Abstract
In general, pro-inflammatory cytokines (PICs) contribute to regulation of epilepsy-associated pathophysiological processes in the central nerve system. In this report, we examined the specific activation of PICs, namely IL-1β, IL-6 and TNF-α in rat brain after kainic acid (KA)-induced status epilepticus (SE). Also, we examined the role played by PICs in regulating expression of GABA transporter type 1 and 3 (GAT-1 and GAT-3, respectively), which are the two important subtypes of GATs responsible for the regulation of extracellular GABA levels in the brain. Our results show that IL-1β, IL-6 and TNF-α were significantly increased in the parietal cortex, hippocampus and amygdala of KA-rats as compared with sham control animals (P < 0.05, KA rats vs. control rats). KA-induced SE also significantly increased (P < 0.05 vs. controls) the protein expression of GAT-1 and GAT-3 in those brain regions. In addition, central administration of antagonists to IL-1β and TNF-α receptors significantly attenuated amplified GAT-1 and GAT-3 (P < 0.05 vs. vehicle control for each antagonist group). However, antagonist to IL-6 receptor failed to attenuate enhancement in expression of GAT-1 and GAT-3 induced by KA-induced SE. Overall, our data demonstrate that PIC pathways are activated in the specific brain regions during SE which thereby selectively leads to upregulation of GABA transporters. As a result, it is likely that de-inhibition of GABA system is increased in the brain. This support a role for PICs in engagement of the adaptive mechanisms associated with epileptic activity, and has pharmacological implications to target specific PICs for neuronal dysfunction and vulnerability related to epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abnormal patterns of highly synchronized recurrent neuronal discharges are observed in one or more brain areas as epilepsy occurs, which often recruit anatomically related structures [28]. Generally, seizures are relatively brief, as in the complex partial seizures of temporal lobe epilepsy; however, in the case of status epilepticus (SE), seizures may persist for minutes or hours, and this may culminate in neuronal damage within several limbic and cortical areas [28]. Nevertheless, neural substrates responsible for epileptic activities remain to be determined.
It has been reported that IL-1β, IL-6 and TNF-α in plasma/cerebrospinal fluid and cells immunoreactive to those proinflammatory cytokines (PICs) in some tissues are increased in patients with TLE [14, 29], this is consistent with a role of PICs in engagement of the pathophysiology of epilepsy and/or brain damage following seizure. Moreover, in animals with SE induced by hippocampal kainic acid (KA) injections or by electrical stimulation increases mRNA expression of hippocampal IL-1β, IL-6 and TNF-α [20, 22]. Because SE induces relatively widespread brain damage, evaluation of the functional protein levels of PICs production in the local brain regions during and following SE is more important. Thus, in this report we specifically examined the protein levels of IL-1β, IL-6 and TNF-α in the representative brain regions related to SE, namely the parietal cortex, hippocampus and amygdala.
It has been reported that the two main subtypes of GATs responsible for the regulation of extracellular GABA levels in the central nervous system are GABA transporter type 1 and 3 (GAT-1 and GAT-3, respectively) [5, 11]. Throughout the brain these transporters are widely expressed in neuronal cell (mainly GAT-1) and glial cells (mainly GAT-3), but published work has largely shown the role played by GATs in regulating GABAa receptor-mediated postsynaptic tonic and phasic inhibition in the cerebral cortex and hippocampus etc. [5, 11]. In light of the key role of GABAergic transmission within the brain regions related to epileptic activity [6, 10], in this study we further examined expression of GAT-1 and GAT-3 in the parietal cortex, hippocampus and amygdala of control rats and KA-animals. In order to better understand the role played by PICs in regulating GABA transporters, we examined expression of GAT-1 and GAT-3 in those brain regions after intracerebroventricular (ICV) infusion of individual PIC receptor antagonists.
There are a number of animal models generally used to study the mechanisms of complex partial epilepsy and development of new antiepileptic drugs [15]. For example, systemically applied drugs (e.g., pilocarpine preceded by lithium treatment) and neurotoxins (e.g., kainic acid) as well as kindling have typically been used to study SE. In our report, a rat model of cerebral KA injection-induced SE [13, 26, 35] was employed to determine the role played by PICs in engagement of epileptic activity. We hypothesized that KA-induced epilepsy increases the levels of IL-1β, IL-6 and TNF-α in the parietal cortex, hippocampus and amygdala and thereby leads to upregulation of GAT-1 and GAT-3, which decreases GABA-mediated inhibitory effects.
Methods
All the animal procedures of this study and their care were conducted in conformity with the institutional guidelines that are in compliance with the Guideline for the Care and Use of laboratory Animals of the U.S. National Health Institute. Fifty-four male Sprague–Dawley rats (250–300 g) were used in our experiments and all animals were maintained under 12 h light/dark-cycle with free access to food and water in a temperature- and humidity-controlled room.
Rats were anesthetized with chloral hydrate (40 mg/kg body weight, i.p.), then immobilized in a stereotaxic apparatus (David Kopf, USA). After making a midline incision, the skull was exposed and one burr hole was drilled. Following this, animals were cannulated with an L-shaped stainless steel cannula aimed at the lateral ventricle according to the coordinates: 3.7 mm posterior to the bregma, 4.1 mm lateral to the midline, and 3.5 mm under the dura. The guide cannula was fixed to the skull using dental zinc cement and jewelers’ screw. After animals recovered from anesthesia, in order to obtain epileptic model they were injected with 1.0 μl of KA [0.5 μg in 1.0 μl of artificial cerebrospinal fluid (aCSF)] in the right lateral ventricle as described previously [35]. The rats were injected with the same of volume of aCSF as controls. The cannula was then connected to an osmotic minipump (Alzet pump brain infusion kit, DURECT Inc., Cupertino, CA) with polycarbonate tubing. The pumps were placed subcutaneously between the scapulae, and loaded with vehicle (aCSF) as control or each PIC receptor antagonists, namely IL-1Ra (IL-1β receptor antagonist) and tocilizumab (IL-6 receptor antagonist) and etanercept (TNF-α receptor antagonist), respectively (Tocris Co., Ellisville, MO, USA). The antagonists in 10 µM of concentration were delivered at 0.25 μl per hour (Alzet Model 1003D/3 day-delivery DURECT Inc., Cupertino, CA, USA). This intervention allowed animals to receive continuous ICV infusion via the osmotic minipumps before brain tissues were removed.
Then, animal behaviors were observed. Consistent with the prior report [35], rats developed status epileptic and convulsive seizures within ~30 min after KA injection, which lasted at least for 1–2 h. Also, during seizure behavior animals showed rolling toward one side, rotating, and stiffing of limbs and tail but no movement during the interphase of spasms. In the control group, rats with aCSF injection did not exhibit any of these abnormal behaviors. It should be noted that all the rats who received KA injection developed those abnormal seizure behaviors and were included in this study.
Rats were divided into (1) epileptic sham control group with aCSF (n = 12); (2) epileptic group induced by KA injection followed by ICV administration of aCSF (n = 12); and (3) epileptic group induced by KA injection followed by ICV infusion of each PIC antagonist (n = 10 in each group). After completion of those treatments, all the animals were anesthetized and sacrificed 3 days after KA-injection. The brains were removed to confirm the placement of the cannula, and then the brain regions were dissected under an anatomical microscope and tissues were taken for the process.
The levels of IL-1β, IL-6 and TNF-α were determined using a two-site immunoenzymatic assay (ELISA, Promega Corp. Madison, WI, USA) according to the provided description and modification. Briefly, polystyrene 96-well microtitel immunoplates were coated with affinity-purified polyclonal rabbit anti-IL-1β, anti-IL-6 and anti-TNF-α antibody. Parallel wells were coated with purified rabbit/mouse IgG for evaluation of nonspecific signal. After overnight incubation at room temperature and 2 h of incubation with the coating buffer, plates were washed. The diluted samples and the each PIC standard solutions were distributed in each plate and left at room temperature overnight. The plates were washed and incubated with anti-IL-1β, anti-IL-6 and anti-TNF-α galactosidase per well. Then, the plates were washed and incubated with substrate solution. After an incubation of 2 h at 37 °C, the optical density was measured using an ELISA reader (Dynatech).
To examine expression of GAT-1 and GAT-3, the brain tissues were processed using a standard western blot procedure. Briefly, total protein was extracted by homogenizing samples of the parietal cortex, hippocampus and amygdala in ice-cold immunoprecipitation assay buffer. The lysates were centrifuged and the supernatants were collected for measurements of protein concentrations. After being denatured in buffer, the supernatant samples containing 20 μg of protein were loaded onto 4–20 % Mini-PROTEAN TGX gels and electrically transferred to a polyvinylidene fluoride membrane. The membrane was blocked in 5 % nonfat milk and was incubated overnight with primary antibodies (mouse anti-GAT-1/anti-GAT-3 at 1:200, Cayman Chemical Co.). Next, the membranes were washed and incubated with an alkaline phosphatase conjugated anti-mouse secondary antibody (1:1,000). The immunoreactive proteins were detected by enhanced chemiluminescence. The bands recognized by the primary antibody were visualized by exposure of the membrane onto an X-ray film. The membrane was stripped and incubated with mouse anti-β-actin to show equal loading of the protein. Then, the film was scanned and the optical density of GAT-1/GAT-3 and β-actin bands was analyzed using the NIH Scion Image software. In order to obtain better control data, the antibody specificity was examined. In this process, pre-incubation with the control peptide antigens were applied for GAT-1 and GAT-3, respectively. Our results showed that no GAT-1/GAT-3 staining was observed after pre-incubation with the control peptide antigens (shown in Figs. 2, 3), suggesting that anti-GAT-1/GAT-3 antibodies used in this study were specific.
All data were presented as mean ± standard deviation (SD). Two-way analysis of variance (ANOVA) was employed for comparison. All statistical analyses were performed by using SAS for Windows (version 5.0, SAS Inc., Cary, NC, USA). For all analyses, statistical significance was set at P < 0.05.
Results
Levels of IL-1β, IL-6 and TNF-α
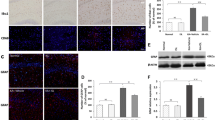
Figure 1 shows that the levels of IL-1β, IL-6 and TNF-α were significantly increased in the parietal cortex, hippocampus and amygdala of KA-rats (P < 0.05 vs. control rats, n = 12) as compared with sham control group (n = 12).
The levels of IL-1β, IL-6 and TNF-α in the parietal cortex (PCX), hippocampus (HIP) and amygdala (AMG). The cytokines were significantly increased in SE-rats 3 days after cerebral KA injection as compared with control animals. Data are expressed as mean ± SD. *P < 0.05, indicated rats with KA-induced SE (n = 12) versus control rats (n = 12)
Protein Expression of GAT-1 and GAT-3
Figure 2 illustrates that expression of GAT-1 in the parietal cortex, hippocampus and amygdala were significantly greater in KA-rats (n = 12, P < 0.05 vs. control animals) than those in control rats (n = 12). Also, attenuating IL-1β (n = 10) and TNF-α receptors (n = 10) by infusion of their individual antagonist significantly recovered the effects induced by KA injection on the expression of GAT-1 in the parietal cortex, hippocampus and amygdala. Insignificant differences were observed in GAT-1 expression between sham control animals and animals that were infused with IL-1β and TNF-α receptors antagonist, respectively. However, antagonist to IL-6 (n = 10) failed to reverse amplified GAT-1 in those brain regions of KA-rats.
Expression of GABA transporter type 1 (GAT-1) in the parietal cortex (PCX), hippocampus (HIP) and amygdala (AMG). GAT-1 was significantly increased in SE-rats 3 days after cerebral KA injection (n = 12) as compared with control animals (n = 12). Attenuating IL-1β and TNF-α receptors by ICV infusion of respective IL-1Ra and etanercept (ETAN) decreased the effects of KA-induced SE on GAT-1. ICV infusion of tocilizumab (TCZB) to block IL-6 receptors failed to alter enhanced GAT-1 induced by KA. Data are expressed as mean ± SD. *P < 0.05 versus control rats and KA injection with the prior IL-1Ra and ETAN; and versus control rats (group with the prior TCZB). The number of KA-rats with the prior PIC antagonists = 10 in each group. Bottom panel (right): typical bands are representative of expression of GAT-1 in the PCX region of three groups of rats. Also, the specificity of ant-GAT-1 antibody was shown by pre-incubation of the control peptide antigen. Molecular weight of GAT-1: 67 kDa
Likewise, Fig. 3 also demonstrates that GAT-3 in the parietal cortex, hippocampus and amygdala was significantly increased by KA–SE (P < 0.05, control vs. KA–SE; n = 12 in each group). Moreover, blocking IL-1β and TNF-α receptors (n = 10 in each group) significantly attenuated the enhancement in GAT-3 induced by KA–SE. ICV infusion of IL-6 antagonist had no significant effects on expression of GAT-3 induced by KA–SE (P > 0.05, n = 10).
Expression of GABA transporter type 3 (GAT-3) in the parietal cortex (PCX), hippocampus (HIP) and amygdala (AMG). GAT-3 was significantly increased in SE-rats 3 days (n = 12) after cerebral KA injection as compared with control animals (n = 12). Also, blocking IL-1β and TNF-α receptors by respective ICV infusion of IL-1Ra (n = 10) and etanercept (ETAN, n = 10) decreased the effects of KA-induced SE on GAT-3. ICV infusion of tocilizumab (TCZB, n = 10) to block IL-6 receptors failed to alter enhanced GAT-3 induced by KA. Data are expressed as mean ± SD. *P < 0.05 versus control rats and KA injection with the prior IL-1Ra and ETAN; and versus control rats (group with the prior TCZB). Bottom panel (right) shows typical bands that are representative of expression of GAT-3 in the PCX region of three groups of rats. Also, the specificity of ant-GAT-3 antibody was shown by pre-incubation of the control peptide antigen. Molecular weight of GAT-3: 70 kDa
Discussion
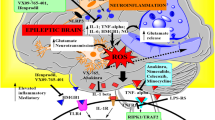
In the previous studies [13, 26, 35], a rat model of cerebral KA injection-induced SE was employed to determine the mechanisms responsible for epileptic disease and antiepileptic drugs. It has also reported that PICs are engaged in later phase alterations of neuronal damage and electronic encephalography related to epileptic activity and significant increases in IL-1β, IL-6 and TNF-α in hippocampus were observed 3 days following KA injection [35]. Using the same rat model, our data demonstrated that KA-induced epilepsy increases the levels of IL-1β, IL-6 and TNF-α in the specific brain regions such as the parietal cortex, hippocampus and amygdala. Moreover, our data showed that GABA transporters, namely GAT-1 and GAT-3 are upregulated by KA-induced SE via receptor mechanisms of IL-1β and TNF-α, but not IL-6. Overall, we suggest that enhancement of IL-1β, IL-6 and TNF-α activity in those brain regions contribute to the neuronal excitability by de-inhibiting GABA system during epileptogenesis.
However, it should be noted that the GATs may also play a role in a reversed direction under certain conditions. For example, the amplified expression and stimulation of GAT-1 and GAT-3 are likely to increase the release of GABA and thereby GABA activity to combat uncontrolled epileptic activity [7]. Indeed, it has been shown that epileptic activity is counteracted by increasing GABAergic activity in animal models [4].
Elevated levels of PICs in the brain are associated with increased seizure vulnerability and seizure-related pathological changes such as neuronal death [2, 31, 32], suggesting that inflammation has been implicated in epileptogenesis. In a rat model of KA-induced epilepsy, neural injuries in the brain occur and this is accompanied by upregulation of PICs including IL-1β, IL-6 and TNF-α in glial cells [30, 35]. Furthermore, neuronal degeneration is observed likely due to increases of PICs [8, 24]. For example, IL-1β can increase seizure susceptibility in rat brains [9]. Intracerebral injection of a high dosage of IL-1β results in limbic seizures in wild type mice, but not in transgenic mice with deficient IL-1β receptors [23]. In addition, antiepileptic activity is observed after using thalidomide to decrease the levels of TNF-α [21]. With respect to the role played by central IL-6 in regulating epileptic activity, prior studies demonstrate that IL-6 can stimulate the synthesis of corticotrophin and glucocorticoids, and initiate an anti-inflammatory feedback loop, suggesting a neuroprotective role played by IL-6 [16, 18]. Nevertheless, it is crucial to determine neural substrates to play a role in the initiation of neuronal excitability responsible for epileptic activities.
GABA is the main inhibitory neurotransmitter in the central nerve system in control of neuronal excitability. After GABA release from presynaptic terminals, GATs play a key role in regulating a rapid removal of extracellular GABA [3, 25], which thereby leads to ending of inhibitory synaptic transmission. Thus, this mechanism is also responsible for GABA spillover to neighboring synapses [3, 19] and GABA homeostasis and then excessive tonic activation of synaptic and extrasynaptic GABA receptors are prevented [3, 27]. In contrast, under certain pathological and physiological conditions the GATs functions are impaired and this is likely to result in the exaggerated GABA release [1, 34]. Results of our current study demonstrated that the levels of IL-1β, IL-6 and TNF-α were significantly increased in the specific brain regions such as parietal cortex, hippocampus and amygdala 3 days after KA-induced SE (Fig. 1). Importantly, our data further showed that protein expression of both GAT1 and GAT3 were greater in those brain regions of KA-animals than them in those regions of control animals. Additionally, attenuating each of IL-1β and TNF-α receptors decreased the effects of KA-induced SE on GAT-1 and GAT-3 (Figs. 2, 3). However, blocking IL-6 receptor did not significantly alter amplified GAT-1 and GAT-3 in those brain regions evoked by KA-induced SE (Figs. 2, 3). Overall, our data suggest that KA-induced epilepsy amplifies the levels of IL-1β, IL-6 and TNF-α in the parietal cortex, hippocampus and amygdala. Our data also suggest that KA-induced epilepsy leads to upregulation of GAT-1 and GAT-3 via stimulation of IL-1β and TNF-α receptors, but not IL-6 receptor. This decreases GABA-mediated inhibitory effects on neuronal activities in those specific brain regions. Since GAT-1 is widely expressed in neuronal cells and GAT-3 is mainly present in glial cells throughout the brain [3, 5], results of the current study suggest that PICs are likely to play a role in regulating a GABA inhibitory system in the neuronal and glial cells after KA-induced SE.
Nevertheless, consistent with the findings of prior studies [12, 17, 33] we have observed increases in the levels of IL-1β, IL-6 and TNF-α in the parietal cortex, hippocampus and amygdala 3 days after KA-induced SE. Also, GAT-1 and GAT-3 are significantly increased by KA-induced SE, but blocking receptors of IL-1β, IL-6 and TNF-α showed that they had different effects on expression of GAT-1 and GAT-3. We suggest that activation of IL-1β and TNF-α receptors, but not IL-6 receptor plays a role in regulating GAT-1 and GAT-3. We assumed that this discrepancy is likely due to intracellular signaling mechanisms for PICs to play a role. A great deal of evidence has demonstrated that IL-1β and TNF-α play a role mainly via p38 MAPK, ERK and JNK signaling transduction pathways, but IL-6 plays a role via JAK–STAT pathways [12, 17, 33]. After activation of those signaling pathways, gene transcription occurs. What different mechanisms by which those signaling transduction pathways are responsible for regulating expression of GAT-1 and GAT-3 via PIC receptors needs to be determined.
In summary, we have provided evidence that KA-induced epileptic activities amplify the levels of IL-1β, IL-6 and TNF-α in the parietal cortex, hippocampus and amygdala. KA-induced epilepsy further leads to upregulation of GABA transporter subtypes GAT-1 and GAT-3 via receptors of IL-1β and TNF-α. These abnormalities are likely to contribute to the enhanced neuronal excitability in those brain regions of epileptic animals. The results may offer promising clues for the development of new therapeutic strategies for managing intractable symptoms observed in patients with epilepsy.
References
Allen NJ, Karadottir R, Attwell D (2004) Reversal or reduction of glutamate and GABA transport in CNS pathology and therapy. Pflug Arch Eur J Physiol 449:132–142
Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, Vezzani A (2008) A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1β. Brain 131:3256–3265
Borden LA (1996) GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int 29:335–356
Boulland JL, Ferhat L, Tallak Solbu T, Ferrand N, Chaudhry FA, Storm-Mathisen J, Esclapez M (2007) Changes in vesicular transporters for gamma-aminobutyric acid and glutamate reveal vulnerability and reorganization of hippocampal neurons following pilocarpine-induced seizures. J Comp Neurol 503:466–485
Conti F, Minelli A, Melone M (2004) GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Rev 45:196–212
da Cruz GM, Felipe CF, Scorza FA, da Costa MA, Tavares AF, Menezes ML, de Andrade GM, Leal LK, Brito GA, da Naffah-Mazzacoratti MG, Cavalheiro EA, de Barros Viana GS (2013) Piperine decreases pilocarpine-induced convulsions by GABAergic mechanisms. Pharmacol Biochem Behav 104:144–153
Dalby NO (2003) Inhibition of gamma-aminobutyric acid uptake: anatomy, physiology and effects against epileptic seizures. Eur J Pharmacol 479:127–137
Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ (2005) Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol 57:152–155
Galic MA, Riazi K, Henderson AK, Tsutsui S, Pittman QJ (2009) Viral-like brain inflammation during development causes increased seizure susceptibility in adult rats. Neurobiol Dis 36:343–351
Hattingen E, Luckerath C, Pellikan S, Vronski D, Roth C, Knake S, Kieslich M, Pilatus U (2014) Frontal and thalamic changes of GABA concentration indicate dysfunction of thalamofrontal networks in juvenile myoclonic epilepsy. Epilepsia 55:1030–1037
Jin XT, Galvan A, Wichmann T, Smith Y (2011) Localization and function of GABA transporters GAT-1 and GAT-3 in the basal ganglia. Front Syst Neurosci 5:63
Kerschensteiner M, Meinl E, Hohlfeld R (2009) Neuro-immune crosstalk in CNS diseases. Neuroscience 158:1122–1132
Kotaria N, Kiladze M, Zhvania MG, Japaridze NJ, Bikashvili T, Solomonia RO, Bolkvadze T (2013) The protective effect of myo-inositol on hippocamal cell loss and structural alterations in neurons and synapses triggered by kainic acid-induced status epilepticus. Cell Mol Neurobiol 33:659–671
Li G, Bauer S, Nowak M, Norwood B, Tackenberg B, Rosenow F, Knake S, Oertel WH, Hamer HM (2011) Cytokines and epilepsy. Seizure 20:249–256
Loscher W (2011) Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 20:359–368
Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, Janigro D (2009) Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis 33:171–181
Miller RJ, Jung H, Bhangoo S, White FA (2009) Cytokine and chemokine regulation of sensory neuron function. In: Canning BJ (ed) Handbook of experimental pharmacology. Springer, Berlin Heidelberg, pp 417–449 SDV
Naitoh Y, Fukata J, Tominaga T, Nakai Y, Tamai S, Mori K, Imura H (1988) Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem Biophys Res Commun 155:1459–1463
Overstreet LS, Westbrook GL (2003) Synapse density regulates independence at unitary inhibitory synapses. J Neurosci 23:2618–2626
Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, Kelly ME, Bureau Y, Anisman H, McIntyre DC (2000) Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Mol Brain Res 75:248–258
Rao RS, Medhi B, Saikia UN, Arora SK, Toor JS, Khanduja KL, Pandhi P (2008) Experimentally induced various inflammatory models and seizure: understanding the role of cytokine in rat. Eur Neuropsychopharmacol 18:760–767
Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A (2008) Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 29:142–160
Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A (2008) Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis 31:327–333
Ravizza T, Rizzi M, Perego C, Richichi C, Veliskova J, Moshe SL, De Simoni MG, Vezzani A (2005) Inflammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia 46(Suppl 5):113–117
Richerson GB, Wu Y (2003) Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol 90:1363–1374
Sakurai M, Kurokawa H, Shimada A, Nakamura K, Miyata H, Morita T (2015) Excitatory amino acid transporter 2 downregulation correlates with thalamic neuronal death following kainic acid-induced status epilepticus in rat. Neuropathology 35:1–9
Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci 27:262–269
Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS (2005) Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia 46:1133–1139
Vezzani A, Balosso S, Ravizza T (2012) Inflammation and epilepsy. Handb Clin Neurol 107:163–175
Vezzani A, Conti M, de Luigi A, Ravizza T, Moneta D, Marchesi F, de Simoni MG (1999) Interleukin-1b immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci 19:5054–5065
Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743
Voutsinos-Porche B, Koning E, Kaplan H, Ferrandon A, Guenounou M, Nehlig A, Motte J (2004) Temporal patterns of the cerebral inflammatory response in the rat lithium–pilocarpine model of temporal lobe epilepsy. Neurobiol Dis 17:385–402
Wolf J, Rose-John S, Garbers C (2014) Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 70:11–20
Wu Y, Wang W, Diez-Sampedro A, Richerson GB (2007) Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 56:851–865
Xie C, Sun J, Qiao W, Lu D, Wei L, Na M, Song Y, Hou X, Lin Z (2011) Administration of simvastatin after kainic acid-induced status epilepticus restrains chronic temporal lobe epilepsy. PLoS One 6:e24966
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Su, J., Yin, J., Qin, W. et al. Role for Pro-inflammatory Cytokines in Regulating Expression of GABA Transporter Type 1 and 3 in Specific Brain Regions of Kainic Acid-Induced Status Epilepticus. Neurochem Res 40, 621–627 (2015). https://doi.org/10.1007/s11064-014-1504-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1504-y