Abstract
Purpose
Glioblastoma (GBM) is the most common and aggressive malignant primary brain tumors in adults. Patients invariably relapse during or after first-line therapy and the median overall survival is 14.6 months. Such poor clinical response is partly ascribed to the activity of ATP-binding cassette (ABC) transporters. The activity of these proteins, severely reduces the amount of therapeutics that penetrates the tumor cells. We hypothesized that ABC transporter expression could correlate with survival surrogates. In this study, we assessed the expression of four commonly expressed ABC transporters in GBM samples and investigated if mRNA levels could serve as a prognostic biomarker.
Methods
Human specimens were analyzed by qPCR to assess ABCB1, ABCC1/3 and ABCG2 expression. Kaplan-Meier and multivariate analyses were then used to evaluate the correlation with overall survival (OS) and progression-free survival (PFS).
Results
Our cohort included 22 non-tumoral samples as well as 159 GBM tumor specimens. ABC transporters were significantly more expressed in GBM samples compared to non-tumoral tissue. Moreover ABCC1 and 3 mRNA expression were significantly increased at recurrence. Statistical analyses revealed that increased expression of either ABCC1 or ABCC3 did not confer a poorer prognosis. However, increased ABCC1 mRNA levels did correlate with a significantly shorter PFS.
Conclusion
In this manuscript, the analyses we conducted suggest that the expression of the four ABC transporters evaluated would not be suitable prognostic biomarkers. We believe that, when estimating prognosis, the plethora of mechanisms implicated in chemoresistance should be analyzed as a multi-facetted entity rather than isolated units.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is a grade IV glioma according to the World Health Organization (WHO) classification and is the most aggressive CNS tumor [1]. Also, it is the most common primary brain tumor [2]. Given its aggressive nature and complicated pathophysiology, its treatment is very challenging. Indeed, these tumors aggressively infiltrate the brain parenchyma and are protected by the blood-brain barrier which greatly limits the entry of chemotherapeutics. These facts, combined with the limited therapeutic index incurred by the tumor brain localization further restrict the effectiveness of each treatment modality. Indeed, the median survival remains a poor 14.6 months despite decades of research .
Currently, GBM is best treated with maximum surgical resection followed by radiotherapy with concomitant and adjuvant chemotherapy as a first line of treatment [3]. Even with recent therapies such as immunotherapy, the survival has not improved significantly and this cancer is still incurable. Another mitigating factor in the successful treatment of GBM is the extreme heterogeneity in the disease, introducing many pathophysiological pathways to negate the effectiveness of a singular therapeutic modality [4]. As a telling example, recent attempts to specifically target EGFRvIII have failed because non-EGFRvIII tumor cells could override the tumor cell population [5, 6].
GBM therapy resistance can either be intrinsic or acquired [7, 8]. As the terms imply, intrinsic resistance is encountered during the initial chemotherapy exposure to a particular chemotherapeutic compound whereas acquired resistance develops following said initial chemotherapy exposure. Many pathways take part in this resistance, but their influence on treatment response is not fully understood. These include drug efflux transporters, tumor induced hypoxic barriers, cancer stem cells and DNA damage repair [9].
Drug efflux is one of the most important mechanisms associated with multidrug resistance in GBM. This mechanism has a particular importance in acquired resistance pathways especially after exposure to alkylating chemotherapies such as temozolomide (TMZ), a common agent used in standard GBM treatments. Drugs efflux can be an ATP-independent mechanism or be the result of ATP-dependent protein activity [10]. ATP-dependent efflux pumps include the ATP-binding cassette (ABC) transporter family which is commonly overexpressed in GBM. The ABC family consists of 49 proteins that actively transport many substrates against their gradients. ABC pumps have a wide spectrum of substrates including antineoplastic medications. Hence, these transports play a major role in chemoresistance. ABC transporters are expressed in many organs. In the CNS, they are expressed by microglia, astrocytes, neurons, pericytes and endothelial cells [11]. The blood brain barrier (BBB) is one of the most protective physiological barriers of the CNS; one of its components is obviously the cerebral vascular endothelium. It expresses ABCB1 (P-gp) and ABCG2 (BCRP) to efflux neurotoxic substances from the brain parenchyma to the bloodstream in baseline conditions [12]. Amongst the ABC transporter family, many proteins involved in efflux and uptake specifically in cancer cells have been described: P-glycoprotein (P-gp), multidrug resistance protein (MRP), and breast cancer resistance protein (BCRP) are such examples [10]. Pglycoprotein (P-gp) is encoded by the MDR1 gene and has been associated with chemotherapy resistance. MDR1 can be upregulated through many pathways after exposure to anti-cancer drugs. MDR1 can also be overexpressed through the presence of heat shock or DNA damaging agents [13].
Henceforth, ABC transporters really create two layers of resistance to the chemotherapeutic treatments of central nervous system (CNS) tumors as they are expressed both at the level of the BBB and at the level of GBM tumor cell membrane [8]. In this study, we assess the expression of four ABC transporters (ABCB1, ABCC1, ABCC3 and ABCG2) on GBM tumor cells and sought to determine whether their expression could serve as a prognostic marker. The study also aimed at improving our understating of GBM relapses and multidrug resistance.
Materials and methods
Human tumor samples
Written informed consent was obtained from patients prior to surgery. All tumor specimens were collected in the operating theatre as described previously [14]. To summarize, tumor samples were extracted from the contrast-enhanced MRI region and contained less than 50% necrosis. Specimens were returned to the culture laboratory where tissues were immediately minced, transferred into a cryotube containing 400–500 µl of RNAlater RNA Stabilization Reagent (QIAGEN) and stored at − 80 °C until further use. All surgeries were achieved between the years 2010 and 2015. Only patients with a diagnosis of GBM were included in this study. The neuropathologist of our team confirmed the pathological diagnosis according to the histologic criteria in accordance with the 2007 WHO classification of brain tumors. Thus, the status for molecular markers such as the IDH1/2, ATRX or MGMT was unavailable for several tumors.
Human non-tumoral samples
For comparison, we included 22 non-tumoral specimens in our analysis of expression levels. These tissue samples were harvested from white matter and were generously provided by the Douglas - Bell Canada Brain Bank (Douglas Mental Health University Institute).
TCGA data analysis
RNASeq and clinical datasets were downloaded from the NIH National Cancer Institute GCD Data Portal from the TCGA-GBM project. We used the Gene Expression Quantification RNASeq data available for 160 GBM cases for our expression and Kaplan-Meier analyses. Overall survival, progression-free survival, and other clinical surrogates were found in the clinical data files. A total of 150 newly diagnosed GBMs were included in our study. For statistical reasons, secondary or recurrent GBM data were not included in our analyses because of the low number of cases in the TCGA repository.
RNA extraction and quality assessment
Total RNA extraction was accomplished as previously described [15]. Briefly, tumor samples weighing 40 to 50 mg were homogenized with TRIzol reagent (Invitrogen) as described by the manufacturer. The aqueous phase was then transferred to a RNeasy Mini Spin Column (QIAGEN) and RNA was isolated and purified according to the manufacturer protocol. Following extraction, the RNA integrity number (RIN) was determined using an RNA Nano Chips with an Agilent 2100 Bioanalyzer (Agilent Technologies) at the RNomics platform at our center (http://rnomics.med.usherbrooke.ca/en/). A cutoff value of 6.5 was used for the RIN, and specimens that didn’t meet the requirement were not used for further analysis. However, to ensure statistical power, the RIN cutoff value was lowered to 6.0 for recurrent GBM samples.
The remaining methods can be found in the supplementary information file.
Results
Patients’ demographics
The extensive listing of our cohort’s demographics can be found in supplementary Table 2. Briefly, the study cohort included 159 patients accrued between the years 2010 and 2015 at the CHUS. We hypothesized that treatment exposure would impact gene expression, which justified the separation of newly diagnosed and recurrent tumors into two distinct subgroups. For the same reason, secondary GBMs were included in the recurrent group. In total, 95 were newly diagnosed patients with a median age of 62 years whereas 64 were recurrent patients with a median age of 53 years. The majority of patients in our cohort were male, that is, 53.7% and 57.8% of the newly diagnosed and recurrent subgroups respectively. Almost all of our cohort underwent at least one line of treatment. Surgical intervention was the first modality of treatment. Gross resection was achieved in 51 newly diagnosed patients (54%) and 16 recurrent patients (25%). The pre-operative KPS was greater than 50 in 82% of newly diagnosed patients and 69% in recurrent patients. However, in some cases, post-operative KPS was too low which made standard chemoradiation therapy unadvisable following surgery. Hence, the Stupp protocol, consisting in radiotherapy and concomitant and adjuvant TMZ, was prescribed for 71 (75%) patients of the newly diagnosed subgroup. The median overall survival (OS) was 16.9 months and the median progression-free survival (PFS) was 6.6 months. When the Stupp protocol was not the first line therapy, newly diagnosed patients (n = 24) received either radiotherapy, short-course radiotherapy, TMZ or no treatment. Thus, the median OS for this subgroup was 5.3 months and the median PFS was 2.7 months. A variety of second-line treatments such as tumor-treating fields (TTF), TMZ, intra-arterial chemotherapy (IAC), etc. were administered at relapse to 58 (61%) patients of the newly diagnosed group and 60 (94%) patients of the recurrent group. For the recurrent patient subgroup, the median OS was 24.5 months whereas the median post-reoperation survival (PRS) and PFS were 5.5 and 3.0 months respectively (supplementary Table 2).
Analysis of ABC pumps expression levels in GBM patients and in non-tumoral brain samples
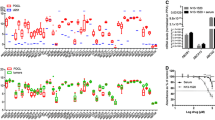
We initially used quantitative real-time PCR to measure the expression levels of the following efflux pumps: ABCB1, ABCC1, ABCC3 and ABCG2. A total of 159 GBM tumor samples and 22 non-tumoral brain samples were analyzed. As seen in Fig. 1, ABCB1 expression was comparable between all three groups of samples. However, ABCC1, ABCC3 and ABCG2 were significantly upregulated in newly diagnosed and recurrent GBM tumors compared to normal samples.
Furthermore, the expression of ABCG2 was more than 150 fold higher than all three other efflux pumps in newly diagnosed tumors (Fig. 2). Likewise, in recurrent tumors, ABCG2 was significantly more expressed than the other ABC transporters. Interestingly, we observed increased expression levels of ABCC1 and ABCC3 in recurrent compared to newly diagnosed tumors suggesting treatment-related or disease progression upregulation. This was not observed with ABCB1 and ABCG2 expression (Fig. 2).
ABC transporter expression. Comparison of mRNA levels (normalized relative quantity) of ABCB1, ABCC1, ABCC3 and ABCG2 in 95 newly diagnosed (a) and in 64 recurrent GBM (b). Graphics “c” and “d” are zooms of graphics “a” and “b” respectively so that differences in the expression levels of ABCB1, ABCC1, ABCC3 are more apparent. **p < 0.01; ***p < 0.001; #p < 0.0001; ns not significant
Interestingly, in 150 newly diagnosed GBM of the TCGA cohort, ABCB1 was significantly less expressed than all three other transporters. Moreover, ABCC3 was the most expressed ABC family member. As expected, in mesenchymal, classical, neural and proneural GBM subtype, expression levels of all four ABC transporters varied considerably (supplementary Fig. 1). Data from recurrent tumors were not analyzed because there were too few.
Correlation of ABC pumps expression and clinical surrogates in newly diagnosed GBM
Expression levels for each efflux pumps were used to segregate the 95 tumor samples into three subclasses: high, moderate, and low expression. Using Kaplan-Meier analysis as well as uni- and multivariate analysis, we investigated whether ABC transporters expression levels had an impact on overall survival and disease progression of newly diagnosed tumors. As can be seen in Figs. 3 and 4 as well as supplementary Tables 3 and 4, ABCB1 did not correlate with the OS nor with the PFS.
While ABCC1 expression did not affect OS, higher levels of that efflux pump were associated with a shorter PFS (HR = 1.533; p = 0.006). Lastly, whereas ABCC3 expression did not affect OS or PFS, higher ABCG2 expression displayed a strong correlation with a better outcome, but had no impact on PFS. However, multivariate analysis revealed this trend was biased by several clinical surrogates and was lacking statistical power (HR = 0.905; p = 0.223).
Moreover, to compare our findings with another cohort, we used RNAseq data from 150 newly-diagnosed GBMs of the TCGA and performed Kaplan-Meier analysis as well as uni- and multivariate analysis. The 75th and 25th percentiles were also used to segregate the cohort into high-, medium- and low-expressing tumors. As in our series, ABC transporters expression levels had no significant impact on overall survival and disease progression of newly diagnosed tumors (supplementary Figs. 4 and 5 as well as supplementary Tables 5 and 6).
Correlation of ABC pumps expression and clinical surrogates in recurrent GBM
The same analyses were performed with the recurrent tumors subgroup data (n = 64 specimens). As for the newly diagnosed subgroup analysis, the specimens were segregated into three subclasses based on the expression levels. However, we used PRS rather than OS. Although our comparison of mRNA levels between both subgroups (newly vs. recurrent) had revealed an increase of ABCC1 and ABCC3 expression at recurrence, no correlation was found between PRS or PFS and expression levels of these ABC transporters (supplementary Figs. 2 and 3). Likewise, ABCG2 expression had no impact on clinical surrogates. Interestingly, Kaplan-Meier analysis showed that low expression ABCB1 correlated with a shorter PFS. As can be seen in the supplementary Tables 7 and 8, Cox regression later revealed that, while the expression of this transporter was still somewhat protective (displayed by a hazard ratio lower than 1), this correlation was not significant.
Discussion
Resistance to chemotherapy is a hallmark of the GBM malignant phenotype [12]. Often coined “multidrug resistance”, it encompasses multilayered mechanisms that include increased drug effluxes via ABC transporters, limited drug entry at tumor cell membrane, detoxifying enzymes at the level of the brain endothelial cells, down regulation of tumor cell apoptotic pathways, and natural barriers, such as the BBB and BTB. These ABC transporters have been described has significant proteins associated with drug resistance and the hindrance of tumor response in gliomas [16].
These features can be present at tumor initiation, and further evolve during the first-line treatment administration of therapeutics which further complicates subsequent therapy at relapse [17]. This is supported by the finding that in our cohort of patients, mRNA levels presented an increase of ABCC1 and ABCC3 expression at recurrence compared to the naïve group, even though this did not translate anyhow in clinical surrogates of survival and tumor response.
As part of these resistance mechanisms, ABC transporters represents a multi-textured barrier, as they are expressed not only at the level of the GBM cells level, but also at the BBB and the BTB; hence, they are major contributors to this inherent and acquired resistance.
In this study, we investigated the impact of the expression of four efflux pumps frequently associated to GBM chemoresistance, namely ABCB1, ABCC1, ABCC3 and ABCG2. We conducted our analyses on 159 fresh tumor samples harvested at our institution as well as with RNAseq data from 150 newly-diagnosed GBMs on the TCGA portal. Although the role of these efflux transporters is well acknowledged, our analyses reveal that their expression levels do not truly associate with disease/patient response to therapy. The only valid association found in this study in multivariate analysis was that ABCC1 mRNA expression levels correlated with a shorter PFS in newly diagnosed GBM patients. Furthermore, our analyses with recurrent patients’ tumor samples did not yield any correlation with OS or PFS. Hence, it appears from our data that the expression of the ABC transporters we tested in this study has no prognostic value as biomarkers in GBM tumors.
Although we convincingly demonstrate an upregulation in ABCC1, ABCC3 and ABCG2 in newly diagnosed as well as relapsing tumors compared to normal samples, this did not influence tumor response to treatment or survival. This lack of association could be explained by the multilayered aspect of the multidrug resistance phenotype depicted by glial tumor cells. Indeed, ABC transporters are but one aspect of this multi-textured resistance mechanism. Hence the combination of all the other obstacles to adequate chemotherapy delivery and treatment resistance encompassing enzyme detoxification at the level of the brain endothelial cells, disruption of the tumor cell apoptotic pathways, as well as the presence of the BBB and BTB, even if partially disrupted, might be sufficient to take over. In this context, it might be counterproductive to view this complex mosaic of resistance mechanisms as a collection of isolated modules; maybe it is best to study it as a whole entity. This could also hint at the fact that the simple blockade of some of these efflux pumps might not be sufficient to really modify the clinical fate of patients bearing malignant gliomas.
As we did in this study, a myriad of other research groups have observed increased expression in GBM tumors (extensively reviewed in [18]). Moreover, Calatozzolo and colleagues reported that overexpression increased with tumor grade [19]. Perplexingly, we could not find any correlation between ABC transporter expression and patient overall survival in newly diagnosed or recurrent GBMs. Interestingly, Kuan and collaborators reported that tumors with a 10-fold overexpression of ABCC3 (compared to normal brain tissue) did correlate with a higher risk factor. Although we did use more clinical surrogates in our multivariate analyses, this is different to our findings [20].
The one transporter that was significantly more expressed, up to 150-fold higher than the others, was ABCG2 (BCRP) in newly diagnosed and recurrent GBM tumors. Interestingly, Bleau et al. hinted at the fact that this ABC transporter was by far the main stem cell-associated transporter [21]. These authors even described a co-expression of Notch, Nestin as well as ABCG2, suggesting a possible role of ABCG2 in the stem like-cell phenotype, linking this protein to the high-resistance observed to treatment modalities in these cells. Indeed, evidence suggests that ABCG2 downregulation inhibits glioma stem cell migration and invasion. It has also been shown that ABCG2 maintains endoplasmic reticulum (ER) homeostasis and suppresses ER stress-induced apoptosis [22].
Conclusion
In conclusion, we investigated the expression of ABCB1, ABCC1, ABCC3 and ABCG2 in newly diagnosed and recurrent GBM tumor sample. Although we did observe increased expression for three of these targets, our multivariate analyses did not reveal any significant correlation with overall survival. However, higher expression of ABCC1 did correlate with a poorer progression-free survival. Taken together, our analyses suggest that the expression of the four ABC pumps investigated in this article would not be suitable prognostic biomarkers. We believe that, when estimating prognosis, the plethora of mechanisms implicated in chemoresistance should be analyzed as a multi-facetted entity rather than isolated units.
Data Availability
The datasets produced and analyzed during the current study are available upon reasonable request from the corresponding author.
References
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of tumors of the central nervous system: a summary. Neurooncology 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Ostrom QT, Cioffi G, Gittleman H et al (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neurooncology 21:v1–v100. https://doi.org/10.1093/neuonc/noz150
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Nicholas MK, Lukas RV, Chmura S et al (2011) Molecular heterogeneity in glioblastoma: therapeutic opportunities and challenges. Semin Oncol 38:243–253. https://doi.org/10.1053/j.seminoncol.2011.01.009
Sampson JH, Heimberger AB, Archer GE et al (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729. https://doi.org/10.1200/jco.2010.28.6963
Weller M (2011) Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med Wkly 141:w13210. https://doi.org/10.4414/smw.2011.13210
Aldape K, Brindle KM, Chesler L et al (2019) Challenges to curing primary brain tumours. Nat Rev Clin Oncol. https://doi.org/10.1038/s41571-019-0177-5
Stupp R, van den Bent MJ, Hegi ME (2005) Optimal role of temozolomide in the treatment of malignant gliomas. Curr Neurol Neurosci 5:198–206. https://doi.org/10.1007/s11910-005-0047-7
Goldie JH (2001) Drug resistance in cancer: a perspective. Cancer Metast Rev 20:63–68. https://doi.org/10.1023/a:1013164609041
Wang Q, Michalak K, Wesolowska O et al (2010) Reversal of multidrug resistance by natural substances from plants. Curr Top Med Chem 10:1757–1768. https://doi.org/10.2174/156802610792928103
Vasiliou V, Vasiliou K, Nebert DW (2009) Human ATP-binding cassette (ABC) transporter family. Hum Genomics 3:281–290. https://doi.org/10.1186/1479-7364-3-3-281
Dréan A, Goldwirt L, Verreault M et al (2016) Blood-brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev Neurother 16:1–16. https://doi.org/10.1080/14737175.2016.1202761
Tsuruo T, Naito M, Tomida A et al (2003) Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci 94:15–21. https://doi.org/10.1111/j.1349-7006.2003.tb01345.x
Roy LO, Poirier MB, Fortin D (2015) Chloroquine inhibits the malignant phenotype of glioblastoma partially by suppressing TGF-beta. Invest New Drugs 33:1020–1031. https://doi.org/10.1007/s10637-015-0275-x
Roy LO, Poirier MB, Fortin D (2018) Differential Expression and clinical significance of transforming growth factor-beta isoforms in GBM tumors. Int J Mol Sci 19:1113–1115. https://doi.org/10.3390/ijms19041113
Decleves X, Amiel A, Delattre JY, Scherrmann J-M (2006) Role of ABC transporters in the chemoresistance of human gliomas. Curr Cancer Drug Tar 6:433–445. https://doi.org/10.2174/156800906777723930
Oliva CR, Nozell SE, Diers A et al (2010) Acquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chain*. J Biol Chem 285:39759–39767. https://doi.org/10.1074/jbc.m110.147504
Gomez-Zepeda D, Taghi M, Scherrmann JM et al (2019) ABC transporters at the blood–brain interfaces, their study models, and drug delivery implications in gliomas. Pharm 12:20. https://doi.org/10.3390/pharmaceutics12010020
Calatozzolo C, Gelati M, Ciusani E et al (2005) Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 AND GST-π in human glioma. J Neuro-oncol 74:113–121. https://doi.org/10.1007/s11060-004-6152-7
Kuan CT, Wakiya K, Herndon JE et al (2010) MRP3: a molecular target for human glioblastoma multiforme immunotherapy. BMC Cancer 10:468–468. https://doi.org/10.1186/1471-2407-10-468
Bleau AM, Huse JT, Holland EC (2009) The ABCG2 resistance network of glioblastoma. Cell Cycle 8:2937–2945. https://doi.org/10.4161/cc.8.18.9504
Mittapalli RK, Chung AH, Parrish KE et al (2016) ABCG2 and ABCB1 limit the efficacy of dasatinib in a PDGF-B–Driven brainstem glioma model. Mol Cancer Ther 15:819–829. https://doi.org/10.1158/1535-7163.mct-15-0093
Acknowledgements
We thank Pr. Roscoe Klinck, Philippe Thibault, Mathieu Durand, Marie-Pierre Garant, Catherine Allard and Samuel Lemaire-Paquette for their consulting during qPCR and statistical data analyses. We also thank The Douglas Bell Canada Brain Bank for kindly providing the non-tumoral brain samples.
Funding
This work was supported by the National Bank research chair for the treatment of brain tumors as well as by the Fondation Coeur en Tête, the Fondation du CHUS and the Fondation de l’Université de Sherbrooke. Written informed consent was obtained from the individual participant included in this study. The authors have no relevant financial or non-financial conflict interests to disclose.
Author information
Authors and Affiliations
Contributions
Conception/design: MBP, MB and DF. Development of methodology: MBP, MB, LOR and DF. Acquisition of data: ML, MB, LOR. Analysis and interpretation of data: LOR, ML, MBP, SA and DF. Writing, editing, and approval of the manuscript: LOR, SA and DF.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial conflict interests to disclose.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roy, LO., Lemelin, M., Blanchette, M. et al. Expression of ABCB1, ABCC1 and 3 and ABCG2 in glioblastoma and their relevance in relation to clinical survival surrogates. J Neurooncol 160, 601–609 (2022). https://doi.org/10.1007/s11060-022-04179-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04179-1