruvate carboxylase is localized to astrocytes [93, 94], and CO2 fixation is inhibited by phenylpyruvate in vitro [44]. However, brain phenylpyruvate levels in the mouse PKU model do not appear to be high enough to cause significant inhibition [95] unless there is local compartmentation of Phe transamination and locally-high levels of this Phe metabolite. Glutamatergic synaptic transmission in cultured hippocampal and cerebrocortical neurons (12–27 days in vitro after harvest from newborn rats or mice) is impaired by acute application of Phe, with apparent competition of Phe for the glycine binding site of NMDA receptors, since higher concentrations of glycine prevented the depressive effects of Phe [96–98]. The expression of NMDA and AMPA glutamate receptor subunits is also altered in brain of Phe-hydroxylase deficient mouse [85, 96, 97, 99]. These findings are quite interesting but the acute in vitro effects of Phe may not be relevant to phenylketonuria, and they need to be extended to evaluate temporal and mechanistic relationships between brain glycine content and glutamatergic neurotransmission in brain tissue of chronically-hyperphenylalanemic rats and mice. Future studies are required to evaluate brain glycine levels in PKU patients, elucidate the mechanism(s) underlying increased brain glycine level and its consequences in developing brain in animal models, and to develop new therapeutic approaches designed to normalize brain glycine level.
Abbreviations
- CMRglc :
-
Cerebral rate of glucose utilization
- DG:
-
2-Deoxy-d-glucose
- GABA:
-
γ-Aminobutyric acid
- pCPhe:
-
p-Chlorophenylalanine
- αMePhe:
-
α-Methylphenylalanine
- PKU:
-
Phenylketonuria
References
Mitchell JJ, Trakadis YJ, Scriver CR (2011) Phenylalanine hydroxylase deficiency. Genet Med 13:697–707
Scriver CR, Eisensmith RC, Woo SL, Kaufman S (1994) The hyperphenylalaninemias of man and mouse. Annu Rev Genet 28:141–165
Knox WE (1972) Phenylketonuria. In: Stanbury JB, Wyngaarden JB, Fredrickson DS (eds) The metabolic basis of inherited disease, 3rd edn. McGraw-Hill, New York, pp 266–295
Tourian A, Sidbury JB (1983) Phenylketonuria and hyperphenylalanemia. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, Brown MS (eds) The metabolic basis of inherited disease, 5th edn. McGraw-Hill, New York, pp 270–286
Camp KM, Parisi MA, Acosta PB, Berry GT, Bilder DA, Blau N, Bodamer OA, Brosco JP, Brown CS, Burlina AB, Burton BK, Chang CS, Coates PM, Cunningham AC, Dobrowolski SF, Ferguson JH, Franklin TD, Frazier DM, Grange DK, Greene CL, Groft SC, Harding CO, Howell RR, Huntington KL, Hyatt-Knorr HD, Jevaji IP, Levy HL, Lichter-Konecki U, Lindegren ML, Lloyd-Puryear MA, Matalon K, MacDonald A, McPheeters ML, Mitchell JJ, Mofidi S, Moseley KD, Mueller CM, Mulberg AE, Nerurkar LS, Ogata BN, Pariser AR, Prasad S, Pridjian G, Rasmussen SA, Reddy UM, Rohr FJ, Singh RH, Sirrs SM, Stremer SE, Tagle DA, Thompson SM, Urv TK, Utz JR, van Spronsen F, Vockley J, Waisbren SE, Weglicki LS, White DA, Whitley CB, Wilfond BS, Yannicelli S, Young JM (2014) Phenylketonuria scientific review conference: state of the science and future research needs. Mol Genet Metab 112:87–122
Blau K (1979) Phenylalanine hydroxylase deficiency: biochemical, physiological, and clinical aspects of phenylketonuria and related phenylalaninemias. In: Youdim MBH (ed) Aromatic amino acid hydroxylases and mental disease. John Wiley & Sons, Chichester, pp 77–139
Kaufman S (1977) Phenylketonuria: biochemical mechanisms. In: Agranoff BW, Aprison MH (eds) Advances in neurochemistry. Plenum Press, New York, pp 1–132
Kaufman S (1989) An evaluation of the possible neurotoxicity of metabolites of phenylalanine. J Pediatr 114:895–900
Loo YH, Potempska A, Wisniewski HM (1985) A biochemical explanation of phenyl acetate neurotoxicity in experimental phenylketonuria. J Neurochem 45:1596–1600
Wen GY, Wisniewski HM, Shek JW, Loo YH, Fulton TR (1980) Neuropathology of phenylacetate poisoning in rats: an experimental model of phenylketonuria. Ann Neurol 7:557–566
Clarke JT, Lowden JA (1969) Hyperphenylalaninemia: effect on the developing rat brain. Can J Biochem 47:291–295
Delvalle JA, Dienel G, Greengard O (1978) Comparison of alpha-methylphenylalanine and p-chlorophenylalanine as inducers of chronic hyperphenylalaninaemia in developing rats. Biochem J 170:449–459
Shedlovsky A, McDonald JD, Symula D, Dove WF (1993) Mouse models of human phenylketonuria. Genetics 134:1205–1210
Lane JD, Schone B, Langenbeck U, Neuhoff V (1980) Characterization of experimental phenylketonuria. Augmentation of hyperphenylalaninemia with alpha-methylphenylalanine and p-chlorophenylalanine. Biochim Biophys Acta 627:144–156
McGeer EG, McGeer PL (1973) Amino acid hydroxylase inhibitors. In: Hochster RM, Kates M, Quastel JH (eds) Metabolic inhibitors A comprehensive treatise. Academic Press, New York, pp 45–105
Udenfriend S, Zaltzman-Nirenberg P, Nagatsu T (1965) Inhibitors of purified beef adrenal tyrosine hydroxylase. Biochem Pharmacol 14:837–845
McDonald JD, Andriolo M, Cali F, Mirisola M, Puglisi-Allegra S, Romano V, Sarkissian CN, Smith CB (2002) The phenylketonuria mouse model: a meeting review. Mol Genet Metab 76:256–261
Dienel GA (1977) Brain development in normal and chronic hyperphenylalanemic rats. PhD thesis, Department of Biological Chemistry, Harvard University
Dienel GA (1981) Chronic hyperphenylalaninemia produces cerebral hyperglycinemia in immature rats. J Neurochem 36:34–43
Benson JV Jr, Patterson JA (1965) Accelerated chromatographic analysis of amino acids commonly found in physiological fluids on a spherical resin of specific design. Anal Biochem 13:265–280
Krebs HA (1950) Manometric determination of l-aspartic acid and l-asparagine. Biochem J 47:605–614
Minard FN, Mushahwar IK (1966) Synthesis of gamma-aminobutyric acid from a pool of glutamic acid in brain after decapitation. Life Sci 5:1409–1413
Yoshino Y, Elliott KA (1970) Incorporation of carbon atoms from glucose into free amino acids in brain under normal and altered conditions. Can J Biochem 48:228–235
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Cruz NF, Duffy TE (1983) Local cerebral glucose metabolism in rats with chronic portacaval shunts. J Cereb Blood Flow Metab 3:311–320
Miquel J, Blasco M (1978) A simple technique for evaluation of vitality loss in aging mice, by testing their muscular coordination and vigor. Exp Gerontol 13:389–396
Barclay LL, Gibson GE, Blass JP (1981) The string test: an early behavioral change in thiamine deficiency. Pharmacol Biochem Behav 14:153–157
Udenfriend S (1962) Fluorescence assays in biology and medicine. Academic Press, New York
Wong PW, O’Flynn ME, Inouye T (1964) Micromethods for measuring phenylalanine and tyrosine in serum. Clin Chem 10:1098–1104
Daniel PM, Moorhouse SR, Pratt OE (1976) Amino acid precursors of monoamine neurotransmitters and some factors influencing their supply to the brain. Psychol Med 6:277–286
Pardridge WM (1977) Kinetics of competitive inhibition of neutral amino acid transport across the blood–brain barrier. J Neurochem 28:103–108
Kreis R, Pietz J, Penzien J, Herschkowitz N, Boesch C (1995) Identification and quantitation of phenylalanine in the brain of patients with phenylketonuria by means of localized in vivo 1H magnetic-resonance spectroscopy. J Magn Reson B 107:242–251
Novotny EJ Jr, Avison MJ, Herschkowitz N, Petroff OA, Prichard JW, Seashore MR, Rothman DL (1995) In vivo measurement of phenylalanine in human brain by proton nuclear magnetic resonance spectroscopy. Pediatr Res 37:244–249
Leuzzi V, Bianchi MC, Tosetti M, Carducci CL, Carducci CA, Antonozzi I (2000) Clinical significance of brain phenylalanine concentration assessed by in vivo proton magnetic resonance spectroscopy in phenylketonuria. J Inherit Metab Dis 23:563–570
Moats RA, Moseley KD, Koch R, Nelson M Jr (2003) Brain phenylalanine concentrations in phenylketonuria: research and treatment of adults. Pediatrics 112:1575–1579
Moller HE, Ullrich K, Weglage J (2000) In vivo proton magnetic resonance spectroscopy in phenylketonuria. Eur J Pediatr 159(Suppl 2):S121–S125
Rupp A, Kreis R, Zschocke J, Slotboom J, Boesch C, Rating D, Pietz J (2001) Variability of blood–brain ratios of phenylalanine in typical patients with phenylketonuria. J Cereb Blood Flow Metab 21:276–284
Pietz J, Rupp A, Ebinger F, Rating D, Mayatepek E, Boesch C, Kreis R (2003) Cerebral energy metabolism in phenylketonuria: findings by quantitative in vivo 31P MR spectroscopy. Pediatr Res 53:654–662
Koch R, Moseley KD, Yano S, Nelson M Jr, Moats RA (2003) Large neutral amino acid therapy and phenylketonuria: a promising approach to treatment. Mol Genet Metab 79:110–113
Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, Bremer HJ (1999) Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest 103:1169–1178
Adriaenssens K, Allen RJ, Lowenthal A, Mardens Y, Tourtellotte WW (1969) Brain and cerebrospinal fluid free amino acids in phenylketonuria. J Genet Hum 17:223–230
McKean CM, Peterson NA (1970) Glutamine in the phenylketonuric central nervous system. N Engl J Med 283:1364–1367
Miller AL, Hawkins RA, Veech RL (1973) Phenylketonuria: phenylalanine inhibits brain pyruvate kinase in vivo. Science 179:904–906
Patel MS (1972) The effect of phenylpyruvate on pyruvate metabolism in rat brain. Biochem J 128:677–684
Land JM, Mowbray J, Clark JB (1976) Control of pyruvate and beta-hydroxybutyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J Neurochem 26:823–830
Castells S, Zischka R, Addo N (1971) Alternation in composition of deoxyribonucleic acid, ribonucleic acid, proteins, and amino acids in brain of rats fed high and low phenylalanine diets. Pediatr Res 5:329–334
O’Brien D, Ibbot FA (1966) Effect of prolonged phenylalanine loading on the free amino-acid and lipid content of the infant monkey brain. Dev Med Child Neurol 8:724–728
Vogel KR, Arning E, Wasek BL, Bottiglieri T, Gibson KM (2013) Characterization of 2-(methylamino)alkanoic acid capacity to restrict blood–brain phenylalanine transport in Pah enu2 mice: preliminary findings. Mol Genet Metab 110(Suppl):S71–S78
Arning E, Bottiglieri T, Sun Q, Jansen EEW, Jakobs C, Lin B, Stetson L, Harding CO, Gibson KM (2009) Metabolic profiling in phenylalanine hydroxylase deficient (PAH −/−) mouse brain reveals decreased amino acid neurotransmitters and preferential alterations of the serotonergic system. Mol Genet Metab 98:21
Perry TL, Urquhart N, MacLean J, Evans ME, Hansen S, Davidson GF, Applegarth DA, MacLeod PJ, Lock JE (1975) Nonketotic hyperglycinemia. Glycine accumulation due to absence of glycerine cleavage in brain. N Engl J Med 292:1269–1273
Applegarth DA, Toone JR (2004) Glycine encephalopathy (nonketotic hyperglycinaemia): review and update. J Inherit Metab Dis 27:417–422
Kikuchi G, Motokawa Y, Yoshida T, Hiraga K (2008) Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proc Jpn Acad Ser B Phys Biol Sci 84:246–263
Banerjee A, Ganji S, Hulsey K, Dimitrov I, Maher E, Ghose S, Tamminga C, Choi C (2012) Measurement of glycine in gray and white matter in the human brain in vivo by 1H MRS at 7.0 T. Magn Reson Med 68:325–331
Schindeler S, Ghosh-Jerath S, Thompson S, Rocca A, Joy P, Kemp A, Rae C, Green K, Wilcken B, Christodoulou J (2007) The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Mol Genet Metab 91:48–54
Kochi H, Hayasaka K, Hiraga K, Kikuchi G (1979) Reduction of the level of the glycine cleavage system in the rat liver resulting from administration of dipropylacetic acid: an experimental approach to hyperglycinemia. Arch Biochem Biophys 198:589–597
Mortensen PB, Kolvraa S, Christensen E (1980) Inhibition of the glycine cleavage system: hyperglycinemia and hyperglycinuria caused by valproic acid. Epilepsia 21:563–569
Martin-Gallardo A, Rodriguez P, Lopez M, Benavides J, Ugarte M (1985) Effects of dipropylacetate on the glycine cleavage enzyme system and glycine levels. A possible experimental approach to non-ketotic hyperglycinemia. Biochem Pharmacol 34:2877–2882
Simila S, von Wendt L, Linna SL, Saukkonen AL, Huhtaniemi I (1979) Dipropylacetate and hyperglycinemia. Neuropadiatrie 10:158–160
Dhamija R, Gavrilova RH, Wirrell EC (2011) Valproate-induced worsening of seizures: clue to underlying diagnosis. J Child Neurol 26:1319–1321
Subramanian V, Kadiyala P, Hariharan P, Neeraj E (2015) A rare case of glycine encephalopathy unveiled by valproate therapy. J Pediatr Neurosci 10:143–145
Kochi H, Seino H, Ono K (1986) Inhibition of glycine oxidation by pyruvate, alpha-ketoglutarate, and branched-chain alpha-keto acids in rat liver mitochondria: presence of interaction between the glycine cleavage system and alpha-keto acid dehydrogenase complexes. Arch Biochem Biophys 249:263–272
Smith CB, Kang J (2000) Cerebral protein synthesis in a genetic mouse model of phenylketonuria. Proc Natl Acad Sci USA 97:11014–11019
Qin M, Smith CB (2007) Regionally selective decreases in cerebral glucose metabolism in a mouse model of phenylketonuria. J Inherit Metab Dis 30:318–325
Burri R, Matthieu JM, Vandevelde M, Lazeyras F, Posse S, Herschkowitz N (1990) Brain damage and recovery in hyperphenylalaninemic rats. Dev Neurosci 12:116–125
Lutz Mda G, Feksa LR, Wyse AT, Dutra-Filho CS, Wajner M, Wannmacher CM (2003) Alanine prevents the in vitro inhibition of glycolysis caused by phenylalanine in brain cortex of rats. Metab Brain Dis 18:87–94
Martynyuk AE, Ucar DA, Yang DD, Norman WM, Carney PR, Dennis DM, Laipis PJ (2007) Epilepsy in phenylketonuria: a complex dependence on serum phenylalanine levels. Epilepsia 48:1143–1150
Hasselbalch S, Knudsen GM, Toft PB, Hogh P, Tedeschi E, Holm S, Videbaek C, Henriksen O, Lou HC, Paulson OB (1996) Cerebral glucose metabolism is decreased in white matter changes in patients with phenylketonuria. Pediatr Res 40:21–24
Yanai K, Iinuma K, Matsuzawa T, Ito M, Miyabayashi S, Narisawa K, Ido T, Yamada K, Tada K (1987) Cerebral glucose utilization in pediatric neurological disorders determined by positron emission tomography. Eur J Nucl Med 13:292–296
Ficicioglu C, Dubroff JG, Thomas N, Gallagher PR, Burfield J, Hussa C, Randall R, Zhuang H (2013) A pilot study of fluorodeoxyglucose positron emission tomography findings in patients with phenylketonuria before and during sapropterin supplementation. J Clin Neurol 9:151–156
Lane JD, Neuhoff V (1980) Phenylketonuria: clinical and experimental considerations revealed by the use of animal models. Naturwissenschaften 67:227–233
Binek PA, Johnson TC, Kelly CJ (1981) Effect of alpha-methylphenylalanine and phenylalanine on brain polyribosomes and protein synthesis. J Neurochem 36:1476–1484
Figlewicz DA, Druse MJ (1980) Experimental hyperphenylalaninemia: effect on central nervous system myelin subfractions. Exp Neurol 67:315–329
Johnson RC, Shah SN (1980) Effects of alpha-methylphenylalanine plus phenylalanine treatment during development on myelin in rat brain. Neurochem Res 5:709–718
Luttges MW, Gerren RA (1979) Postnatal alpha-methylphenylalanine treatment effects on adult mouse locomotor activity and avoidance learning. Pharmacol Biochem Behav 11:493–498
Nigam MP, Labar DR (1979) The effect of hyperphenylalaninemia on size and density of synapses in rat neocortex. Brain Res 179:195–198
Diamond A, Ciaramitaro V, Donner E, Djali S, Robinson MB (1994) An animal model of early-treated PKU. J Neurosci 14:3072–3082
Sourkes TL, Murphy GF, Chavez B, Zielinska M (1961) The action of some alpha-methyl and other amino acids on cerebral catecholamines. J Neurochem 8:109–115
Torchiana ML, Porter CC, Stone CA, Hanson HM (1970) Some biochemical and pharmacological actions of-methylphenylalanine. Biochem Pharmacol 19:1601–1614
Dos Reis EA, Rieger E, de Souza SS, Rasia-Filho AA, Wannmacher CM (2013) Effects of a co-treatment with pyruvate and creatine on dendritic spines in rat hippocampus and posterodorsal medial amygdala in a phenylketonuria animal model. Metab Brain Dis 28:509–517
Iacobas DA, Iacobas S, Urban-Maldonado M, Scemes E, Spray DC (2008) Similar transcriptomic alterations in Cx43 knockdown and knockout astrocytes. Cell Commun Adhes 15:195–206
Iacobas DA, Iacobas S, Urban-Maldonado M, Spray DC (2005) Sensitivity of the brain transcriptome to connexin ablation. Biochim Biophys Acta 1711:183–196
Surtees R, Blau N (2000) The neurochemistry of phenylketonuria. Eur J Pediatr 159(Suppl 2):S109–S113
Ribas GS, Sitta A, Wajner M, Vargas CR (2011) Oxidative stress in phenylketonuria: What is the evidence? Cell Mol Neurobiol 31:653–662
de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ (2010) Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab 99(Suppl 1):S86–S89
Martynyuk AE, van Spronsen FJ, Van der Zee EA (2010) Animal models of brain dysfunction in phenylketonuria. Mol Genet Metab 99(Suppl 1):S100–S105
Sarkissian CN, Gamez A, Scriver CR (2009) What we know that could influence future treatment of phenylketonuria. J Inherit Metab Dis 32:3–9
van Spronsen FJ, Hoeksma M, Reijngoud DJ (2009) Brain dysfunction in phenylketonuria: Is phenylalanine toxicity the only possible cause? J Inherit Metab Dis 32:46–51
Moura AP, Grings M, Marcowich GF, Bumbel AP, Parmeggiani B, de Moura Alvorcem L, Wajner M, Leipnitz G (2014) Evidence that glycine induces lipid peroxidation and decreases glutathione concentrations in rat cerebellum. Mol Cell Biochem 395:125–134
Moura AP, Grings M, Dos Santos Parmeggiani B, Marcowich GF, Tonin AM, Viegas CM, Zanatta A, Ribeiro CA, Wajner M, Leipnitz G (2013) Glycine intracerebroventricular administration disrupts mitochondrial energy homeostasis in cerebral cortex and striatum of young rats. Neurotox Res 24:502–511
Seminotti B, Knebel LA, Fernandes CG, Amaral AU, da Rosa MS, Eichler P, Leipnitz G, Wajner M (2011) Glycine intrastriatal administration induces lipid and protein oxidative damage and alters the enzymatic antioxidant defenses in rat brain. Life Sci 89:276–281
Busanello ENB, Moura AP, Viegas CM, Zanatta Â, da Costa Ferreira G, Schuck PF, Wajner M (2010) Neurochemical evidence that glycine induces bioenergetical dysfunction. Neurochem Int 56:948–954
Waniewski RA, Martin DL (1998) Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci 18:5225–5233
Shank RP, Bennett GS, Freytag SO, Campbell GL (1985) Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res 329:364–367
Yu AC, Drejer J, Hertz L, Schousboe A (1983) Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem 41:1484–1487
Sarkissian CN, Scriver CR, Mamer OA (2000) Measurement of phenyllactate, phenylacetate, and phenylpyruvate by negative ion chemical ionization–gas chromatography/mass spectrometry in brain of mouse genetic models of phenylketonuria and non-phenylketonuria hyperphenylalaninemia. Anal Biochem 280:242–249
Martynyuk AE, Glushakov AV, Sumners C, Laipis PJ, Dennis DM, Seubert CN (2005) Impaired glutamatergic synaptic transmission in the PKU brain. Mol Genet Metab 86(Suppl 1):S34–S42
Glushakov AV, Dennis DM, Sumners C, Seubert CN, Martynyuk AE (2003) L-phenylalanine selectively depresses currents at glutamatergic excitatory synapses. J Neurosci Res 72:116–124
Glushakov AV, Glushakova O, Varshney M, Bajpai LK, Sumners C, Laipis PJ, Embury JE, Baker SP, Otero DH, Dennis DM, Seubert CN, Martynyuk AE (2005) Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain 128:300–307
Imperlini E, Orru S, Corbo C, Daniele A, Salvatore F (2014) Altered brain protein expression profiles are associated with molecular neurological dysfunction in the PKU mouse model. J Neurochem 129:1002–1012
Schulz JB, Wree A, Schleicher A, Zilles K (1992) Plasticity in the rat hippocampal formation following ibotenic acid lesion of the septal region: a quantitative [14C]deoxyglucose and acetylcholinesterase study. J Cereb Blood Flow Metab 12:1007–1021
Wree A, Schleicher A, Zilles K, Beck T (1988) Local cerebral glucose utilization in the Ammon’s horn and dentate gyrus of the rat brain. Histochemistry 88:415–426
Acknowledgments
This work was supported, in part, by NIH Grants GM 00451, CA 08676, and AM-16739.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Special Issue: 40th Year of Neurochemical Research.
Rights and permissions
About this article
Cite this article
Dienel, G.A., Cruz, N.F. Biochemical, Metabolic, and Behavioral Characteristics of Immature Chronic Hyperphenylalanemic Rats. Neurochem Res 41, 16–32 (2016). https://doi.org/10.1007/s11064-015-1678-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1678-y