Abstract
Purpose
To summarize the clinical features and outcomes of petroclival meningioma patients treated with stereotactic radiosurgery (SRS) as either a primary or an adjuvant modality.
Methods
Relevant articles were retrieved from PubMed, Scopus, Web of Science, and Cochrane. A systematic review and meta-analysis of treatment outcomes comparing primary and adjuvant SRS was conducted.
Results
Seven articles comprising 722 cases were included. The mean tumor marginal dose was 13.5 Gy. After SRS, symptoms improved in 28.7%, remained unchanged in 61.3%, and worsened in 10.0% of the cohort. Tumor control was achieved in 94.8% of patients. The mean tumor volume change was −6.4 cm3. The 5-year and 10-year progression-free survival (PFS) rates were 91–100% and 69.6–89.9%, respectively. Overall, 61.9% of patients underwent primary radiosurgery, and 38.1% had adjuvant radiosurgery. Patients who had primary SRS reported higher rates of tumor control (94.3% vs. 88.2%) and fewer SRS-related complications (3.7% vs. 10.3%) than those who received adjuvant SRS (not accounting for microsurgical complications). The functional status of patients who had primary SRS was more likely to improve or remain unchanged, with an effect size of 1.12 (95% CI 1.1–1.25; I2 = 0). Neither group displayed superiority in worsening functional outcomes or tumor control rate.
Conclusion
SRS of petroclival meningiomas was associated with excellent long-term PFS and local tumor control rates. Primary SRS was highly effective for patients with smaller volume lesions without clinically symptomatic mass effect. In patients who warrant initial resection, adjuvant radiosurgery remains an important modality to prevent regrowth while maintaining postresection function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Petroclival meningiomas (PCMs) arise from the upper two-thirds of the clivus at the petroclival junction, and medial to the trigeminal nerve [1]. PCMs pose significant surgical challenges because of their proximity to the brainstem and vital neurovascular structures. Thus, achieving gross total resection may be challenging surgically and associated with high morbidity and mortality rates [2].
Until a few decades ago, these tumors were mostly considered untreatable [3, 4]; however, the management paradigm has evolved rapidly and now includes improved microsurgical techniques, earlier detection using imaging, and improved alternative management modalities and strategies [4]. Stereotactic radiosurgery (SRS) has been widely documented as an important treatment option for newly diagnosed, incompletely resected, or recurrent meningiomas [5]. The primary goal of SRS is to provide long-term tumor control and minimize treatment-related complications, especially in surgically challenging lesions such as PCMs [17].
Despite the recent interest in SRS for the management of skull base meningiomas, controversy still exists regarding its role in managing PCMs [6]. Although primary SRS is recommended for small PCMs and adjuvant SRS for large (defined as tumor diameter of > 3 cm or tumor volume of > 10 cm3), recurrent, or incompletely resected lesions, few studies have investigated the clinical and radiological outcomes in both patient cohorts (primary or adjuvant SRS) [7,8,9,10,11]. In this systematic review and meta-analysis, we report the clinical and radiological outcomes as well as progression-free survival (PFS) rates after primary or adjuvant SRS for the management of PCMs.
Methods
Literature search
A systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. The PubMed, Scopus, Web of Science, and Cochrane databases were searched from inception to July 26, 2021, operating the combination of the Boolean operators “OR” and “AND” and terms “petroclival” and “meningioma.” Articles were uploaded to Mendeley, and duplicates were removed.
Study selection
Predetermined inclusion and exclusion criteria were set. Studies were included if they: (1) involved ≥ 5 patients aged ≥ 18 years with meningiomas located within the petroclival region, as clearly mentioned within the text, and treated with either primary or adjuvant SRS; (2) reported data on clinical features, SRS protocols, and outcomes; and (3) were written in English. Studies were excluded if they: (1) were literature reviews, case reports, technical notes, abstracts, or autopsy reports; (2) did not clearly differentiate data of patients with PCMs from data of patients with different tumors or with meningiomas in different locations; or (3) contained insufficient data on treatments and outcomes. One of the included articles (i.e., Kim et al. [9]) captured patients between 15 and 74 years.
Two authors (O.B.A. and P.P.) independently reviewed the titles and abstracts of all extracted citations and then appraised the full text of articles that met the inclusion criteria. Two authors (A.N.M. and H.A.) settled any disagreements. Eligible studies were included, and references were screened to retrieve additional relevant studies.
Data extraction
One author (O.B.A.) extracted data from included articles, confirmed by three independent authors (P.P., A.N.M., and H.A.) to ensure accuracy. Missing data were either not reported or could not be differentiated from other data. Data included: author and year of study, patients’ ages and sexes, symptoms, cranial nerve involvement, invaded structures, imaging features, lesion size, treatment modality, marginal radiation dose, post-radiosurgery outcomes and tumor volume change, PFS, and follow-up duration. SRS protocols were categorized as (1) primary SRS if patients underwent initial radiosurgery only, or (2) adjuvant if patients underwent SRS after initial microsurgical resection.
Data synthesis and quality assessment
The primary outcomes of interest were clinical characteristics, SRS protocols, PFS, and clinical/radiological outcomes. The level of evidence of each article was evaluated following the 2011 Oxford Centre for Evidence-Based Medicine guidelines. The risk of bias was independently assessed for each article by two authors (P.P. and O.B.A.) using the Joanna Briggs Institute checklists for case series [13].
Statistical analysis
R (Version 4.1.1, 2021.09.0 RStudio, Inc. URL: http://www.R-project.org/; R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses. Continuous variables are summarized as medians or means and ranges, while categorical variables are summarized as frequencies and percentages.
A meta-analysis of pooled data was done according to the types of study obtained. A statistically significant difference was considered as P < 0.05 and ratios that are not crossing 1, the value of no effect. Data from all studies were combined to estimate the relative ratio with 95% confidence intervals (CIs) for treatment strategy. The χ2 test and the Higgins I2 test were used to assess heterogeneity. Data were pooled in all studies and were conducted using random-effects models.
Results
Study selection
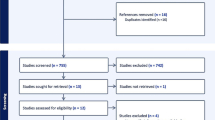
The initial literature search yielded 466 citations from PubMed, 435 from Scopus, 578 from Web of Science, and one article from Cochrane. After going through the study selection process displayed in Fig. 1, seven articles categorized as level IV evidence were included in this systematic review based on the prespecified criteria. (Table 1; Supplementary Table 1) [8,9,10, 14,15,16,17]. Table 1 describes the SRS clinical outcomes reported by each article, providing an overview comparison across the reported heterogeneous data. Risk of bias assessment resulted in “good” quality (i.e., low risk of bias) for all included studies (Supplementary Table 2).
Demographics and clinical characteristics of the overall cohort
A total of 722 PCMs treated with Gamma Knife SRS were included (Table 2). The mean age of patients was 55.1 years (range, 15–90 years), with female predominance (75.6%). The most common presenting symptoms were ataxia (20.0%), trigeminal neuralgia (17.6%) and headache (16.6%). Trigeminal, facial, and vestibulocochlear nerves were affected in 26.5%, 26.1%, and 19.4% of cases, respectively. The cavernous sinus was the most invaded adjacent structure (37%), followed by brainstem (23.9%) and Meckel’s cave (21.7%). The mean tumor size at presentation was 8.1 cm3 (range, 0.13–64.9 cm3).
The WHO grade of 188 meningiomas was reported. One hundred and eighty-three (97.3%) were WHO grade I, three (1.6%) were grade II, and two (1.1%) were grade III. The five cases of grades II and III were reported by Flannery et al. [17], who observed that ultimately patients with atypical (WHO grade II) and anaplastic meningiomas (WHO grade III) clinically deteriorated with evidence of tumor progression at a median interval of 36 months after SRS. The mean radiosurgical isodose prescribed to the tumor margin was 49.3 (range, 30–60), and the mean marginal radiation dose was 13.5 Gy (range, 9–40 Gy).
Management strategies and survival outcomes
At last follow-up, 149 patients (28.7%) showed improved symptoms, 319 (61.3%) had unchanged symptoms, and 52 (10.0%) demonstrated worsened symptoms. Tumor volume decreased or remained stable in 661 (94.8%) patients and increased in 36 (5.2%) patients (Table 2). The mean tumor volume change was −6.4% (range −82% to 225%). The mean clinical follow-up time was 59.6 months (range 6–254 months), while the mean radiological follow-up time was 56.5 months (range 3–204 months). The 5-year PFS rate ranged from 91 to 100%, and the 10-year PFS rate ranged from 69.6 to 89.9%. After SRS, 76 patients with a total of 86 complications were encountered among both treatment strategies (Table 2). CN V deficit was the most common complication (15.1%), followed by hydrocephalus (9.3%), ataxia (8.1%) and dizziness (8.1%).
Primary vs. adjuvant radiosurgery
Seven articles encompassed clinical characteristics and outcomes of patients treated with primary (n = 447; 61.9%) and adjuvant SRS (n = 275; 38.1%) (Table 3; Fig. 2A) [8,9,10, 14,15,16,17]. Patients who underwent primary SRS were older (mean age 55.9 vs. 48.6 years for adjuvant SRS), with comparable female patient rates (78.3% vs. 80.6%). At clinical presentation, the mean tumor volume was 7.4 cm3 in the primary SRS group and 12.1 cm3 in the adjuvant SRS group. The mean marginal radiosurgery doses were comparable: 15.1 Gy (range 11–16 Gy) in the primary SRS group and 11.2 Gy (range 8–17 Gy) in the adjuvant SRS group. The indications for adjuvant SRS after surgical resection were residual tumor in 203 (73.8%), recurrent tumor in 41 (14.9%), and not specified in 31 (11.2%) patients.
Bar chart comparing the outcomes of primary vs. adjuvant SRS (A). Forest plots for (B) improved/stable functional status, (C) worsening functional status, and (D) tumor control rate between primary and adjuvant SRS. CI, confidence interval; RR, relative risk; priEvents, primary group events; priTotal, primary group total subjects; adjEvents, adjuvant group events; adjTotal, adjuvant group total subjects
Functional status improvement and stability were evaluated at the end of the follow-up period in 127 patients in the primary SRS group and 114 in the adjuvant SRS group (Table 3). A total of 109 out of 127 (85.8%) in the primary SRS group and 89 out of 114 (78.1%) in the adjuvant SRS group demonstrated functional status improvement or stability at last follow-up. Additionally, our meta-analysis showed an effect size of functional improvement of 1.12 (95% CI 1.01–1.25; I2 = 0%) in favor of the primary SRS group with statistical significance (P < 0.05; Fig. 2B). The primary SRS patients were also less likely to develop worsening functional status: eighteen out of 127 (14.2%) in the primary SRS group and 25 out of 114 (21.9%) in the adjuvant SRS group. However, our meta-analysis illustrated an effect size of 0.56 (95% CI 0.31–1.01; I2 = 0%), which failed to reach statistical significance (Fig. 2C).
At last follow-up, primary SRS resulted in decreased or stable tumor volumes in 94.3% of patients with available data compared with 88.2% for adjuvant SRS patients (Table 3). The random effect size was 1.03 (95% CI 0.85–1.25; I2 = 85%) in favor of the primary strategy; however, substantial heterogeneity was present, and the effect size was not statistically significant (Fig. 2D). An increase in tumor volume was reported in 5.7% after primary SRS and 11.8% after adjuvant SRS. The average clinical follow-up duration was 58.3 and 62.8 months in the primary and adjuvant SRS groups, respectively, and the mean radiological follow-up duration was 57.1 and 55.9 months, respectively (Table 3).
Of studies reporting complications of the primary SRS group, a total of eight complications were reported (Table 3). These complications included trigeminal neuralgia (2 patients), ataxia (2 patients), vestibulocochlear neuropathy (1 patient), facial pain (1 patient), tinnitus (1 patient), and hemiplegia (1 patient). Four patients developed complications after adjuvant SRS (not accounting for microsurgical complications), including three who developed cyst formation and one who developed hemiplegia.
Discussion
PCMs are among the most challenging skull base tumors because of their close anatomical relationship to critical neurovascular structures. Microsurgical resection has been associated with high rates of morbidity ranging from 28% to 76% and mortality ranging from 3.7% to 17% as well as incomplete resection in most patients [18,19,20,21,22]. Large case series and high-quality articles are limited in the literature, especially on the role that SRS may play in managing these lesions. In this systematic review and meta-analysis on PCMs managed with primary or adjuvant SRS, we described the clinical characteristics and management outcomes and presented an outcome comparison between primary and adjuvant SRS. Our meta-analysis showed that primary SRS was correlated with a higher rate of improved/stable functional status compared with adjuvant SRS. In the studies we identified, we found no statistical difference in worsening functional status or tumor control rate between primary and adjuvant SRS.
Patients and clinical characteristics
We found that PCMs were predominant in females (75.6%), and the mean age of patients at presentation was 55.1 years, both consistent with the reported data on intracranial and skull base meningiomas [23, 24]. The most commonly involved cranial nerves were V, VII, and VIII. PCMs often directly compress the trigeminal nerve or invade adjacent structures, such as Meckel’s cave, resulting in Gasserian ganglia compression and trigeminal neuralgia, as reported in 17.6% of cases. These features are in concordance with other petroclival lesions, reflecting the proximity of the petroclival region to cranial nerves and other vital neurovascular structures and indicating the necessity for early intervention to avoid permanent neurological deficits and debilitating long-term outcomes [25, 26].
The mean tumor size at presentation was 8.1 cm3 (range 0.13–64.9 cm3), which is comparable with other skull base meningiomas, probably because of their similar threshold sizes above which skull base tumors compress critical structures and become symptomatic [27].
In our systematic review, the mean isodose prescribed to the tumor margin was 49.3% (range 30–60%), similar to the mean 51.5% isodose described in other intracranial meningiomas [28]. Additionally, the mean marginal radiation doses of the cohort in this review was 13.5 Gy, similar to other studies on skull base, parasagittal, and falcine meningiomas (mean doses ranging from 13 to 16 Gy) [7, 29].
Management and tumor outcomes
At last follow-up after radiosurgery, 94.8% of the described cohort experienced decreased or stable tumor volume, which shows a comparable tumor growth control rate with other volumetric studies investigating intracranial meningioma control rates after SRS [30].
Of the whole cohort, the majority (61.3%) had no change in functional status after management (primary and adjuvant SRS), with 28.7% of patients reporting functional improvement and 10% reporting worsening functional status. In contrast, studies describing outcomes of microsurgical resection reported a higher rate of symptomatic relief along with a greater risk of new neurological dysfunction [31, 32]. We ascribe this difference to the distinct therapeutic goals and selection bias of each treatment, as resection aims to achieve initial relief of mass effect with decompression of neurovascular structures, and thus immediate improvement in functional status, whereas SRS aims to offer long-term local tumor control and prevent tumor growth and condition deterioration.
Our 5-year and 10-year PFS rates (91–100% and 69.6–89.9%, respectively) are comparable with those of other skull base meningiomas treated with SRS (88.9–100%, and 77–92%, respectively), likely suggesting that SRS for PCMs achieves comparable tumor control rates with other skull base meningiomas not limited by their anatomical location [33,34,35].
Primary vs. adjuvant radiosurgery
Resection remains the first option for large symptomatic PCMs in patients suitable for surgery, with adjuvant SRS being reserved for residual and recurrent tumors. These tumors tend to be complex, invading multiple neurovascular structures. Many authors advise that maximal safe resection should be undertaken for eligible patients with symptomatic PCMs followed by adjuvant SRS for residual disease to achieve high tumor control and minimal complication rates [33, 36, 37]. Included articles scarcely reported on adjuvant SRS-related complications and described an overall 4 new complications related to adjuvant SRS among 39 patients with available data (not accounting for microsurgical complications). At present, insufficient data exists to endorse aggressive resection compared with maximal safe resection followed by SRS [31, 32].
Almefty et al. [32] reported that 64% of 64 patients with PCMs underwent gross total resection, 65.6% of whom developed immediate postoperative new cranial nerve deficits, often resulting in persistent cranial nerve dysfunction. Similarly, Li et al. [38] found a 66.8% rate of cranial nerve dysfunction along with a 2% rate of surgical mortality in 199 patients with surgically managed PCMs. A consensus seems to suggest that resection of PCMs should be primarily focused on debulking the tumor and decompression of the brainstem and cranial nerves followed by SRS to maximize tumor control and minimize complication rates.
Primary SRS resulted in lower complication rates (3.7% vs. 10.3%), and our meta-analysis indicated better functional outcomes in the primary SRS group compared to the adjuvant SRS group. This might be attributed to the fact that most adjuvant SRS articles did not report specific SRS-related complications and instead grouped them together with microsurgical complications and functional outcomes, which limited our analysis. Although we found a worsened functional status rate difference between the primary and adjuvant SRS groups (14.2% vs. 21.9%), our meta-analysis failed to show any significant difference. In a study investigating the long-term results of Gamma Knife radiosurgery for parasellar meningiomas, Williams et al. [39] did not find any significant predictive difference between primary SRS and SRS following prior resection. Similarly, in their study investigating Gamma Knife radiosurgery for benign intracranial meningiomas, Ge et al. [40] demonstrated a Hazard ratio of 2.30 in cases with prior resection; however, their multivariate analysis did not reach statistical significance. Thus, it may be challenging to explicitly distinguish the complications of microsurgical resection from SRS in the adjuvant group and to rule out the synergistic effect of resection on the patients’ status even after the SRS, especially when most literature does not explicitly report patients’ status prior to SRS. Therefore, we believe that the causality between complications and SRS in the adjuvant group remains unclear and necessitates further and more controlled investigation.
Although primary SRS offers a minimally invasive modality for the management of small and asymptomatic PCMs, concerns related to the long-term effect of radiation in the management of meningiomas exist [41, 42]. The publication of the IMPASSE study [43] further indicated that very few meningioma patients who underwent primary SRS developed neurological or radiation-related complications. Similarly, the present meta-analysis indicated that patients who underwent primary SRS were more likely to achieve improved or stable functional status and had a lower rate of worsening functional status.
The present report found that patients who underwent primary SRS had a higher rate of tumor control and a lower rate of tumor progression compared with patients who underwent adjuvant SRS (94.3% vs. 88.2% and 5.7% vs. 11.8%, respectively). The meta-analysis on tumor control, however, showed no superiority of either strategy. Tumor control and tumor volume change rates were different between the primary and adjuvant SRS groups and the overall cohort. This is primarily related to the inclusion of more articles reporting overall tumor control data indistinctively compared with articles reporting on specific treatment modality (e.g. post-SRS volume change: primary SRS group, n = 283; adjuvant SRS group, n = 153; overall cohort, n = 697). In addition, there is likely a significant selection bias between primary and adjuvant SRS-treated tumors, which may impact the overall results. These data further emphasize the need for large, multicentric controlled studies investigating the functional outcomes and tumor control rates between both treatment modalities.
Limitations
This study was potentially affected by multiple limitations. First is the heterogeneity of reported data, which challenged the consistency of different variables. Different studies adopted different definitions and treatment protocols, including, but not limited to, radiation dose, radiation technique, and indications for treatment. Furthermore, the availability of several variables such as histological grade, invasion of adjacent structures, and mean volume change after treatment was limited.
Conclusion
Radiosurgery, either primary or adjuvant, represents a safe and effective strategy for the management of PCMs. It is associated with improved or stable long-term functional outcomes and high tumor control rates in many patients, along with a low complication profile. Primary SRS may be preferred for patients with small PCMs, whereas adjuvant SRS may be recommended for larger and/or recurrent PCMs to achieve higher tumor control rates while minimizing complications.
Data availability
All authors confirm the appropriateness of all dataset and software used for supporting the conclusion. All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
References
Zhao Z, Yuan X, Yuan J et al (2020) Treatment strategy for petroclival meningiomas based on a proposed classification in a study of 168 cases. Sci Rep 10:4655. https://doi.org/10.1038/s41598-020-61497-y
Little KM, Friedman AH, Sampson JH et al (2005) Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery 56:546–559. https://doi.org/10.1227/01.neu.0000153906.12640.62
Castellano F, Ruggiero G (1953) Meningiomas of the posterior fossa. Acta Radiol Suppl 104:1–177
Maurer AJ, Safavi-Abbasi S, Cheema AA et al (2014) Management of petroclival meningiomas: a review of the development of current therapy. J Neurol Surg B Skull Base 75:358–367. https://doi.org/10.1055/s-0034-1373657
Davidson L, Fishback D, Russin JJ et al (2007) Postoperative gamma knife surgery for benign meningiomas of the cranial base. Neurosurg Focus 23(4):E6. https://doi.org/10.3171/FOC-07/10/E6
Giammattei L, di Russo P, Starnoni D et al (2021) Petroclival meningiomas: update of current treatment and consensus by the EANS skull base section. Acta Neurochir 163:1639–1663. https://doi.org/10.1007/s00701-021-04798-z
Jumah F, Narayan V, Samara A et al (2020) Efficacy and safety of gamma knife radiosurgery for posterior cranial fossa meningioma: a systematic review. Neurosurg Rev 43:1089–1099. https://doi.org/10.1007/s10143-019-01144-x
Ha MH, Jang WY, Jung TY et al (2020) Treatment outcome of gamma knife radiosurgery for petroclival meningiomas: retrospective analysis of a single institution experience. Brain Tumor Res Treat 8:83–92
Kim JW, Kim DG, Se Y-B et al (2017) Gamma knife radiosurgery for petroclival meningioma: long-term outcome and failure pattern. Stereotact Funct Neurosurg 95:209–215. https://doi.org/10.1159/000475763
Sadik ZHA, Te LS, Leenstra S, Hanssens PEJ (2018) Volumetric changes and clinical outcome for petroclival meningiomas after primary treatment with Gamma Knife radiosurgery. J Neurosurg 129:1623–1629. https://doi.org/10.3171/2017.7.JNS17380
Han M-S, Jang W-Y, Moon K-S et al (2017) Is fractionated gamma knife radiosurgery a safe and effective treatment approach for large-volume (>10 cm3) intracranial meningiomas? World Neurosurg 99:477–483. https://doi.org/10.1016/j.wneu.2016.12.056
Page MJ, McKenzie JE, Bossuyt PM, et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372
Joanna Briggs Institute Case Series (2017) Checklist for case series. https://jbi.global/critical-appraisal-tools
Starke R, Kano H, Ding D et al (2014) Stereotactic radiosurgery of petroclival meningiomas: a multicenter study. J Neurooncol 119:169–176. https://doi.org/10.1007/s11060-014-1470-x
Subach BR, Lunsford LD, Kondziolka D et al (1998) Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery 42:435–437. https://doi.org/10.1097/00006123-199803000-00001
Roche P-H, Pellet W, Fuentes S, et al (2003) Gamma knife radiosurgical management of petroclival meningiomas results and indications. Acta Neurochir 145:883–888; discussion 888. https://doi.org/10.1007/s00701-003-0123-1
Flannery TJ, Kano H, Lunsford LD et al (2010) Long-term control of petroclival meningiomas through radiosurgery: clinical article. J Neurosurg 112:957–964. https://doi.org/10.3171/2009.8.JNS09695
Nanda A, Javalkar V, Banerjee AD (2011) Petroclival meningiomas: study on outcomes, complications and recurrence rates: clinical article. J Neurosurg 114:1268–1277. https://doi.org/10.3171/2010.11.JNS10326
Al-Mefty O, Fox JL, Smith RR (1988) Petrosal approach for petroclival meningiomas. Neurosurgery 22:510–517. https://doi.org/10.1227/00006123-198803000-00010
Mayberg MR, Symon L (1986) Meningiomas of the clivus and apical petrous bone. Report of 35 cases. J Neurosurg 65:160–167. https://doi.org/10.3171/jns.1986.65.2.0160
Harsh GR 4th, Sekhar LN (1992) The subtemporal, transcavernous, anterior transpetrosal approach to the upper brain stem and clivus. J Neurosurg 77:709–717. https://doi.org/10.3171/jns.1992.77.5.0709
Couldwell WT, Fukushima T, Giannotta SL, Weiss MH (1996) Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg 84:20–28. https://doi.org/10.3171/jns.1996.84.1.0020
Lu VM, Goyal A, Rovin RA (2018) Olfactory groove and tuberculum sellae meningioma resection by endoscopic endonasal approach versus transcranial approach: a systematic review and meta-analysis of comparative studies. Clin Neurol Neurosurg 174:13–20. https://doi.org/10.1016/j.clineuro.2018.08.029
Teng H, Liu Z, Yan O et al (2021) Lateral ventricular meningiomas: clinical features, radiological findings and long-term outcomes. Cancer Manag Res 13:6089–6099. https://doi.org/10.2147/CMAR.S320651
Barresi V, Caffo M, Alafaci C et al (2012) Intradural chordoma of the Meckel’s cave: a challenging differential diagnosis. Neuropathology 32:577–582. https://doi.org/10.1111/j.1440-1789.2011.01295.x
AlOtaibi F, Guiot M-C, Muanza T, Di Maio S (2014) Giant petroclival primary intradural chordoma: case report and systematic review of the literature. J Neurol Surg Rep 75:e160–e169. https://doi.org/10.1055/s-0034-1378157
Flannery T, Poots J (2019) Gamma knife radiosurgery for meningioma. Prog Neurol Surg 34:91–99. https://doi.org/10.1159/000493054
Balagamwala EH, Suh JH, Barnett GH et al (2012) The importance of the conformality, heterogeneity, and gradient indices in evaluating Gamma Knife radiosurgery treatment plans for intracranial meningiomas. Int J Radiat Oncol Biol Phys 83:1406–1413. https://doi.org/10.1016/j.ijrobp.2011.10.024
Hasegawa T, Kida Y, Yoshimoto M et al (2011) Gamma knife surgery for convexity, parasagittal, and falcine meningiomas: clinical article. J Neurosurg 114:1392–1398. https://doi.org/10.3171/2010.11.JNS10112
Harrison G, Kano H, Lunsford LD et al (2016) Quantitative tumor volumetric responses after Gamma Knife radiosurgery for meningiomas. J Neurosurg 124:146–154. https://doi.org/10.3171/2014.12.JNS141341
Li D, Hao S-Y, Wang L et al (2013) Surgical management and outcomes of petroclival meningiomas: a single-center case series of 259 patients. Acta Neurochir 155:1367–1383. https://doi.org/10.1007/s00701-013-1795-9
Almefty R, Dunn IF, Pravdenkova S et al (2014) True petroclival meningiomas: results of surgical management. J Neurosurg 120:40–51. https://doi.org/10.3171/2013.8.JNS13535
Nicolato A, Foroni R, Pellegrino M et al (2001) Gamma knife radiosurgery in meningiomas of the posterior fossa. Experience with 62 treated lesions. Minim Invasive Neurosurg 44:211–217. https://doi.org/10.1055/s-2001-19934
Sheehan JP, Starke RM, Kano H et al (2015) Gamma Knife radiosurgery for posterior fossa meningiomas: a multicenter study. J Neurosurg 122:1479–1489. https://doi.org/10.3171/2014.10.JNS14139
Starke RM, Nguyen JH, Rainey J et al (2011) Gamma Knife surgery of meningiomas located in the posterior fossa: factors predictive of outcome and remission. J Neurosurg 114:1399–1409. https://doi.org/10.3171/2010.11.JNS101193
Nakao N, Ohkawa T, Miki J et al (2010) Surgical treatment and outcome of skull base meningiomas with extracranial extensions. Clin Neurol Neurosurg 112:40–46. https://doi.org/10.1016/j.clineuro.2009.10.003
Patibandla MR, Lee C-C, Tata A et al (2018) Stereotactic radiosurgery for WHO grade I posterior fossa meningiomas: long-term outcomes with volumetric evaluation. J Neurosurg 129:1249–1259. https://doi.org/10.3171/2017.6.JNS17993
Li D, Tang J, Ren C, et al (2016) Surgical management of medium and large petroclival meningiomas: a single institution’s experience of 199 cases with long-term follow-up. Acta Neurochir 158:409–425; discussion 425. https://doi.org/10.1007/s00701-015-2671-6
Williams BJ, Yen CP, Starke RM et al (2011) Gamma Knife surgery for parasellar meningiomas: long-term results including complications, predictive factors, and progression-free survival. J Neurosurg 114:1571–1577. https://doi.org/10.3171/2011.1.JNS091939
Ge MDY, Liu MDD, Zhang MDZ et al (2019) Gamma Knife radiosurgery for intracranial benign meningiomas: follow-up outcome in 130 patients. Neurosurg Focus 46(6):1–9. https://doi.org/10.3171/2019.3.FOCUS1956
Bledsoe JM, Link MJ, Stafford SL et al (2010) Radiosurgery for large-volume (> 10 cm3) benign meningiomas. J Neurosurg 112:951–956. https://doi.org/10.3171/2009.8.JNS09703
DiBiase SJ, Kwok Y, Yovino S et al (2004) Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 60:1515–1519. https://doi.org/10.1016/j.ijrobp.2004.05.073
Sheehan J, Pikis S, Islim A et al (2022) An international multicenter matched cohort analysis of incidental meningioma progression during active surveillance or after stereotactic radiosurgery: the IMPASSE study. Neuro Oncol 24:116–124. https://doi.org/10.1093/neuonc/noab132
Acknowledgements
We thank Kristin Kraus, MSc, for her editorial assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design: OBA, HA. Literature search, articles screen, and data extraction: OBA, PP, ANM, HA. Data analysis: OBA, PP, ANM, HA, MAL. Manuscript draft: OBA, HA. All authors critically revised and edited the first draft and commented on all versions of the manuscript. The project was supervised by PAG, WTC, LDL, HA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bin Alamer, O., Palmisciano, P., Mallela, A.N. et al. Stereotactic radiosurgery in the management of petroclival meningiomas: a systematic review and meta-analysis of treatment outcomes of primary and adjuvant radiosurgery. J Neurooncol 157, 207–219 (2022). https://doi.org/10.1007/s11060-021-03934-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03934-0