Abstract
Purpose
To investigate whether type-specific sex differences in survival exist independently of clinical and molecular factors in adult-type diffuse gliomas according to the 2021 World Health Organization (WHO) classification.
Methods
A retrospective chart and imaging review of 1325 patients (mean age, 54 ± 15 years; 569 females) with adult-type diffuse gliomas (oligodendroglioma, IDH-mutant, and 1p/19q-codeleted, n = 183; astrocytoma, IDH-mutant, n = 211; glioblastoma, IDH-wildtype, n = 800; IDH-wildtype diffuse glioma, NOS, n = 131) was performed. The demographic information, extent of resection, imaging data, and molecular data including O6-methylguanine-methyltransferase promoter methylation (MGMT) promotor methylation were collected. Sex differences in survival were analyzed using Cox analysis.
Results
In patients with glioblastoma, IDH-wildtype, female sex remained as an independent predictor of better overall survival (hazard ratio = 0.91, P = 0.031), along with age, histological grade 4, MGMT promoter methylation status, and gross total resection. Female sex showed a higher prevalence of MGMT promoter methylation (40.2% vs 32.0%, P = 0.017) but there was no interaction effect between female sex and MGMT promoter methylation status (P-interaction = 0.194), indicating independent role of female sex. The median OS for females were 19.2 months (12.3–35.0) and 16.2 months (10.5–30.6) for males. No sex difference in survival was seen in other types of adult-type diffuse gliomas.
Conclusion
There was a female survival advantage in glioblastoma, IDH-wildtype, independently of clinical data or MGMT promoter methylation status. There was no sex difference in survival in other types of adult-type diffuse gliomas, suggesting type-specific sex effects solely in glioblastoma, IDH-wildtype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recently published 2021 World Health Organization (WHO) classification, followed by the previous 2016 WHO classification, consistently emphasizes the role of molecular markers in the classification of adult-type diffuse gliomas, namely isocitrate dehydrogenase (IDH) mutation and 1p/19q codeletion status [1]. In the 2021 classification, there are three types of adult-type diffuse gliomas: oligodendroglioma, IDH-mutant, and 1p/19q-codeleted, astrocytoma, IDH-mutant, and glioblastoma, IDH-wildtype [1]. WHO grade 2 and 3 IDH-wildtype diffuse gliomas which lack testing of molecular markers sufficient for molecular glioblastoma are designated as IDH-wildtype diffuse gliomas, not otherwise specified (NOS) [2, 3]. This new classification emphasizes different prognosis within each type rather than comparing prognosis among different types within each WHO grade in the previous 2016 WHO classification.
Patient sex is recognized as a biologically relevant factor not only for cancer incidence but also for survival [4]. Sex differences have been consistently recognized by epidemiological studies in patients with gliomas [5, 6]. Majority of studies focused on incidence of glioma, reporting a higher incidence of glioma in males [7, 8, 9, –11]. However, less information on sex differences in survival of patients with glioma is available [6, 12]. While several large population-based studies have shown female survival advantage in glioblastoma [6, 13, 14, –16], these datasets lacked important molecular markers such as IDH mutation or O6-methylguanine-methyltransferase (MGMT) promoter methylation status. While both IDH mutation and MGMT promoter methylation status are important prognostic markers in glioblastoma [17, 18, 19, 20, –22], whether there is an independent effect of sex over well-acknowledged prognostic molecular markers is unknown. Moreover, survival difference according to sex among types of adult-type diffuse glioma other than glioblastoma is yet to be known; a recent study showed that non-glioblastoma patients revealed no sex difference in survival, but this study lacked type-specific analysis according to the molecular markers in the 2021 WHO classification [16].
Exploring sex differences in survival is important as this can be incorporated in implementing personalized treatment strategies and designing clinical trials. In addition, type-specific effects of sex in survival is also relevant as personalized treatment strategies can be tailored according to types of adult-type diffuse gliomas. Current overrepresentation of male in the clinical trials of adult-type diffuse gliomas may obscure key elements of sexual dimorphism in survival [23]. Hence, an integrated clinical and molecular analysis is warranted to elucidate type-specific sex differences in survival in adult-type diffuse gliomas.
Therefore, this study aimed to investigate whether type-specific sex differences in survival exist independently of clinical and molecular factors in adult-type diffuse gliomas according to the 2021 WHO classification.
Methods
Patient enrollment
Between January 2005 and October 2021, 1,458 patients with adult-type diffuse glioma from our institution were recruited. The inclusion criteria were as follows: (a) gliomas confirmed by histopathology, (b) known IDH mutation and 1p/19q codeletion status, and (c) age over 18 years. The exclusion criteria were as follows: (a) follow-up loss within 3 months excluding death (n = 92), (b) presence of H3 K27 alteration leading to the diagnosis of diffuse midline glioma, H3 K27-altered (n = 36), and (c) insufficient tissue for molecular diagnosis (n = 5). A total of 1325 patients were included in this study. Figure 1 shows the patient inclusion process.
Molecular classification
Diagnoses were made according to the WHO classification [24]. Immunohistochemical analysis was performed to detect IDH1 R132H mutation, and IDH1/2 status was confirmed by peptide nucleic acid-mediated clamping polymerase chain reaction in IDH1-negative patients on immunohistochemical analysis. Fluorescent in situ hybridization analysis was conducted to detect 1p/19q codeletion. The H3 K27 mutant protein was detected by immunohistochemistry analysis using polyclonal antibodies for the histone H3.3 tail. MGMT promoter methylation status was determined by methylation-specific polymerase chain reaction in all patients (n = 1325, 100%).
Targeted next-generation sequencing using the Illumina TruSight Tumor 170 panel was performed in 844 patients (63.7%) [25, 26]. Epidermal growth factor receptor (EGFR) amplification was considered when genes were with ≥ twofold-change relative to the average level. For telomerase reverse transcriptase promoter (TERTp), C228T and C250T mutations were evaluated using a pyrosequencing assay [27]. Combined gain of entire chromosome 7 and loss of entire chromosome 10 (chromosome + 7/− 10) was also analyzed. EGFR amplification, TERTp mutation, and + 7/− 10 chromosome copy number status were available in 565 (42.6%), 844 (63.7%), and 417 patients (31.5%), respectively. WHO grade 2 and 3 IDH-wildtype diffuse gliomas lacking testing of these molecular markers sufficient for molecular glioblastoma were assigned as IDH-wildtype diffuse gliomas, NOS [2, 3].
MRI protocol
Brain MRI scans, including T1-weighted image, T2-weighed image, pre-and postcontrast fluid-attenuated inversion recovery (FLAIR), and postcontrast 3D T1-weighted images were acquired with a 3T unit (Achieva or Ingenia; Philips Healthcare, Best, Netherlands).
Data collection
Data including age at initial diagnosis, sex, histological grade (grade 2, 3, or 4 based on histological features), molecular markers, treatment (such as radiation, temozolomide, or PCV [procarbazine, lomustine, and vincristine] therapy), date of death or last follow-up were collected. All patients underwent preoperative MRI examination for initial evaluation and postoperative MRI examination performed within 48 h of operation. The tumor location of geographic epicenter on the preoperative MRI was determined with the largest component of either contrast-enhancing or non-contrast-enhancing tumor. Lobar location included frontal lobe, temporal lobe, parietal lobe, occipital lobe, and insula, while nonlobar location included basal ganglia, thalamus, brainstem, cerebellum and corpus callosum [28, 29]. The extent of resection based on pre- and postoperative MRI (gross total resection, subtotal [tumor removal of ≥ 75% but < 100%], partial [tumor removal of < 75%] or biopsy) was determined by independent review of two neuroradiologists (M.K. and Y.W.P., with 8 and 11 years of experience, respectively). In the rare case of ambiguity, a senior neuroradiologist (S.S.A, with 18 years of experience) was consulted for the final decision.
Overall survival (OS) was defined as the time from the day of initial diagnosis of glioma until death or last follow-up.
Statistical analysis
The clinical and imaging characteristics of patients were compared according to types of adult-type diffuse gliomas (namely oligodendroglioma, IDH-mutant, and 1p/19q-codeleted, astrocytoma, IDH-mutant, glioblastoma, IDH-wildtype, and IDH-wildtype diffuse glioma, NOS), using the Chi-square for categorical variables and independent samples t-test or Mann–Whitney U test for continuous variables according to normality. The clinical and imaging characteristics were compared according to sex within each type of adult-type diffuse gliomas.
Cox regression analysis was performed to determine predictors of OS. Variables of interest in the univariable analysis were included in the multivariable models using backward elimination according to the likelihood ratio with a variable selection criterion of P < 0.05. The proportional hazards assumption was met in all models except for glioblastoma, IDH-wildtype, particularly for MGMT promoter methylation status (P = 0.047). However, as there was no significant interaction of MGMT promoter methylation status with time (P = 0.288), Cox analyses was pursued. As all glioblastoma, IDH-wildtype patients were included after 2005 and underwent Stupp protocol [5], treatment was not included in the Cox regression analysis in this type. Survival rates were determined using the unadjusted and adjusted Kaplan–Meier method, and curves were compared using the log-rank test. In glioblastoma, IDH-wildtype patients, an interaction between female sex and MGMT promoter methylation status was evaluated to assess whether the effect of female sex on survival was dependent of MGMT promoter methylation status. In addition, a likelihood ratio test was used in the context of Cox models to assess the contribution of sex in predicting survival beyond that provided by other predictors in the multivariable Cox model including MTMT promoter methylation status. The variance inflation factor was used to detect multicollinearity between variables; all variables included in the multivariable model showed a variance inflation factor < 10. Statistical analysis was performed using R statistical software (R version 4.0.2, R Core Team, Vienna, Austria). Statistical significance was set at P < 0.05. A biostatistician (with 15 years of experience) was consulted for statistical analysis.
Results
Patient characteristics
This study included 1325 patients with adult-type diffuse glioma (mean age ± standard deviation [SD], 54.0 ± 15.0 years) consisting of 569 females (42.9%) and 756 males (57.1%) with a median follow-up period of 19.3 months (interquartile range [IQR], 11.0–40.5). There were 183 patients (13.8%) with oligodendroglioma, IDH-mutant, and 1p/19q-codeleted, 211 patients (15.9%) with astrocytoma, IDH-mutant, 800 patients (60.4%) with glioblastoma, IDH-wildtype and 131 patients (9.9%) with IDH-wildtype diffuse glioma, NOS. The median OS was 29.9 months (IQR, 14.2–164.4), and 701 patients (52.9%) expired. Amongst all types of adult-type diffuse gliomas (oligodendroglioma, IDH mutant, 1p/19q-codeleted, astrocytoma, IDH mutant, glioblastoma, IDH-wildtype and IDH-wildtype diffuse glioma, NOS), there were significant differences in the age (P < 0.001), sex (P = 0.038), histological grade (P < 0.001), molecular markers (P < 0.001 for MGMT promoter methylation, EGFR amplification, TERTp mutation, and chromosome + 7/− 10), tumor location (P < 0.001 for frontal and nonlobar location, and P = 0.008 for infratentorial location), treatment (P < 0.001 for gross total resection, radiation therapy, temozolomide, and PCV therapy), and death (P < 0.001). Patients with glioblastoma, IDH-wildtype had the shortest median OS (17.3 months; IQR 10.6–33.0) followed by IDH-wildtype diffuse glioma, NOS (33.0 months; IQR 16.8–59.4), and both astrocytoma, IDH-mutant and oligodendroglioma, IDH mutant, and 1p/19q-codeleted did not reach median OS within the study period (P < 0.001). The clinical characteristics of the study patients are presented in Table 1. The Kaplan–Meier curves of different types of adult-type diffuse glioma is presented in Supplementary Fig. 1.
Difference of patient characteristics according to sex in each type
The clinical characteristics according to sex in each type of adult-type diffuse gliomas are presented in Supplementary Table 1. In glioblastoma, IDH-wildtype, female sex showed significantly higher prevalence of MGMT promoter methylation (40.2% vs 32.0%, P = 0.017) and nonlobar location (27.9% vs 21.3%, P = 0.030) than male sex. No significant differences were observed in other characteristics in glioblastoma, IDH-wildtype. In astrocytoma, IDH-mutant, female sex showed a significantly higher prevalence of MGMT promoter methylation (82.8% vs 70.2%, P = 0.037). In other types of adult-type diffuse gliomas, there were no significant differences in characteristics according to sex.
Overall survival in patients with glioblastoma, IDH-wildtype according to sex
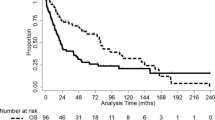
In univariable analysis, female sex was significantly associated with better OS (hazard ratio [HR] = 0.81, 95% confidence interval [CI] = 0.68–0.96; P = 0.014). Age, histological grade 4, MGMT promoter methylation status, nonlobar tumor location, and gross total resection were also identified to be significant predictors of OS. In multivariable analysis, female sex remained as an independent predictor of OS (HR 0.91, 95% CI 0.83–0.99; P = 0.031), along with age, histological grade 4, MGMT promoter methylation status, and gross total resection. The interaction between female sex and MGMT promoter methylation status was not significant (P-interaction = 0.194) indicating that female sex impacts survival independently of MGMT methylation status. In addition, the likelihood ratio test showed additional effect of sex in model fitness (P = 0.015). Results of univariable and multivariable analyses for determining the predictor of OS in patients with glioblastoma, IDH-wildtype are summarized in Table 2. The unadjusted and adjusted Kaplan–Meier curves showed a significant difference in OS by sex (log-rank test, P = 0.009 and P = 0.031 respectively) (Fig. 2). The median OS for females were 19.2 months (IQR, 12.3–35.0) and 16.2 months (IQR, 10.5–30.6) for males.
Overall survival in patients with astrocytoma, IDH-mutant according to sex
In univariable analysis, there was no statistical difference in OS according to sex (HR 0.92; 95% CI 0.49–1.70; P = 0.781). In univariable analysis, nonlobar tumor location, gross total resection, and temozolomide therapy were identified to be significant predictors of OS. In multivariable analysis, gross total resection and temozolomide therapy were identified to be significant predictors of OS. Results of univariable and multivariable analysis for determining predictors of overall survival in patients with astrocytoma, IDH mutant are summarized in Supplementary Table 2. The Kaplan–Meier curves did not show a significant difference in OS according to sex (log-rank test, P = 0.781) in astrocytoma, IDH-mutant (Supplementary Fig. 2).
Overall survival in patients with oligodendroglioma, IDH-mutant, and 1p/19q-codeleted according to sex
In univariable analysis, there was no statistical difference in OS according to sex (HR 0.68, 95% CI 0.24–1.82; P = 0.442). In univariable analysis, age and histological grade 3 were identified to be significant predictors of OS. In multivariable analysis, age and histological grade 3 continued to be significant predictors of OS. Results of univariable and multivariable analysis for determining predictors of overall survival in patients with oligodendroglioma, IDH mutant, and 1p/19q-codeleted are summarized in Supplementary Table 3. The Kaplan–Meier curves did not show a significant difference in OS according to sex (log-rank test, P = 0.439) in oligodendroglioma, IDH mutant, and 1p/19q-codeleted (Supplementary Fig. 2).
Overall survival in patients with IDH-wildtype diffuse glioma, NOS according to sex
In univariable analysis, there was no statistical difference in OS according to sex (HR 1.03, 95% CI 0.67–1.58; P = 0.904). In univariable analysis, age, histological grade 3, and gross total resection were identified to be significant predictors of OS. In multivariable analysis, age, histological grade 3, and gross total resection continued to be significant predictors of OS. Results of univariable and multivariable analysis for determining predictors of OS in patients with IDH-wildtype diffuse glioma, NOS are summarized in Supplementary Table 4. The Kaplan–Meier curves did not show a significant difference in OS according to sex (log-rank test, P = 0.904) in IDH-wildtype diffuse glioma, NOS (Supplementary Fig. 2).
Discussion
Sex as a prognostic factor in adult-type diffuse gliomas may deserve more attention and systematic investigation in the molecular era. In this study, we undertook an integrated clinical and molecular analysis to investigate whether type-specific sex differences in survival exist independently of clinical and molecular factors in adult-type diffuse gliomas according to the 2021 WHO classification. In glioblastoma, IDH-wildtype, female sex remained as an independent prognostic factor even after adjusting for MGMT promoter methylation status, suggesting that significant sexual dimorphism in survival exists. Sex disparity in survival was not observed in other types of adult-type diffuse gliomas, indicating that there are type-specific sex differences in survival in adult-type diffuse gliomas. Therefore, sex may be considered as a relevant factor in designing clinical trials and planning treatment strategies to avoid over- or under-treatment of glioblastoma, IDH-wildtype, but not in other types of adult-type diffuse gliomas.
Previous studies that had reported female survival advantage in patients with glioblastoma were performed prior to the 2021 WHO classification and included patients with both IDH-wildtype and IDH-mutant glioblastomas.[6, 13, 14, –16] The previous so-called IDH-mutant glioblastoma is now classified as astrocytoma, IDH-mutant, grade 4, which is a completely different type from glioblastoma, IDH-wildtype in the new classification, albeit representing a small proportion of previous glioblastoma. Also, the effect of MGMT promoter methylation status, which is a crucial prognostic marker in glioblastoma, was not taken into consideration in the previous studies proposing sex disparity in survival [30, 31].
In this study, we were able to demonstrate that female survival advantage in glioblastoma, IDH-wildtype was independent of MGMT promotor methylation status. There was a higher proportion of MGMT methylation status in females in glioblastoma, IDH wildtype which was in accordance with the results of previous studies in glioblastoma [31, 32], but sex remained as a significant prognostic factor with no interaction between female sex and MGMT promoter methylation status. Our study showed overall slightly lower level of MGMT promoter methylation as only IDH-wildtypes were included while astrocytoma, IDH-mutant, grade 4 were included as glioblastomas in the previous studies [3, 31, 32]. Interestingly, MGMT promoter methylation does not seem to occur uniformly in sex-bound fashion in all cancer types. A meta-analysis of the role of MGMT promoter methylation in non-small cell lung cancer showed no correlation with sex [33]. This suggests that the higher proportion of MGMT promoter methylation and better response to alkylating treatment seen in females in glioblastoma, IDH-wildtype is a tumor-specific phenomenon.
While female survival advantage in cancer has been attributed to a variety of environmental, genetic, immunologic, and hormonal factors [34, 35], sex differences in survival was only observed in glioblastoma, IDH-wildtype, and not in other types of adult-type diffuse gliomas. IDH-wildtype and IDH-mutant gliomas are thought to be distinct diseases with different pathogenesis and genetic profiles. IDH mutation with or without 1p/19q codeletion occurs in the early stage of gliomagenesis of IDH-mutant gliomas, and are thought to be the driver mutation [36]. On the other hand, IDH-wildtype gliomas undergo completely different driver events with a cascade of core signaling pathways [37]. It may be postulated that sex modulates specific tumorigenic pathways that bear prognostic implication in glioblastoma, IDH-wildtype. However, to fully uncover hidden elements of pathophysiology in the type-specific sex effects in survival, an integrated multilevel and transdisciplinary research approach, involving molecular cell biology, preclinical and clinical studies, needs to be undertaken [38, 39].
In our study, IDH-wildtype diffuse gliomas, NOS, did not show female survival advantage, which may be explained by the heterogeneous nature of this group. IDH-wildtype, NOS encompasses WHO grade 2 and 3 astrocytic tumors without the results of the relevant molecular markers in the 2021 WHO classification. Previous studies reported the incidence of molecular GBM with a wide range of 44.0–81.6% within IDH-wildtype grade 2 or 3 patients [40, 41, 42, 43], while the remainders in this group may be classified to diverse types such as diffuse astrocytoma, MYB- or MYBL1-altered, diffuse low-grade glioma, MAPK pathway-altered, or diffuse hemispheric glioma, H3 G34-mutant [3]. This heterogeneity probably accounts for female sex not exerting prognostic effect in IDH-wildtype diffuse gliomas, NOS.
There were several limitations in this study. First, this was a single-center, retrospective study with patients enrolled over a long period. There were many changes in diagnoses and treatment strategies of glioma patients other than glioblastoma, IDH-wildtype. Multicenter analysis is warranted to generalize the findings of our study. Second, Karnofsky performance status was not included in the analysis. However, clinical significance of Karnofsky performance status is limited due to its retrospective and subjective nature [44].
In conclusion, female survival advantage was seen in glioblastoma, IDH-wildtype but not in other types of adult-type diffuse gliomas, suggesting type-specific sex effects solely in glioblastoma, IDH-wildtype. This may have implications for designing personalized treatment strategies in glioblastoma, IDH-wildtype but not necessarily in other types of adult-type diffuse gliomas.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106%JNeuro-Oncology
Louis DN, Wesseling P, Paulus W, Giannini C, Batchelor TT, Cairncross JG, Capper D, Figarella-Branger D, Lopes MB, Wick W, van den Bent M (2018) cIMPACT-NOW update 1: not otherwise specified (NOS) and not elsewhere classified (NEC). Acta Neuropathol 135:481–484. https://doi.org/10.1007/s00401-018-1808-0
Brat DJ, Aldape K, Bridge JA, Canoll P, Colman H, Hameed MR, Harris BT, Hattab EM, Huse JT, Jenkins RB, Lopez-Terrada DH, McDonald WC, Rodriguez FJ, Souter LH, Colasacco C, Thomas NE, Yount MH, van den Bent MJ, Perry A (2022) Molecular biomarker testing for the diagnosis of diffuse gliomas. Arch Pathol Lab Med. https://doi.org/10.5858/arpa.2021-0295-CP
Clocchiatti A, Cora E, Zhang Y, Dotto GP (2016) Sexual dimorphism in cancer. Nat Rev Cancer 16:330–339. https://doi.org/10.1038/nrc.2016.30
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Ostrom QT, Rubin JB, Lathia JD, Berens ME, Barnholtz-Sloan JS (2018) Females have the survival advantage in glioblastoma. Neuro Oncol 20:576–577. https://doi.org/10.1093/neuonc/noy002
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2017) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 19:v1–v88. https://doi.org/10.1093/neuonc/nox158
Shinojima N, Kochi M, Hamada J, Nakamura H, Yano S, Makino K, Tsuiki H, Tada K, Kuratsu J, Ishimaru Y, Ushio Y (2004) The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme. J Neurosurg 101:219–226. https://doi.org/10.3171/jns.2004.101.2.0219
Ho VK, Reijneveld JC, Enting RH, Bienfait HP, Robe P, Baumert BG, Visser O (2014) Changing incidence and improved survival of gliomas. Eur J Cancer 50:2309–2318. https://doi.org/10.1016/j.ejca.2014.05.019
Dubrow R, Darefsky AS (2011) Demographic variation in incidence of adult glioma by subtype, United States, 1992–2007. BMC Cancer 11:325. https://doi.org/10.1186/1471-2407-11-325
Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS (2018) Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol 4:1254–1262. https://doi.org/10.1001/jamaoncol.2018.1789
Claus EB, Black PM (2006) Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973–2001. Cancer 106:1358–1363. https://doi.org/10.1002/cncr.21733
Yang W, Warrington NM, Taylor SJ, Whitmire P, Carrasco E, Singleton KW, Wu N, Lathia JD, Berens ME, Kim AH, Barnholtz-Sloan JS, Swanson KR, Luo J, Rubin JB (2019) Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aao5253
Beig N, Singh S, Bera K, Prasanna P, Singh G, Chen J, Saeed Bamashmos A, Barnett A, Hunter K, Statsevych V, Hill VB, Varadan V, Madabhushi A, Ahluwalia MS, Tiwari P (2021) Sexually dimorphic radiogenomic models identify distinct imaging and biological pathways that are prognostic of overall survival in glioblastoma. Neuro Oncol 23:251–263. https://doi.org/10.1093/neuonc/noaa231
Tavelin B, Malmström A (2022) Sex differences in glioblastoma-findings from the Swedish national quality registry for primary brain tumors between 1999–2018. J Clin Med 11:486. https://doi.org/10.3390/jcm11030486
Gittleman H, Ostrom QT, Stetson LC, Waite K, Hodges TR, Wright CH, Wright J, Rubin JB, Berens ME, Lathia J, Connor JR, Kruchko C, Sloan AE, Barnholtz-Sloan JS (2019) Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neuro-Oncol Pract 6:451–462. https://doi.org/10.1093/nop/npz019
Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JWM, Boots-Sprenger SHE, Wesseling P, Hulsebos TJM, Troost D, van Tilborg AA, Leenstra S, Vandertop WP, Bardelli A, van Noorden CJF, Bleeker FE (2014) The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol 16:1263–1273. https://doi.org/10.1093/neuonc/nou005
Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG (2000) Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343:1350–1354. https://doi.org/10.1056/nejm200011093431901
Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JEC, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. https://doi.org/10.1056/NEJMoa043331
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120:707–718. https://doi.org/10.1007/s00401-010-0781-z
Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27:4150–4154. https://doi.org/10.1200/jco.2009.21.9832
Wagner AD, Oertelt-Prigione S, Adjei A, Buclin T, Cristina V, Csajka C, Coukos G, Dafni U, Dotto GP, Ducreux M, Fellay J, Haanen J, Hocquelet A, Klinge I, Lemmens V, Letsch A, Mauer M, Moehler M, Peters S, Özdemir BC (2019) Gender medicine and oncology: report and consensus of an ESMO workshop. Ann Oncol 30:1914–1924. https://doi.org/10.1093/annonc/mdz414
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Park YW, Ahn SS, Park CJ, Han K, Kim EH, Kang SG, Chang JH, Kim SH, Lee SK (2020) Diffusion and perfusion MRI may predict EGFR amplification and the TERT promoter mutation status of IDH-wildtype lower-grade gliomas. Eur Radiol 30:6475–6484. https://doi.org/10.1007/s00330-020-07090-3
Na K, Kim HS, Shim HS, Chang JH, Kang SG, Kim SH (2019) Targeted next-generation sequencing panel (TruSight Tumor 170) in diffuse glioma: a single institutional experience of 135 cases. J Neurooncol 142:445–454. https://doi.org/10.1007/s11060-019-03114-1
Hirsch FR, Varella-Garcia M, McCoy J, West H, Xavier AC, Gumerlock P, Bunn PA Jr, Franklin WA, Crowley J, Gandara DR (2005) Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol 23:6838–6845. https://doi.org/10.1200/jco.2005.01.2823
Park YW, Han K, Ahn SS, Bae S, Choi YS, Chang JH, Kim SH, Kang SG, Lee SK (2018) Prediction of IDH1-mutation and 1p/19q-codeletion status using preoperative MR imaging phenotypes in lower grade gliomas. AJNR Am J Neuroradiol 39:37–42. https://doi.org/10.3174/ajnr.A5421
Nicolasjilwan M, Hu Y, Yan C, Meerzaman D, Holder CA, Gutman D, Jain R, Colen R, Rubin DL, Zinn PO, Hwang SN, Raghavan P, Hammoud DA, Scarpace LM, Mikkelsen T, Chen J, Gevaert O, Buetow K, Freymann J, Kirby J, Flanders AE, Wintermark M (2015) Addition of MR imaging features and genetic biomarkers strengthens glioblastoma survival prediction in TCGA patients. J Neuroradiol 42:212–221. https://doi.org/10.1016/j.neurad.2014.02.006
Schiffgens S, Wilkens L, Brandes AA, Meier T, Franceschi E, Ermani M, Hartmann C, Sandalcioglu IE, Dumitru CA (2016) Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget 7:55169–55180. https://doi.org/10.18632/oncotarget.10465
Smits A, Lysiak M, Magnusson A, Rosell J, Söderkvist P, Malmström A (2021) Sex disparities in MGMT promoter methylation and survival in glioblastoma: further evidence from clinical cohorts. J Clin Med 10:556. https://doi.org/10.3390/jcm10040556
Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, Anjum S, Wang J, Manyam G, Zoppoli P, Ling S, Rao AA, Grifford M, Cherniack AD, Zhang H, Poisson L, Carlotti CG Jr, Tirapelli DP, Rao A, Mikkelsen T, Lau CC, Yung WK, Rabadan R, Huse J, Brat DJ, Lehman NL, Barnholtz-Sloan JS, Zheng S, Hess K, Rao G, Meyerson M, Beroukhim R, Cooper L, Akbani R, Wrensch M, Haussler D, Aldape KD, Laird PW, Gutmann DH, Noushmehr H, Iavarone A, Verhaak RG (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164:550–563. https://doi.org/10.1016/j.cell.2015.12.028
Chen L, Wang Y, Liu F, Xu L, Peng F, Zhao N, Fu B, Zhu Z, Shi Y, Liu J, Wu R, Wang C, Yao S, Li Y (2018) A systematic review and meta-analysis: association between MGMT hypermethylation and the clinicopathological characteristics of non-small-cell lung carcinoma. Sci Rep 8:1439–1439. https://doi.org/10.1038/s41598-018-19949-z
Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, Zambon P, Gatta G, De Angelis R (2009) The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer 45:1017–1027. https://doi.org/10.1016/j.ejca.2008.11.008
Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R (2009) EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 45:931–991. https://doi.org/10.1016/j.ejca.2008.11.018
Turkalp Z, Karamchandani J, Das S (2014) IDH mutation in glioma: new insights and promises for the future. JAMA Neurol 71:1319–1325. https://doi.org/10.1001/jamaneurol.2014.1205
Chen J, McKay RM, Parada LF (2012) Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149:36–47. https://doi.org/10.1016/j.cell.2012.03.009
Choi KS, Sunwoo L (2022) Artificial Intelligence in Neuroimaging: Clinical Applications. Investig Magn Reson Imaging 26:1–9. https://doi.org/10.13104/imri.2022.26.1.1
Park YW, Lee N, Ahn SS, Chang JH, Lee S-K (2021) Radiomics and Deep Learning in Brain Metastases: Current Trends and Roadmap to Future Applications. Investig Magn Reson Imaging 25:266–280. https://doi.org/10.13104/imri.2021.25.4.266
Tesileanu CMS, Dirven L, Wijnenga MMJ, Koekkoek JAF, Vincent A, Dubbink HJ, Atmodimedjo PN, Kros JM, van Duinen SG, Smits M, Taphoorn MJB, French PJ, van den Bent MJ (2020) Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol 22:515–523. https://doi.org/10.1093/neuonc/noz200
Aibaidula A, Chan AK, Shi Z, Li Y, Zhang R, Yang R, Li KK, Chung NY, Yao Y, Zhou L, Wu J, Chen H, Ng HK (2017) Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol 19:1327–1337. https://doi.org/10.1093/neuonc/nox078
Park YW, Park JE, Ahn SS, Kim EH, Kang SG, Chang JH, Lee SK (2021) Magnetic resonance imaging parameters for noninvasive prediction of epidermal growth factor receptor amplification in isocitrate dehydrogenase-wild-type lower-grade gliomas: a multicenter study. Neurosurg 89(2):257–265
Park YW, Kim S, Park CJ, Ahn SS, Han K, Kang SG, Lee SK (2022) Adding radiomics to the 2021 WHOupdates may improve prognostic prediction for current IDH-wildtype histological lower-grade gliomas with known EGFR amplification and TERT promoter mutation status. Eur Radiol 1–10
Wang CW, Lai JC (2017) Reporting functional status in UNOS: the weakness of the Karnofsky Performance Status Scale. Clin Transpl. https://doi.org/10.1111/ctr.13004
Funding
This research received funding from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A1A01071648).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MK, YWP, SK, and KH. The first draft of the manuscript was written by MK and YWP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the institutional review board of the Severance Hospital (Approval Number 4-2022-0319). The requirement for patient consent was waived owing to the retrospective study design. The study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M., Kim, S., Park, Y.W. et al. Sex as a prognostic factor in adult-type diffuse gliomas: an integrated clinical and molecular analysis according to the 2021 WHO classification. J Neurooncol 159, 695–703 (2022). https://doi.org/10.1007/s11060-022-04114-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04114-4