Abstract
Rapid eye movement sleep (REMS) modulates Na-K ATPase activity and maintains brain excitability. REMS deprivation (REMSD)-associated increased Na-K ATPase activity is mediated by noradrenaline (NA) acting on α1-adrenoceptor (AR) in the brain. It was shown that NA-induced increased Na-K ATPase activity was due to allosteric modulation as well as increased turnover of the enzyme. Although the former has been studied in detail, our understanding on the latter was lacking, which we have studied. Male Wistar rats were REMS deprived for 4-days by classical flower-pot method; suitable control experiments were conducted. In another set, α1-AR antagonist prazosin (PRZ) was i.p. injected 48 h REMSD onward. At the end of experiments rats were sacrificed by cervical dislocation and brains were removed. Synaptosomes prepared from the brains were used to estimate Na-K ATPase activity as well as protein expressions of different isoforms of the enzyme subunits using western blot. REMSD significantly increased synaptosomal Na-K ATPase activity and that was due to differential increase in the expressions of α1-, α2- and α3-isoforms, but not that of β1- and β2-isoforms. PRZ reduced the REMSD-induced increased Na-K ATPase activity and protein expressions. We also observed that the increased Na-K ATPase subunit expression was not due to enhanced mRNA synthesis, which suggests the possibility of post-transcriptional regulation. Thus, the findings suggest that REMSD-associated increased Na-K ATPase activity is due to elevated level of α-subunit of the enzyme and that is induced by NA acting on α1-AR mediated mRNA-stabilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid eye movement sleep (REMS) is a unique stage of sleep that is expressed in all higher-order vertebrates including mammals [1, 2], although its quantity varies with the progression of age [3]. REMS has been proposed to serve house-keeping function of the brain by maintaining the threshold of excitability of neurons to an optimum level so that the physiological processes can be optimally and dynamically controlled by the brain to maintain homeostasis [4, 5]. It also plays an important role in regulating several other physiological processes including ontogeny, brain maturation, synaptogenesis and interconnectivity between brain areas [6–8]. REMS disturbance is reported in several neurodegenerative and psychiatric disorders [9–11]. REMS also plays a crucial role in the development and integrity of the central nervous system, while its sustained loss has been associated with many signs and symptoms including increased anxiety, aggression, irritability, confusion, loss of concentration, reduced memory consolidation, increased sensitiveness to tactile stimuli and decreased threshold for electroconvulsive shock in both animal and human subjects [12–15].
The transmembrane potential gradient is responsible for neuronal excitability, which is reflected due to differential ionic distribution across the plasma membrane. We hypothesized that one of the functions of REMS is to maintain brain excitability, and it is achieved by modulation of the neuronal membrane-bound Na-K ATPase activity [16, 17]. The Na-K ATPase extrudes 3Na+ and simultaneously imports 2 K+ at the cost of energy released by hydrolysis of one ATP molecule in each cycle and maintains transmembrane potential gradient for optimum functioning of the neurons [18, 19]. The importance of Na-K ATPase in maintaining the ionic gradient is further emphasized by the findings that disruption of the enzyme activity caused hyper-excitability and severe alterations in neuronal functioning [20, 21]. The Na-K ATPase is a transmembrane tetramer having two each of α- and β-subunits that form a heterodimer. The α-subunit possesses binding sites for Na+, K+, ATP and ouabain (a specific Na-K ATPase inhibitor) and plays catalytic role, whereas the β-subunit is the regulatory subunit that helps in proper folding, assembly and trafficking of the enzyme to the plasma membrane [22–24]. Both these subunits have a number of isoforms which express in tissue and development specific manner. It has been suggested that due to higher excitability the brain tissue expresses most isoforms of α-and β-subunits viz. α1, α2, α3 and β1, β2 [25–27]. Therefore, it is likely that in brain the expressions of Na-K ATPase subunits are likely to vary due to changes in conditions and in a tissue-specific manner, which in turn would affect the brain excitability.
REMS deprivation (REMSD) associated elevated noradrenaline (NA) acts on α1-adrenoceptor (AR) and stimulates Na-K ATPase activity [28, 29]. The intracellular mechanism of its allosteric regulation has been worked out to a reasonable extent [5]. However, as REMSD affected both the Km as well as the Vmax of the enzyme [30], REMSD associated sustained increase in NA is likely to modulate the Na-K ATPase transcription as well. As various isoforms of Na-K ATPase are expressed in the brain, we investigated if REMSD differentially affected specific isoforms of Na-K ATPase. Further, we also investigated if the effects were mediated by NA and the subtype of the AR involved in mediating the response.
Materials and Method
Chemicals
Tris-Base, noradrenaline (NA); α1-AR antagonist, prazosin (PRZ); β-AR antagonist, propranolol (PRN); adenosine tri-phosphate (ATP); Na-K ATPase inhibitor, ouabain; N, N-dimethyl acetamide (N,N-DA) procured from Sigma–Aldrich, USA. Trizol® reagent; RNA-zap; TURBO DNA-free kit (Ambion); power SYBER-PCR master mix (Applied Biosystems); Superscript-III first strand cDNA synthesis kit (Invitrogen); were obtained from Life Technologies, USA. Tricholoroacetic acid (TCA) and other chemicals used were of analytical grade.
Experimental Procedures
The experiments were carried out on healthy inbred male wistar rats (220–250 g) obtained from the Central Animal House Facility of the Jawaharlal Nehru University. The experimental as well as each of the control groups had five rats each and a total of 46 rats were used in this study. The rats were housed in polypropylene cages with stainless steel net lids, maintained under 12:12 light–dark cycle (lights on at 7:30 AM) at controlled temperature (25 ± 1 °C) and provided with free access to food and water ad libitum. National Institute of Health guidelines for care and use of laboratory animals were followed and the experimental procedures were approved by the Institutional Animal Ethics Committee of the Jawaharlal Nehru University. Every effort was made to reduce pain and discomfort to the animals and to minimize the use of number of rats necessary to complete the study.
REMSD by the Classical Flower Pot Method
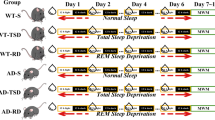
Rats were REMS deprived by the flower pot method, which has been extensively used across the globe for such studies in cats and rats [16, 31–33]. This method exploits the muscle atonia exhibited by the antigravity muscles during REMS [34]. We have standardised the method in rats and have been using it for more than two decades. The details of the method and its advantages and disadvantages have been reported and reviewed earlier [5, 15]. In brief, the experimental rats were maintained on a 6.5 cm diameter island single platform surrounded by water in individual tanks. To rule out the effects due to non-specific factors a group of animals were maintained on an island of larger platform of 12.5 cm diameter surrounded by water. Thus, these large platform control (LPC) animals were maintained in identical condition as that of the experimental rats except that the platform size was a little larger so that the LPC rats could experience both the non-REMS as well as REMS. The diameters of the platforms were selected based on animal weight [16, 35]. A control set included rats deprived of REMS for 4 days (96 h) and then they were allowed to recover from REMS loss for 3 days in their normal home cages, the recovery control (REC). In another control group of normal rats were kept in the same room in semi transparent cages, the free moving control (FMC) rats. All rats had easy and ad libitum access to food and water throughout the period of experiment. The water tanks, used in the experiments for REMSD and controls rats, were washed and cleaned one at a time daily at the same time and the water replaced. The rats were maintained individually in dry cages for about 10 min while respective tank was cleaned; the rats were monitored to remain active throughout this washing period as a sign that they did not rest and fell sleep during this period.
Intraperitoneal (i.p.) Injection of α- and β-AR Antagonists
The rats were REMS deprived for 4 days. After 2 days of REMSD while deprivation was continuing, on the third and fourth day of deprivation 0.5 ml of either prazosin (PRZ) (4 mg/kg) or propranolol (PRN) (10 mg/kg), an α1- or a β-AR antagonist was i.p. injected into the rats. PRZ was dissolved in 20 % N,N-dimethylacetamide (N, N-DA), while PRN was dissolved in saline.
Synaptosome Preparation
The synaptosomes were prepared from the rat’s whole brain as has been described earlier in detail [29]. Briefly, at the end of the experiments the REMS-deprived as well as the control rats were quickly decapitated following cervical dislocation. The brains were immediately removed and homogenized in 10 volumes of ice-cold buffer containing 0.32 M sucrose, 12 mM Tris–HCl buffer (pH 7.4) and 1 mM EDTA. The homogenate was centrifuged at 3000×g (6000 rpm) for 5 min and the supernatant was centrifuged for 20 min at 11,000×g (12,000 rpm). The pellet obtained was resuspended in 1 ml of homogenizing buffer and layered on to discontinuous sucrose gradient of 1.2 M and 0.8 M sucrose (4 ml each) and subjected to ultracentrifugation at 25,000 rpm (105,000×g) for 2 h. After ultracentrifugation the band obtained at the interface of 1.2 M and 0.8 M sucrose was carefully retrieved, diluted with homogenizing buffer and used as synaptosomes for further studies. The protein concentration in the isolated synaptosomal fraction from control and experimental animals was determined by Lowry’s method using bovine serum albumin (1 mg/ml) as standard.
Estimation of Na-K ATPase Activity
The ouabain sensitive Na-K ATPase activity in the synaptosomes prepared from control and experimental rat brains were estimated by the method reported earlier in detail [16, 29]. In brief, the reaction mixture contained 100 mM NaCl, 20 mM KCl, 5 mM MgCl2, 3 mM ATP and 50 mM Tris (pH7.4). An aliquot (30–40 μg protein) of the synaptosome membrane fractions isolated each from control and experimental (REMS deprived) rat brains was separately incubated with the reaction mixture at 37 °C for 20 min; ATP was used as the substrate, while ouabain (1 mM) was used as a specific blocker of Na-K ATPase. The reaction was stopped by adding 1 ml of 10 % ice-cold TCA and the mixture was centrifuged at 800×g for 5 min. The supernatant was collected for estimation of released inorganic phosphate following the method of Fiske and Subbarow [36] using UV–Vis spectrophotometer (Perkin-Elmer, USA). The released phosphate was an estimate of ouabain sensitive Na-K ATPase activity, which has been expressed as µmoles of Pi released/mg protein/h. The relative percentage change in specific Na-K ATPase activity in the brains of rats deprived of REMS with reference to all controls was calculated and represented as bar diagram.
Western Blotting
REMSD induced changes in relative abundance of Na-K ATPase subunit isoforms in the synaptosomes prepared from rat brain were estimated by western blotting and compared statistically. Equal amounts (40 μg) of synaptosomal protein from control and REMSD rats were separated on polyacrylamide gel and transferred onto 0.45 μm nitrocellulose membrane (mdi, India) using a semi-dry transfer apparatus (Bio-Rad, Australia). The membranes were blocked in 5 % non-fat dry milk in Tris-buffered saline (TBS) containing 0.1 % Tween-20 (TBS-T) for 2 h. This was followed by 2 h incubation of the membrane with Na-K ATPase subunit-specific rabbit polyclonal primary antibodies (Upstate, USA); the concentration of the latter varied from 1:1000 to 1:3000 while standardization. Anti-GAPDH (ab2302, polyclonal chicken antibody, Millipore, USA, 1:2000) was used to as detect GAPDH in the same blot to rule out possible loading error (loading control). The membranes were then washed three times with TBS-T and probed with horseradish peroxidase conjugated secondary antibody (goat anti-rabbit, sc-3837, Santa Cruz Biotechnology, USA, 1:10,000; rabbit anti-chicken, A9046, Sigma, USA, 1:10,000) for 1.5 h. All incubation and washings were done at room temperature. The membranes were then washed 3-times with TBS-T followed by with TBS and subsequently developed following enhanced chemiluminescence method using Clarity (Bio-Rad, USA). The relative changes in the band intensities of the desired Na-K ATPase subunits were densitometrically estimated using alpha image software (Alpha Innotech, USA).
RNA Isolation and Quantitative PCR (qPCR)
Total RNA from the controls and experimental (REMSD) rat brains was extracted using Trizol® reagent. The contaminating DNA was removed from the isolated RNA by DNase treatment using TURBO DNA-free Kit. Equal (1 μg) amount of total RNA was reverse transcribed using Superscript-ΙΙΙ first strand cDNA synthesis kit (Invitrogen, USA). qPCR for different α-subunit isoforms of Na-K ATPase was performed with power SYBR-PCR master mix. Equal amount (1 µg) of cDNA, gene specific primers (Table 1) and master mix were subjected to qPCR using ABI Prism 7500 FAST Real-Time PCR system (Applied Biosystems, USA). Fold change analysis was applied using the 2−ΔΔCt method by averaging the mean values normalized against GAPDH and TBP reference genes. The relative abundance of each mRNA was calculated and expressed as treated versus control in comparison with reference genes (GAPDH and TBP). The qPCR experiments were repeated at least four times with three replicates each.
Data Analysis
Statistical analyses were carried out using SigmaStat 3.5 (Jandel Scientific, San Rafael, CA, USA). For each experiment the FMC value was taken as 100 % and relative changes in all other controls (viz. LPC and REC) and experimental samples (REMSD, REMSD + PRZ, REMSD + PRN) were calculated. The percentage changes after REMSD (i.e. experimental) as compared to FMC were statistically compared with that of percentage changes of other control groups viz. LPC and REC using one-way analysis of variance (ANOVA). Significance levels were evaluated by applying Post-hoc Holm-Sidak Test; p at least < 0.05 was taken significant.
Results
Na-K ATPase Activity in the Rat Brain was Increased After REMSD
After REMSD the Na-K ATPase activity significantly increased in the rat brain synaptosomes compared to FMC (F (1,18) = 21.66, p < 0.001) and LPC (F (1,14) = 11.39, p < 0.01). The increase in activity returned to baseline level (i.e. recovered) if the REMS deprived rats were allowed to live in normal cages for 3 days to recover from the lost REMS (F (1,14) = 16.20, p < 0.01) (Fig. 1). The specific enzyme activities in rat brain synaptosomes in controls, after REMSD and after various treatments have been shown in Table 2.
Percent changes in ouabain-specific Na-K ATPase activity in synaptosomes prepared from REMSD, LPC, REC, REMSD + PRZ (α1-AR antagonist) and REMSD + PRN (β-AR antagonist) rat brains as compared with FMC taken as 100 % are shown. The Na-K ATPase activities of REMSD and REMSD + PRN group are statisticaly significant as compared to FMC and other groups. ***p < 0.001, **p < 0.01 significant compared with FMC and other controls. Abbreviations are as in the text
REMSD Induced Increased Na-K ATPase Activity was Mediated by NA Acting Through α1-AR
To evaluate if REMSD associated increased Na-K ATPase activity was induced by NA and to identify the subtypes of AR mediating the action of NA, the Na-K ATPase activity was estimated in control as well as PRZ (α1-AR antagonist) and PRN (β-AR antagonist) treated REMSD rats. The synaptosomal Na-K ATPase activity of REMSD + PRZ-treated group was comparable with that of FMC, whereas the REMSD + PRN-treated rats showed Na-K ATPase activity significantly higher than FMC rats (F (1,12) = 62.83, p < 0.001) (Fig. 1; Table 2). The results confirmed that elevated Na-K ATPase activity upon REMSD was induced by NA acting on α1-AR.
Effect of REMSD on Protein Abundance of Na-K ATPase Subunit Isoforms
The Na-K ATPase is a tetramer consisting of α- and β-heterodimers and each has more than one isoforms. We extended our study to evaluate the REMSD induced differential and/or specific changes in protein abundance(s) of different Na-K ATPase subunits and their isoforms. Protein levels of regulatory β-subunit isoforms, β1 and β2, remained unaffected after 96 h of REMSD in comparison to FMC and other controls (Fig. 2). However, in contrast the catalytic α-subunit isoforms, α1, α2 and α3, were found to be significantly increased by 1.4-fold (F (4,25) = 3.526, p < 0.01), twofold (F (4,25) = 3.844, p < 0.01) and 1.4-fold (F (4,25) = 6.989, p < 0.01), respectively, as compared to that of the FMC. This increase in the expression of α-subunit isoforms of Na-K ATPase was prevented if the rats undergoing REMSD were treated with α1-AR antagonist, PRZ (Fig. 3). The expressions of various subunits of the Na-K ATPase in LPC group were comparable to that of the FMC. The expression pattern of the REC group although showed tendency of reduction compared to the REMSD samples, it remained elevated compared to that of the FMC suggesting the need of more recovery sleep for the Na-K ATPase subunits to return to baseline i.e. FMC level.
Protein expressions of a β1- and b β2-Na-K ATPase isoforms in brain samples of rats under various conditions are shown. Upper panel shows a representative western blot of each isoform, while the histogram in the respective lower panel shows percent changes in the mean (±SEM) band densities of the blots from 5-sets of experiments as compared to FMC taken as 100 %. Abbreviations are as in the text
Protein expressions of a α1-, b α2-, and c α3-isoforms of Na-K ATPase α-subunit in the brain samples of rats under various conditions are shown. Upper panel shows a representative western blot of each isoform, while the histogram in the respective lower panel shows percent changes in the mean (±SEM) band densities of the blots from 5-sets of experiments as compared to FMC taken as 100 %. ***p < 0.001; **p < 0.01, significant as compared to FMC and other control. Abbreviations are as in the text
Effect of REMSD on mRNA (gene) Expressions of Na-K ATPase Isoforms
We evaluated whether REMSD associated increase in the protein level of α-subunit of Na-K ATPase was due to its enhanced synthesis (transcription). Total RNA was extracted from controls and experimental rat brains after 96 h of REMSD and relative changes in mRNA expressions of various isoforms of Na-K ATPase α-subunit were estimated using qPCR. Interestingly, the mRNA expression of none of the isoforms of α-subunit of Na-K ATPase was significantly increased in samples prepared from 96 h REMS deprived rat brains (Fig. 4a); the mRNA expressions in all the controls were also comparable to FMC.
Relative fold changes in mRNA level (obtained by qPCR) of various isoforms of Na-K ATPase α-subunit in the rat brains under various conditions as compared to FMC taken as 1 are shown. The values have been presented after normalization against GAPDH and TBP from 5-sets of experiments. Abbreviations are as in the text. a Changes in mean (±SEM) mRNA expression in various controls and after REMSD are shown. b Changes in mean (±SEM) mRNA expression in FMC and after REMSD for varying durations are shown
Effect of Duration of REMSD on Na-K ATPase Isoform gene Expressions
To rule out temporal compensatory effect due to rebound, if any, for not observing significant changes in the gene expressions described above, we extended our study to estimate mRNA expressions of all the isoforms of α-subunit of Na-K ATPase after subjecting different groups of rats to long and short period of REMSD. The rats were REMS deprived for 24, 48, 96 and 144 h and total RNA was extracted from brains of REMS deprived and FMC groups of rats. Relative changes in various isoforms of α-subunit of Na-K ATPase were estimated by qPCR. However, no significant change in mRNA expression of any of the α-subunit isoforms (α1, α2 and α3) of Na-K ATPase was observed in any of the samples studied (Fig. 4b).
Discussion
REMS is one of the complex processes exhibited by the animals higher in evolution. Disturbed or loss of REMS has been associated with several psycho-somatic disorders and pathological conditions including schizophrenia, epilepsy, mood disorder, memory loss, etc. [37–40]. Experimental REMS loss has been correlated with loss of concentration and memory consolidation, increased irritability, fighting behaviour and altered neuronal firing rate [15, 41–43]. These led us to propose a unified hypothesis that REMSD alters neuronal excitability and as a corollary the function of REMS is to maintain brain excitability [4, 18]; however, the underlying cellular mechanism for executing such changes was unknown. As the Na-K ATPase plays a fundamental role in maintaining neuronal transmembrane potential, the basis of neuronal excitability [44–46], and disruption of this enzyme activity has been reported to alter neuronal excitability [21]. Therefore, the Na-K ATPase activity has been considered as a marker and an estimate of altered brain excitability [5].
Although we do not know the precise function of REMS, why has it evolved at least in higher animals and why it continues to be expressed through evolution, consistent research at least has given us reasonable insight on the mechanism of neural regulation of REMS. REMS and its loss (REMSD) are influenced by many factors including hormones as well as neurotransmitters. Loss of REMS has been shown to affect many physiological processes [47], cell survival by inducing apoptosis and ionic imbalance [48–50]. Findings from such studies led us to propose that REMS maintains brain level of NA, which by modulating Na-K ATPase activity maintains brain excitability and thus, serves house-keeping function of the brain [5]. The level of NA is elevated in the brain after REMSD (or REMS loss) because (i) the activities of the NA-ergic REM-OFF neurons in the LC, which normally cease firing during REMS, continue to remain active during REMSD [51]; (ii) experimental activation (or non-cessation of firing) of those neurons prevented REMS [52–54]; (iii) inactivation of LC neurons increased REMS [55]; (iv) expression of tyrosine hydroxylase (TH), which synthesizes NA, is increased [56, 57]; (v) activity of monoamine oxidase activity, which degrades NA, is decreased after REMSD [58]. The elevated NA has been reported to induce many of the REMSD-associated changes in the body, including increased Na-K ATPase activity [5, 51]. As the REMSD-associated increase in Na-K ATPase activity was prevented by α1-AR antagonist, prazosin, it was concluded that the effects were mediated by elevated level of NA as reported earlier [5, 16]. Isolated reports are available that other neurotransmitters also change in different brain regions in relation to REMS and REMSD [Reviewed in Ref#51], and such neurotransmitters may affect the Na-K ATPase activity; however, correlation among them needs systematic studies.
Adya and Mallick [30] reported that REMSD-associated elevated NA increased Km as well as Vmax of the Na-K ATPase in the rat brain, thereby suggesting the possibility of allosteric as well as transcriptional regulation of the enzyme. Consistent and systematic studies although have led us to understand the intracellular molecular mechanism of NA induced α1-adrenoceptor mediated stimulation of Na-K ATPase activity to a reasonable extent [5, 28, 59, 60], the mechanism of REMSD-associated NA-induced transcriptional regulation of the Na-K ATPase was unknown. It is important to understand because the knowledge is likely to advance our understanding on an underlying cause of expressions of pathophysiological changes and symptoms especially upon chronic REMSD. Therefore, this study was carried out to decipher the molecular mechanism of REMSD-associated NA-induced increase in turnover of brain Na-K ATPase molecules which ultimately would alter the excitability level of the neurons and the brain at large.
REMSD affects the brain as well as most of the physiological processes controlled by the brain globally; it also increased the Na-K ATPase activity as well as its α1-subunit expression in particular in different regions in the brain [61, 62]. Therefore, we continued our study in synaptosomes prepared from the whole brain. The rats were deprived of REMS by the classical flower-pot method. This is the best available method for long-term REMSD as the method employs the REMS-specific inherent physiological property, the muscle atonia of the subject undergoing REMSD. Nevertheless, like most other behavioural studies, this method also suffers from some limitations; however, those limitations could be reasonably overcome by designing the control experiments. Quite importantly, suitable controls like FMC, LPC, and REC could be carried out to rule out the effects due to non-specific confound. Details advantages and disadvantages of this method have been dealt with in earlier reviews [5, 15]. The nerve terminal enriched synaptic-membrane fraction [63], the synaptosomes, were prepared from REMSD and control rat brains and used for estimating the Na-K ATPase activity. We observed increased synaptosomal Na-K ATPase activity after 96 h of REMSD as compared to controls, and this increase was mediated by NA acting on the α1-AR and not on the β-AR. Thus, the results confirmed our earlier findings [5, 28, 61].
Na-K ATPase is a tetramer consisting of α- and β-subunit heterodimer, each subunit having many isoforms, which are expressed in tissue and developmental specific manner. In brain α1-, α2-, α3-isoforms of catalytic α-subunit and β1-, β2-isoforms of regulatory β-subunit are expressed. Out of theses isoforms the α1- and β1- are ubiquitous, α2- and β2- are mostly glia specific although some neurons may also express them [64] and α3-isoform is restricted to neurons. The α2- and α3-Na-K ATPase together are known to comprise approximately 67 % of the total catalytic mRNA population and their inactivation results in 72–86 % inhibition of total Na-K ATPase activity in the rat brain [65, 66]. We observed that after REMSD although there was significant increase in protein abundance of catalytic α1-, α2- and α3-subunits as compared to that of their level in FMC and other controls, no significant change was seen in the abundance of β1- and β2-subunits. This increased expression of Na-K ATPase isoforms was mediated by NA acting on α1-AR. The involvement of α1-AR was confirmed by the fact that PRZ prevented this REMSD induced effect. It needs to be highlighted that the REMSD-induced increase in Na-K ATPase activity and protein expression both were modulated by NA acting on α1-AR.
The results suggest that REMSD differentially regulates the expression of α- and β-subunit isoforms of the Na-K ATPase in the rat brain. The functional Na-K ATPase requires synthesis, assembly and transport of both α- and β-subunit to the plasma membrane; however, these subunits are not always synthesized in a coordinated fashion [67, 68]. In our study the β-subunit expression remained unaltered after 96 h of REMSD. This can be explained by earlier reports that the α- and β-subunit isoforms are encoded by separate genes [69, 70], they mature separately, assemble in the endoplasmic reticulum (ER) and then are transported to the target site, the plasma membrane [71]. The mRNA abundance of different Na-K ATPase subunit isoforms varies during development [65]. Comparative stoichiometric estimate of total α- and β-subunit isoform mRNA levels showed that the latter exceeded by 40–75 % that of the former in most rat tissues [65].
It has been proposed that possibly adequate amount of β-subunit exists in intracellular pools that can be used as reservoirs to form new functional αβ-complexes to form active Na-K ATPase [72]. Laughery et al. [73] studied in baculovirus-infected SF9 cells that when α-subunit was expressed in the absence of β-subunit, the former was retained in the ER; however, in contrast, if the β-subunit was expressed in the absence of α-subunit, it was delivered to the plasma membrane. They also observed fourfold higher expression of β-subunit in comparison to α-subunit in the plasma membrane of wild-type αβ-expressing SF9 cells. Therefore, it has been proposed that the significant amount of β-subunit observed in the plasma membrane is likely to be in free form and not associated as heterodimer form [73]. Rajasekaran et al. [74] observed that the MDCK cells expressing α1-subunit expressed low level of endogenous β1-subunit. This prompted them to suggest that a low-level expression of β1-subunit was sufficient for dimerization with the α1-subunit. Based on such observations it was proposed that possibly sufficient β-subunit was available to assemble with increased abundance of α-subunit [75, 76] and that supports our findings.
The increased turnover of α-subunit of the enzyme can be achieved by either or combination of the following; by inducing its mRNA synthesis, by increasing its translation efficiency or by increasing surface abundance (up-regulation) of the enzyme molecules. To confirm if the increased α-subunit expression was due to increased mRNA synthesis, we estimated the relative mRNA level of the α-subunit isoforms. The qPCR results showed that REMSD did not affect mRNA expressions of any of the α-subunit isoforms of the Na-K ATPase. Further, as neither short nor long period of REMSD (24–144 h) affected the mRNA expression, it suggests the possibility of increased translation efficiency, possibly by increasing mRNA stability and/or decreasing its degradation, which needs to be explored. Our contention may be supported by earlier report that although there was twofold induction in the protein level of α- and β-subunit in the LLC-PK cells incubated in K+ depleted medium, the increase in α-subunit protein level was not due to increased expression but due to decreased degradation of the mRNA [76]. It may also be supported by the observations that increased Na-K ATPase α1-subunit expression in β1-overexpressing cells was due to increased translation efficiency of α1-subunit in the endoplasmic reticulum (ER). The authors proposed that increased efficiency was by either recruitment of more ribosome or by retaining already bound ribosome on the α-subunit transcript on the ER membrane during its synthesis and thus, facilitating the efficient synthesis of this polytopic protein [74]. Additionally, it has been reported that the concentration of synaptosomal (intracellular) Ca2+ is reduced [77], brain NA level is expected to rise after REMSD [5, 78] and this elevated NA reduces Ca2+ influx [60]. Therefore, we propose that reduced intracellular Ca2+ may have some role to play in reducing the mRNA synthesis and stabilising the available lot. Our contention may be further supported by the fact that elevation of Ca2+ has been reported to stimulate mRNA level of Na-K ATPase α1-subunit in rat kidney, which is partly due to the enhanced rate of transcription [79].
In summary, we conclude that REMSD induced elevated NA by acting on the α1-AR differentially modulates subunits of Na-K ATPase; increasing expressions of the α-subunit isoforms, without affecting the β-subunit in the rat brain. This is likely to be due to stabilization of the mRNA; however, the molecular mechanism of mRNA stabilization needs further study. This elevated level of α-subunits of the Na-K ATPase supports REMSD-associated increased Na-K ATPase activity and neuronal excitability [28]. This increased Na-K ATPase in turn modulates the brain functions leading to expression of REMS loss-associated symptoms, especially under chronic conditions. Thus, the findings support our hypothesis that REMS maintains brain excitability and serves as housekeeping function of the brain [5].
References
Zepelin H, Siegel JM, Tobler I (2005) Mammalian sleep. In: Kryger MH, Roth T, Dement WC (eds) Principles and practices of sleep medicine. Saunders, New York, pp 91–100
McNamara P, Capellini I, Harris E, Nunn CL, Barton RA, Preston B (2008) The phylogeny of sleep database: a new resource for sleep scientists. Open Sleep J 1:11–14. doi:10.2174/1874620900801010011
Vogel GW, Feng P, Kinney GG (2000) Ontogeny of REM sleep in rats: possible implications for endogenous depression. Physiol Behav 68(4):453–461. doi:10.1016/S0031-9384(99)00207-3
Mallick BN, Adya HV, Thankachan S (1999) REM sleep deprivation alters factors affecting neuronal excitability: role of norepinephrine and its possible mechanism of action. In: Mallick BN, Inoue S (eds) Rapid eye movement sleep. Marcel Dekker, New York, pp 338–354
Mallick BN, Singh A (2011) REM sleep loss increases brain excitability: role of noradrenaline and its mechanism of action. Sleep Med Rev 15(3):165–178. doi:10.1016/j.smrv.2010.11.001
Shaffery JP, Sinton CM, Bissette G, Roffwarg HP, Marks GA (2002) Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience 110(3):431–443. doi:10.1016/S0306-4522(01)00589-9
Shaffery JP, Allard JS, Manaye KF, Roffwarg HP (2012) Selective rapid eye movement sleep deprivation affects cell size and number in kitten locus coeruleus. Front Neurol 3(69):1–9. doi:10.3389/fneur.2012.00069
Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP (1995) A functional role for REM sleep in brain maturation. Behav Brain Res 69(1–2):1–11. doi:10.1016/0166-4328(95)00018-O
Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T (1995) Sleep in Alzheimer’s disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep 18(3):145–148
Gagnon JF, Montplaisir J, Bedard MA (2002) Rapid-eye-movement sleep disorders in Parkinson’s disease. Rev Neurol (Paris) 158(2):135–152
Vogel GW (1999) REM sleep deprivation and behavioral changes. In: Mallick BN, Inoue S (eds) Rapid eye movement sleep. Marcel Dekker, New York, pp 355–366
Ackermann S, Rasch B (2014) Differential effects of non-REM and REM sleep on memory consolidation. Curr Neurol Neurosci Rep 14(2):430. doi:10.1007/s11910-013-0430-8
Cohen HB, Dement WC (1965) Sleep: changes in threshold to electroconvulsive shock in rats after deprivation of “paradoxical” phase. Science 150(3701):1318–1319. doi:10.1126/science.150.3701.1318
Dement W (1960) The effect of dream deprivation. Science 131(3415):1705–1707. doi:10.1126/science.131.3415.1705
Gulyani S, Majumdar S, Mallick BN (2000) Rapid eye movement sleep and significance of its deprivation studies: a review. Sleep Hypnosis 2(2):49–68
Gulyani S, Mallick BN (1993) Effect of rapid eye movement sleep deprivation on rat brain Na-K ATPase activity. J Sleep Res 2(1):45–50. doi:10.1111/j.1365-2869.1993.tb00060.x
Mallick BN, Thakkar M, Gulyani S (1994) Rapid eye movement sleep deprivation induced alteration in neuronal excitability: possible role of norepinephrine. In: Mallick BN, Singh R (eds) Environment and physiology. Narosa, New Delhi, pp 196–203
Glynn IM (1993) Annual review prize lecture. ‘All hands to the sodium pump’. J Physiol 462:1–30. doi:10.1113/jphysiol.1993.sp019540
Skou JC (1990) The fourth Datta lecture. The energy coupled exchange of Na+ for K+ across the cell membrane. The Na+, K+-pump. FEBS Lett 268(2):314–324. doi:10.1016/0014-5793(90)81278-V
McCarren M, Alger BE (1987) Sodium-potassium pump inhibitors increase neuronal excitability in the rat hippocampal slice: role of a Ca2+-dependent conductance. J Neurophysiol 57(2):496–509
Vaillend C, Mason SE, Cuttle MF, Alger BE (2002) Mechanisms of neuronal hyperexcitability caused by partial inhibition of Na+-K+-ATPases in the rat CA1 hippocampal region. J Neurophysiol 88(6):2963–2978. doi:10.1152/jn.00244.2002
Chow DC, Forte JG (1995) Functional significance of the beta-subunit for heterodimeric P-type ATPases. J Exp Biol 198(Pt 1):1–17
Blanco G, DeTomaso AW, Koster J, Xie ZJ, Mercer RW (1994) The alpha-subunit of the Na, K-ATPase has catalytic activity independent of the beta-subunit. J Biol Chem 269(38):23420–23425
Lingrel JB, Kuntzweiler T (1994) Na+, K+-ATPase. J Biol Chem 269(31):19659–19662
Lingrel JB, Orlowski J, Shull MM, Price EM (1990) Molecular genetics of Na, K-ATPase. Prog Nucleic Acid Res Mol Biol 38:37–89. doi:10.1016/s0079-6603(08)60708-4
Sweadner KJ (1989) Isozymes of the Na +/K + -ATPase. Biochim Biophys Acta 988(2):185–220. doi:10.1016/0304-4157(89)90019-1
Blanco G, Mercer RW (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol 275(5):F633–F650
Mallick BN, Adya HV, Faisal M (2000) Norepinephrine-stimulated increase in Na+, K+-ATPase activity in the rat brain is mediated through alpha1A-adrenoceptor possibly by dephosphorylation of the enzyme. J Neurochem 74(4):1574–1578. doi:10.1046/j.1471-4159.2000.0741574.x
Mallick BN, Adya HV (1999) Norepinephrine induced alpha-adrenoceptor mediated increase in rat brain Na-K ATPase activity is dependent on calcium ion. Neurochem Int 34(6):499–507. doi:10.1016/S01970186(99)00025-X
Adya HV, Mallick BN (2000) Uncompetitive stimulation of rat brain Na-K ATPase activity by rapid eye movement sleep deprivation. Neurochem Int 36(3):249–253. doi:10.1016/S0197-0186(99)00121-7
Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ (1974) The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav 2(4):553–556. doi:10.1016/0091-3057(74)90018-5
Hicks RA, Okuda A, Thomsen D (1977) Depriving rats of REM sleep: the identification of a methodological problem. Am J Psychol 90(1):95–102. doi:10.2307/1421644
Oniani T, Lortkipanidze N, Maisuradze L, Oniani L (1988) Neurophysiological analysis of the effects of partial paradoxical sleep deprivation. Neurophysiology 20(1):15–21. doi:10.1007/BF02198420
Jouvet D, Vimont P, Delorme F (1964) Study of selective deprivation of the paradoxal phase of sleep in the cat. J Physiol (Paris) 56(3):381
Yanik G, Radulovacki M (1987) REM sleep deprivation up-regulates adenosine A1 receptors. Brain Res 402(2):362–364. doi:10.1016/0006-8993(87)90046-1
Fiske CH, Subbarow Y (1925) Determination of inorganic phosphate. J Biol Chem 66:375–400
Monti JM, Monti D (2005) Sleep disturbance in schizophrenia. Int Rev Psychiatry 17(4):247–253. doi:10.1080/09540260500104516
Walker MP, Stickgold R (2006) Sleep, memory, and plasticity. Annu Rev Psychol 57:139–166. doi:10.1146/annurev.psych.56.091103.070307
Gottesmann C, Gottesman I (2007) The neurobiological characteristics of rapid eye movement (REM) sleep are candidate endophenotypes of depression, schizophrenia, mental retardation and dementia. Prog Neurobiol 81(4):237–250. doi:10.1016/j.pneurobio.2007.01.004
Benca RM (2001) Consequences of insomnia and its therapies. J Clin Psychiatry 62(Suppl 10):33–38
Morden B, Connera R, Mitchell G, Dement W, Levine S (1968) Effect of rapid eye movement (REM) sleep deprivation on shock-induced fighting. Physiol Behav 3(3):425–432. doi:10.1016/0031-9384(68)90073-5
Dement W, Fisher C (1963) Experimental interference with the sleep cycle. Can Psychiatr Assoc J 257:400–405
Sampson H (1966) Psychological effects of deprivation of dreaming sleep. J Nerv Ment Dis 143(4):305–317. doi:10.1097/00005053-196610000-00002
Trachtenberg M, Packey D, Sweeney T (1981) In vivo functioning of the Na+, K+-activated ATPase. Curr Top Cell Regul 19:159–217. doi:10.1016/b978-0-12-152819-5.50022-1
Horisberger JD, Lemas V, Kraehenbuhl JP, Rossier BC (1991) Structure-function relationship of Na, K-ATPase. Annu Rev Physiol 53:565–584. doi:10.1146/annurev.ph.53.030191.003025
Pavlov KV, Sokolov VS (2000) Electrogenic ion transport by Na+, K+-ATPase. Membr Cell Biol 13(6):745–788
Mallick BN, Madan V, Faisal M (2005) Biochemical change. In: Kushida CA (ed) Sleep deprivation basic science, physiology and behavior. Marcel Dekker, New York, pp 339–357
Biswas S, Mishra P, Mallick BN (2006) Increased apoptosis in rat brain after rapid eye movement sleep loss. Neuroscience 142(2):315–331. doi:10.1016/j.neuroscience.2006.06.026
Majumadar S, Mallick BN (2005) Cytomorphometric changes in rat brain neurons after rapid eye movement sleep deprivation. Neuroscience 135(3):679–690. doi:10.1016/j.neuroscience.2005.06.085
Das G, Mallick BN (2008) Noradrenaline acting on alpha1-adrenoceptor mediates REM sleep deprivation-induced increased membrane potential in rat brain synaptosomes. Neurochem Int 52(4–5):734–740. doi:10.1016/j.neuint.2007.09.002
Mallick BN, Singh A, Khanday MA (2012) Activation of inactivation process initiates rapid eye movement sleep. Prog Neurobiol 97(3):259–276. doi:10.1016/j.pneurobio.2012.04.001
Singh S, Mallick BN (1996) Mild electrical stimulation of pontine tegmentum around locus coeruleus reduces rapid eye movement sleep in rats. Neurosci Res 24(3):227–235. doi:10.1016/0168-0102(95)00998-1
Kaur S, Panchal M, Faisal M, Madan V, Nangia P, Mallick BN (2004) Long term blocking of GABA-A receptor in locus coeruleus by bilateral microinfusion of picrotoxin reduced rapid eye movement sleep and increased brain Na-K ATPase activity in freely moving normally behaving rats. Behav Brain Res 151(1–2):185–190. doi:10.1016/j.bbr.2003.08.011
Jaiswal MK, Dvela M, Lichtstein D, Mallick BN (2010) Endogenous ouabain-like compounds in locus coeruleus modulate rapid eye movement sleep in rats. J Sleep Res 19:183–191. doi:10.1111/j.1365-2869.2009.00781.x
Cespuglio R, Gomez ME, Faradji H, Jouvet M (1982) Alterations in the sleep-waking cycle induced by cooling of the locus coeruleus area. Electroencephalogr Clin Neurophysiol 54(5):570–578. doi:10.1016/0013-4694(82)90042-6
Majumdar S, Mallick BN (2003) Increased levels of tyrosine hydroxylase and glutamic acid decarboxylase in locus coeruleus neurons after rapid eye movement sleep deprivation in rats. Neurosci Lett 338(3):193–196. doi:10.1016/S0304-3940(02)01404-0
Porkka-Heiskanen T, Smith SE, Taira T, Urban JH, Levine JE, Turek FW, Stenberg D (1995) Noradrenergic activity in rat brain during rapid eye movement sleep deprivation and rebound sleep. Am J Physiol 268:R1456–R1463
Thakkar M, Mallick BN (1993) Effect of rapid eye movement sleep deprivation on rat brain monoamine oxidases. Neuroscience 55(3):677–683. doi:10.1016/0306-4522(93)90433-G
Adya HV, Mallick BN (1998) Comparison of Na-K ATPase activity in rat brain synaptosome under various conditions. Neurochem Int 33(4):283–286. doi:10.1016/S0197-0186(98)00043-6
Das G, Gopalakrishnan A, Faisal M, Mallick BN (2008) Stimulatory role of calcium in rapid eye movement sleep deprivation-induced noradrenaline-mediated increase in Na-K-ATPase activity in rat brain. Neuroscience 155(1):76–89. doi:10.1016/j.neuroscience.2008.04.069
Gulyani S, Mallick BN (1995) Possible mechanism of rapid eye movement sleep deprivation induced increase in Na-K ATPase activity. Neuroscience 64(1):255–260. doi:10.1016/0306-4522(94)00333-Z
Majumdar S, Faisal M, Madan V, Mallick BN (2003) Increased turnover of Na-K ATPase molecules in rat brain after rapid eye movement sleep deprivation. J Neurosci Res 73(6):870–875. doi:10.1002/jnr.10710
Gray E, Whittaker V (1962) The isolation of nerve endings from brain: an electron microscopic study of cell fragments derived by homogenization and centrifugation. J Anat 96(1):79–88
Moseley AE, Lieske SP, Wetzel RK, James PF, He S, Shelly DA, Paul RJ, Boivin GP, Witte DP, Ramirez JM, Sweadner KJ, Lingrel JB (2003) The Na, K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice. J Biol Chem 278(7):5317–5324. doi:10.1074/jbc.M211315200
Orlowski J, Lingrel JB (1988) Tissue-specific and developmental regulation of rat Na, K-ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem 263(21):10436–10442
Matsuda T, Iwata H, Cooper JR (1984) Specific inactivation of alpha (+) molecular form of (Na++ K+)-ATPase by pyrithiamin. J Biol Chem 259(6):3858–3863
Gick GG, Hatala MA, Chon D, Ismail-Beigi F (1993) Na, K-ATPase in several tissues of the rat: tissue-specific expression of subunit mRNAs and enzyme activity. J Membr Biol 131(3):229–236. doi:10.1007/BF02260111
Factor P, Senne C, Dumasius V, Ridge K, Jaffe HA, Uhal B, Gao Z, Sznajder JI (1998) Overexpression of the Na+, K+-ATPase alpha1 subunit increases Na+, K+-ATPase function in A549 cells. Am J Respir Cell Mol Biol 18(6):741–749. doi:10.1165/ajrcmb.18.6.291
Kent RB, Fallows DA, Geissler E, Glaser T, Emanuel JR, Lalley PA, Levenson R, Housman DE (1987) Genes encoding alpha and beta subunits of Na, K-ATPase are located on three different chromosomes in the mouse. Proc Natl Acad Sci 84(15):5369–5373. doi:10.1073/pnas.84.15.5369
Yang-Feng TL, Schneider JW, Lindgren V, Shull MM, Benz EJ Jr, Lingrel JB, Francke U (1988) Chromosomal localization of human Na+, K+-ATPase alpha- and beta-subunit genes. Genomics 2(2):128–138. doi:10.1016/0888-7543(88)90094-8
Geering K (2008) Functional roles of Na, K-ATPase subunits. Curr Opin Nephrol Hypertens 17(5):526–532. doi:10.1097/MNH.0b013e3283036cbf
Lavoie L, Levenson R, Martin-Vasallo P, Klip A (1997) The molar ratios of alpha and beta subunits of the Na+-K+-ATPase differ in distinct subcellular membranes from rat skeletal muscle. Biochemistry 36(25):7726–7732. doi:10.1021/bi970109s
Laughery MD, Todd ML, Kaplan JH (2003) Mutational analysis of alpha-beta subunit interactions in the delivery of Na, K-ATPase heterodimers to the plasma membrane. J Biol Chem 278(37):34794–34803. doi:10.1074/jbc.M302899200
Rajasekaran SA, Gopal J, Willis D, Espineda C, Twiss JL, Rajasekaran AK (2004) Na, K-ATPase beta1-subunit increases the translation efficiency of the alpha1-subunit in MSV-MDCK cells. Mol Biol Cell 15(7):3224–3232. doi:10.1091/mbc.E04-03-0222
Lescale-Matys L, Hensley CB, Crnkovic-Markovic R, Putnam DS, McDonough AA (1990) Low K+ increases Na, K-ATPase abundance in LLC-PK1/Cl4 cells by differentially increasing beta, and not alpha, subunit mRNA. J Biol Chem 265(29):17935–17940
Lescale-Matys L, Putnam DS, McDonough AA (1993) Na+-K+ ATPase alpha 1- and beta 1-subunit degradation: evidence for multiple subunit specific rates. Am J Physiol 264(3 Pt 1):C583–C590
Mallick BN, Gulyani S (1996) Alterations in synaptosomal calcium concentrations after rapid eye movement sleep deprivation in rats. Neuroscience 75(3):729–736. doi:10.1016/0306-4522(96)00177-7
Mallick BN, Majumdar S, Faisal M, Yadav V, Madan V, Pal D (2002) Role of norepinephrine in the regulation of rapid eye movement sleep. J Biosci 27(5):539–551. doi:10.1007/BF02705052
Rayson BM (1991) [Ca2+]i regulates transcription rate of the Na+/K+ ATPase alpha 1 subunit. J Biol Chem 266(32):21335–21338
Acknowledgments
MA received UGC-fellowship. Research funding from DBT-BUILDER; JC Bose Fellowship; PURSE; UGC and UPOE to BNM are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amar, M., Mallick, B.N. Rapid Eye Movement Sleep Deprivation Associated Increase in Na-K ATPase Activity in the Rat Brain is Due to Noradrenaline Induced α1-Adrenoceptor Mediated Increased α-Subunit of the Enzyme. Neurochem Res 40, 1747–1757 (2015). https://doi.org/10.1007/s11064-015-1660-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1660-8