Abstract
Purpose
Management of CNS involvement in leukemia may include craniospinal irradiation (CSI), though data on CSI efficacy are limited.
Methods
We retrospectively reviewed leukemia patients who underwent CSI at our institution between 2009 and 2021 for CNS involvement. CNS local recurrence (CNS-LR), any recurrence, progression-free survival (PFS), CNS PFS, and overall survival (OS) were estimated.
Results
Of thirty-nine eligible patients treated with CSI, most were male (59%) and treated as young adults (median 31 years). The median dose was 18 Gy to the brain and 12 Gy to the spine. Twenty-five (64%) patients received CSI immediately prior to allogeneic hematopoietic cell transplant, of which 21 (84%) underwent total body irradiation conditioning (median 12 Gy). Among 15 patients with CSF-positive disease immediately prior to CSI, all 14 assessed patients had pathologic clearance of blasts (CNS-response rate 100%) at a median of 23 days from CSI start. With a median follow-up of 48 months among survivors, 2-year PFS and OS were 32% (95% CI 18–48%) and 43% (95% CI 27–58%), respectively. Only 5 CNS relapses were noted (2-year CNS-LR 14% (95% CI 5–28%)), which occurred either concurrently or after a systemic relapse. Only systemic relapse after CSI was associated with higher risk of CNS-LR on univariate analysis. No grade 3 or higher acute toxicity was seen during CSI.
Conclusion
CSI is a well-tolerated and effective treatment option for patients with CNS leukemia. Control of systemic disease after CSI may be important for CNS local control. CNS recurrence may reflect reseeding from the systemic space.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Involvement of the central nervous system (CNS) by leukemia at initial diagnosis is relatively uncommon and observed in approximately 5–15% of patients with acute lymphoblastic leukemia (ALL) [1] and 3% of patients with acute myeloid leukemia (AML) [2]. In contrast, it is more frequently noted as a site of relapse during or after treatment [3]. Regardless, CNS involvement in leukemia is a poor prognostic factor.
In patients with CNS disease, treatment is challenged by the blood brain barrier, which can result in suboptimal penetration of chemotherapy. Though intrathecal (IT) administration of chemotherapy into the cerebrospinal fluid (CSF) space may circumvent the blood brain barrier, IT therapy is limited by inadequate drug diffusion into the spine and brain parenchyma. Moreover, the CNS microenvironment may induce chemoresistance to leukemic cells through different mechanisms [4]. Adequate control of the craniospinal axis is important not only for disease remission, but also to prevent or mitigate symptoms from CNS involvement, including encephalopathy, headaches, diplopia, vision loss, weakness, sensory changes, and bowel and/or bladder incontinence.
Focal radiotherapy has been utilized for CNS leukemia given the radio-responsiveness of this disease, including solid tumor deposits like chloromas [5]. Varied field sizes to treat the craniospinal axis can be employed, including involved field radiation, whole brain radiation (WBRT), and craniospinal irradiation (CSI). Larger, more comprehensive fields are associated with improved CNS progression-free survival (PFS) [6,7,8]. However, treatment of the craniospinal axis with photons is limited by acute toxicities including myelosuppression, nausea, decreased appetite, and/or esophagitis, in addition to late toxicity to thoracic and abdominal organs. Proton therapy, which is associated with a rapid dose fall-off distal to the targeted tissue, has been associated with reduced acute toxicity compared to photons in patients with medulloblastoma treated with CSI [9] and a favorable toxicity profile among patients with CNS leukemia [7]. With increased availability of proton therapy, improved therapeutic ratio, and observation that the CSF circulates beyond the brain including the spinal canal, CSI is likely to be increasingly considered, especially in patients treated with definitive intent [10]. Despite this, there is a paucity of data on the outcome and durability of response after CSI.
Herein, we aimed to evaluate the outcomes of leukemia patients who receive CSI and to determine factors predicting CNS recurrence after CSI.
Methods and materials
Patient cohort and eligibility
After approval from the institutional review board, we retrospectively reviewed consecutive pediatric and adult leukemia patients that underwent CSI for CNS involvement between 2009 and 2021 at our institution. Eligible patients included those with AML, ALL, and chronic myelogenous leukemia (CML) in blast crisis who had CNS involvement confirmed pathologically by presence of any blasts on CSF flow cytometry. Active systemic disease was defined as the presence of ≥ 5% blasts in the bone marrow or presence of extramedullary disease. Minimal residual disease (MRD) was defined as detectable disease (> 0%) but < 5% leukemic blasts in the blood and marrow. AML patients were risk stratified according to the European Leukemia Net (ELN) 2017 criteria [11].
Craniospinal irradiation (CSI)
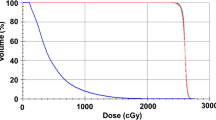
Use of CSI was at the discretion of the treating radiation oncologist and generally occurred after a multi-disciplinary discussion including medical and/or neuro-oncology. Indications for treatment included cytoreduction of persistent disease for symptom control, minimization of residual disease prior to transplant, and/or consolidation after systemic therapy. Radiation technique—photons versus proton therapy—was also at the discretion of the treating radiation oncologist; of note, proton therapy was not available at our institution until 2013. The radiation dose and fractionation delivered to the brain and spine were recorded individually. In circumstances where different doses were delivered to the upper and lower spine, the lower of the two doses was recorded. In cases where CSI was delivered in tandem with allogeneic hematopoietic cell transplantation (HCT), a portion of the intended dose to the craniospinal axis was delivered with total body irradiation (TBI) conditioning (e.g., 12 Gy delivered via CSI and 12 Gy via TBI).
Toxicity assessment
Toxicity was evaluated using the common terminology criteria for adverse events version 5 (CTCAE v5.0) [12]. To minimize the confounding effect of HCT and challenges with attributing toxicity from CSI versus HCT, acute toxicity analysis was performed separately for patients treated with CSI alone versus CSI and HCT.
Assessment of response and disease recurrence
Among patients with flow cytometric evidence of blasts in the CSF immediately prior to CSI, CSF evaluation was performed after CSI, though the timing of CSF evaluation was not standardized. These patients with CSF-positive disease were eligible for evaluation of CNS response rate (CNS-RR). Response was defined as no evidence of blasts by CSF flow cytometry. Other endpoints of interest included CNS local recurrence (CNS-LR), defined as flow cytometric evidence of blasts after CSF clearance. Progression-free survival (PFS) was defined as the time from CSI start to any first recurrence (systemic, CNS), progression, or death.
Follow-up
Patients who received HCT immediately after CSI were followed up at least weekly until 100 days after HCT. Thereafter, they were routinely followed up at 6 months, 1 year and 2 years. Patients with chronic complications were followed more frequently and for a longer duration. Patients that received CSI alone had weekly visits until 30 days after CSI; visits were then gradually spaced out per patients’ condition and at the discretion of the physician.
Statistical analysis
All end points were calculated from the start of CSI. The probability of CNS-LR and any recurrence were summarized using a cumulative incidence estimate, where death without recurrence was considered a competing risk. PFS, CNS PFS, and overall survival (OS) were calculated using the Kaplan-Meier method. Exploratory analyses for predictors for CNS-LR were evaluated using univariate Cox proportional-hazards regression. The following disease and treatment characteristics were evaluated: age, sex, systemic relapse after CSI, time from diagnosis to first CNS disease, positive CSF at the time of CSI, active systemic disease present prior to CSI, prior CNS relapse before CSI, and HCT immediately after CSI. Use of HCT and systemic relapse after CSI were each modeled as a time-varying covariate. Age, time from diagnosis to CNS disease, and number of additional CNS relapses prior to CSI were all modeled as continuous linear variables. P < 0.05 was considered statistically significant. SAS version 9.4 was used for statistical analysis.
Results
Patient demographics and CNS disease
Thirty-nine patients formed the cohort for analysis. Patients were generally young at the time of CSI (median 31 years, range 7–67) with either ALL or AML (Table 1). Three patients had CML, and all were in blast-phase (2 in lymphoid and 1 in myeloid blast crisis). Consistent with prior observations [2], most CNS disease was noted after initial diagnosis (median 20.8 months); only 17.9% had CNS involvement at initial diagnosis. Prior to CSI, patients received a variety of CNS-directed systemic therapy, most frequently administered as IT. However, intraventricular and high dose systemic therapy were also used (Table 2). CNS-directed systemic therapy was given in close proximity to CSI, with a median of 0.6 months (range 0–4) between last administration of systemic therapy and CSI start, suggesting that CSI was typically used in scenarios of higher acuity.
CSI
Most patients received CSI at the time of their first CNS diagnosis (29, 74.4%); 9 (23%) and 1 (2.6%) patients received CSI after 1 and 2 CNS relapses, respectively. Patients treated with CSI generally had poor prognostic features, including 25.6% patients having active systemic disease and 38.5% of patients with blasts in the CSF at the time of CSI (Table 2). Twenty-five (64%) patients received CSI immediately prior to allogeneic HCT, of which 21 (84%) had TBI (median 12 Gy, range 2-13.2) as part of the conditioning regimen (Table 2). Patients treated with CSI alone received a higher CSI dose (median 18 Gy; range 10.8–24) than those proceeding to HCT (median 12 Gy; range 2-13.2), likely reflecting that most patients undergoing HCT also received dose to the craniospinal axis with TBI. CSI was delivered using protons in 21 (54%) and photons in 18 (46%) of patients.
Outcomes
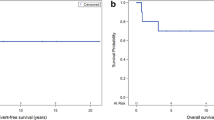
Fifteen (38.5%) patients had CSF-positive disease immediately prior to CSI; all 14 of those assessed for response had confirmed clearance of blasts at a median of 23 days (range 7–53) from CSI start (CNS-RR 100%). With a median follow-up of 48 months (range 0.4–123) for survivors, the estimated 2-year PFS and OS rates were 32% (95% CI, 18–48%) and 43% (95% CI, 27–58%), respectively (Fig. 1A). HCT after CSI was not a requisite for long-term CNS remission. Among 2-year survivors free of any progression, just over half (53.8%) had received CSI in tandem with HCT, whereas the remainder were treated with CSI alone. When focusing on the AML and ALL subgroups, the 2-year point estimates of CNS PFS were 28.2% (95% CI, 9.6–50.5%) and 39.5% (16.3-62.1%), respectively, while the 2-year point estimates of OS were 28.2% (9.6–50.5%) and 52.6% (26.9–73.1%), respectively.
Most relapses—including CNS recurrence (Fig. 1B)—occurred within the first 2 years after CSI and were associated with mortality. Only 5 CNS relapses occurred during follow up, with an estimated 2-year CNS-LR rate of 14% (95% CI, 5–28%). All CNS relapses occurred concurrent with (i.e. <1 month at 0, 0.1 and 0.6 months, n = 3) or after (n = 2) a systemic relapse at 1.2 and 17.5 months.
Predictors for CNS relapse were evaluated with Cox regression (Table 3). Notably, patients with systemic relapse after CSI were more likely to experience CNS relapse. There were 5 CNS relapses among 15 patients who had systemic relapse versus 0 CNS relapses among 24 patients without systemic relapse; therefore, there was an infinite hazard ratio with systemic relapse modeled as a time-varying covariate, P < 0.0001. Associations with CNS relapse for other factors are summarized in Table 3. Since there were only 5 CNS local recurrences, a multivariable analysis was not performed. Due to the modest patient numbers, we did not perform a subset analysis comparing pediatric and adult patients. However, increasing age as a continuous variable was numerically associated with an increased risk of relapse (HR: 1.02; 95%CI 0.99–1.04; P = 0.06). All 5 patients who experienced local recurrence after CSI were older than 20.
Toxicity
CSI was well tolerated with no grade 3 or higher toxicity in the 14 patients who received CSI alone (Table 4). The most common side effects were fatigue and nausea. The toxicity profile was similar between the proton and photon groups. Toxicities for the 25 patients who received HCT immediately after CSI are summarized in Table 5. The most common toxicity was mucositis (23 patients, 92%), followed by neutropenic fever and graft versus host disease (17 patients each, 68%). Notably, lower rates of diarrhea (0 vs. 60%) and esophagitis (6.7% vs. 40%) were observed in the proton group among patients who received HCT.
Discussion
In this retrospective series of patients with CNS leukemia, we demonstrate that CSI is associated with high rates of response that occurs relatively quickly after treatment. Additional, novel findings include the observation that systemic control after CSI is associated with CNS control and that this systemic control does not have to be exclusively mediated by HCT. At least for ALL patients, who receive CNS-directed therapy as part of initial treatment [13], CNS involvement of ALL that is chemotherapy-resistant does not predict decreased responsiveness to radiotherapy.
Our findings confirm the efficacy of CSI reported by other single-institution studies. Only 14% of subjects in our cohort had CNS recurrence by 2 years, though this may be an underestimate as there was a high competing risk of death preceded by systemic relapse. CSF clearance was noted in all assessed patients, similar to the high response rate (86.7%) reported among 15 adult AML or ALL patients with CNS recurrence that underwent CSI at MD Anderson [14]. Within this same cohort, no CNS recurrences were noted, though median PFS and OS were only 3 and 4 months following CSI, respectively. Additional CSI experiences for CNS leukemia are noted in pediatric ALL, though it is uncertain whether these results are generalizable to adult counterparts. In a single-center study at Stanford, 5-year disease-free survival was 67% among 41 pediatric patients with ALL (80% with B-cell ALL) treated with CSI prior to a TBI-based conditioning HCT (median brain 24 Gy, spine 18 Gy) [8]. This numerically higher PFS, as compared to our cohort, may in part reflect a cohort exclusively comprised of pediatric ALL, which is associated with a more favorable prognosis (particularly B-cell ALL) compared to adult ALL and AML. Rates of relapse-free survival (8-year 40%) were lower among 39 pediatric patients with CNS leukemia treated at Memorial Sloan Kettering Cancer Center (MSKCC) in the 1970s with IT therapy, 6–9 Gy CSI consolidation, and intraventricular chemotherapy maintenance [15]. The inferior outcomes compared to the Stanford cohort may stem in part from different treatment eras, CSI dose, and/or use of HCT consolidation, though our study suggests that long-term remission can be attained outside of HCT consolidation.
CNS disease control was strongly associated with systemic disease control after CSI in our cohort and was not associated with HCT consolidation. These novel findings raise consideration that CNS recurrence may reflect reseeding from the systemic space and highlight the importance of any adequate systemic control. Indeed, this is suggested in a study from MSKCC [15], among patients with leukemic meningitis treated with CSI and intraventricular chemotherapy, time to systemic or testicular relapse was shorter than time to CNS relapse (median, 9 vs. 19 months). It is unknown whether CNS-directed therapy can influence systemic disease control. Though not evaluable within our study as all patients were treated with CSI, in a randomized study from the Pediatric Oncology Group, fewer bone marrow or testicular relapses were noted among patients randomized to CSI compared to cranial radiation and maintenance IT chemotherapy [16]; no differences in CNS relapse were noted between the two arms. Though radiation modality was not explicitly detailed, conceivably all patients were treated with photons for CSI. With photons, there may be “off target” effect of the exiting radiation dose to the vertebral bodies and/or sacrum.
In our study, the decision to deliver radiation with protons or photons was primarily driven by year of treatment. With availability of proton therapy at our institution starting in 2013, proton therapy was increasingly utilized in attempts to minimize toxicity. Indeed, patients that underwent CSI with photons were treated between 2009 and 2016, whereas those that underwent proton CSI were treated between 2015 and 2021. Favorable toxicity profiles with proton CSI (30 Gy in 10 fractions) have been recently reported among patients with leptomeningeal disease from solid tumors [17]. At least among patients with adult medulloblastoma (median 30.6 Gy), proton CSI is significantly associated with less weight loss, grade 2 nausea and vomiting, esophagitis, and reduction in blood counts compared to photon CSI [9]. Of note, these differences in toxicity may be more difficult to observe in leukemia patients given lower doses of CSI used either alone (18–24 Gy) or with TBI-based conditioning for HCT (12 Gy).
CSI was well tolerated, with no acute grade 3 or higher toxicity noted. Given the small number of patients and heterogenous therapies (i.e. CSI alone and CSI with HCT), we were unable to determine differences in toxicity between patients treated with photons versus proton therapy among patients who did not proceed to HCT. Nonetheless, lower rates of diarrhea (0 vs. 60%) and esophagitis (6.7% vs. 40%) were observed in the proton group among patients who received HCT after CSI. Notably, it is hard to determine whether this finding is due to higher CSI dose given with photons or from the proton steep dose fall-off. Gunther et al. [7] reported lower rates of grade 1–3 mucositis with proton CSI compared to photon CSI in adult patients with leukemia and lymphoma, but no differences were noted between the 2 groups in terms of other toxicities during CSI or until day 100 post-HCT. Whether there are differences in acute toxicity between photons and protons requires further validation in other cohorts.
Several limitations should be noted. Due to the retrospective nature of our study, follow-up was not standardized, particularly in patients treated with CSI alone. Conceivably, lower grade toxicity (e.g. grade 1 or 2) may have been missed. However, as grade 3 or higher toxicity generally require medical intervention, there was a lower likelihood that these toxicities would be missed as part of retrospective review. CSF evaluation was not routinely performed in the absence of symptoms and/or IT therapy. Therefore, CNS involvement in asymptomatic patients may have been missed. There likely was a selection bias of which patients received CSI—in particular, those with good performance status and well controlled or no systemic disease. In contrast, patients with poor performance were instead offered focal RT (e.g. base of skull, whole brain, spine) or no RT at all. Last, our cohort is relatively heterogenous based on subsequent treatments received after CSI (e.g. HCT). While this limits our ability to comment on radiation toxicity by technique, it provides a unique opportunity to evaluate whether HCT influences CNS control, which could not be evaluated in the prior retrospective studies mentioned above.
In conclusion, CSI for CNS leukemia is well tolerated and provides durable response. Control of systemic disease after CSI is strongly associated with CNS local control. Our findings require validation in larger and ideally prospective cohorts and highlight the unmet need for novel systemic therapy in patients with relapsed/refractory CNS leukemia.
References
Paul S, Short NJ (2022) Central nervous system involvement in adults with acute leukemia: diagnosis, prevention, and management. Curr Oncol Rep 24:427–436. https://doi.org/10.1007/s11912-022-01220-4
Shihadeh F, Reed V, Faderl S et al (2012) Cytogenetic profile of patients with acute myeloid leukemia and central nervous system disease. Cancer 118:112–117. https://doi.org/10.1002/cncr.26253
Pui C-H, Howard SC (2008) Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol 9(3):257–268. https://doi.org/10.1016/S1470-2045(08)70070-6
Jonart LM, Ebadi M, Basile P et al (2020) Disrupting the leukemia niche in the central nervous system attenuates leukemia chemoresistance. Haematologica 105:2130–2140. https://doi.org/10.3324/haematol.2019.230334
Bakst R, Wolden S, Yahalom J (2012) Radiation therapy for chloroma (granulocytic sarcoma). Int J Radiat Oncol Biol Phys 82:1816–1822. https://doi.org/10.1016/j.ijrobp.2011.02.057
Walker GV, Shihadeh F, Kantarjian H et al (2014) Comprehensive craniospinal radiation for controlling central nervous system leukemia. Int J Radiat Oncol Biol Phys 90:1119–1125. https://doi.org/10.1016/j.ijrobp.2014.08.004
Gunther JR, Rahman AR, Dong W et al (2017) Craniospinal irradiation prior to stem cell transplant for hematologic malignancies with CNS involvement: effectiveness and toxicity after photon or proton treatment. Pract Radiat Oncol 7:e401–e408. https://doi.org/10.1016/j.prro.2017.05.002
Hiniker SM, Agarwal R, Modlin LA et al (2014) Survival and neurocognitive outcomes after cranial or craniospinal irradiation plus total-body irradiation before stem cell transplantation in pediatric leukemia patients with central nervous system involvement. Int J Radiat Oncol Biol Phys 89:67–74. https://doi.org/10.1016/j.ijrobp.2014.01.056
Brown AP, Barney CL, Grosshans DR et al (2013) Proton Beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys 86:277–284. https://doi.org/10.1016/j.ijrobp.2013.01.014
Pinnix CC, Yahalom J, Specht L, Dabaja BS (2018) Radiation in Central Nervous System Leukemia: Guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 102:53–58. https://doi.org/10.1016/j.ijrobp.2018.05.067
Döhner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 26(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
National Cancer Institute (2017) Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 2 December 2022
Kopmar NE, Cassaday RD (2023) How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia. Blood 23;141(12):1379–1388. https://doi.org/10.1182/blood.2022017035
Sanders KE, Ha CS, Cortes-Franco JE et al (2004) The role of craniospinal irradiation in adults with a central nervous system recurrence of leukemia. Cancer 100:2176–2180. https://doi.org/10.1002/cncr.20280
Steinherz P, Jereb B, Galicich J (1985) Therapy of CNS leukemia with intraventricular chemotherapy and low-dose neuraxis radiotherapy. J Clin Oncol 3(9):1217–1226. https://doi.org/10.1200/JCO.1985.3.9.1217
Land VJ, Thomas PRM, Boyett JM et al (1985) Comparison of maintenance treatment regimens for first central nervous system relapse in children with acute lymphocytic leukemia. A Pediatric Oncology Group study. Cancer 56:81–87. https://doi.org/10.1002/1097-0142(19850701)56:1%3C81::AID-CNCR2820560114%3E3.0.CO;2-2
Yang Jonathan T, Wijetunga NA, Yamada J et al (2021) Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol 23:134–143. https://doi.org/10.1093/neuonc/noaa152
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
ME: collected and analyzed the data ; wrote the main manuscript, MM: collected the data, TG: analyzed the data, RE, LH, JG, SL, JY, MB, RDC, MEP, LT and VV: provided constructive feedback and revisions, YDT: Designed and led the study and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebadi, M., Morse, M., Gooley, T. et al. Craniospinal irradiation for CNS leukemia: rates of response and durability of CNS control. J Neurooncol 166, 351–357 (2024). https://doi.org/10.1007/s11060-023-04501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04501-5