Abstract
Purpose
Immunotherapy has demonstrated efficacy in treatment of intracranial metastasis from melanoma, calling into question the role of intracranial radiotherapy (RT). Herein, we assessed the utilization patterns of intracranial RT in patients with melanoma brain metastasis and compared outcomes in patients treated with immunotherapy alone versus immunotherapy in addition to intracranial RT.
Methods
We queried the National Cancer Database (NCDB) for patients with melanoma brain metastases treated with immunotherapy and intracranial RT or immunotherapy alone. Multivariable logistic regression identified variables associated with increased likelihood of receiving immunotherapy alone. Multivariable Cox regression was used to identify co-variates predictive of overall survival (OS). Propensity matching was used to account for indication bias.
Results
We identified 528 and 142 patients that were treated with combination therapy and immunotherapy alone, respectively. Patients with lower comorbidity score were more likely to receive immunotherapy alone. Extracranial disease and treatment at a non-academic center were associated with worse OS. Median OS for all patients was 11.0 months. Treatment with stereotactic radiosurgery (SRS) in addition to immunotherapy was superior to immunotherapy alone, median OS of 19.0 versus 11.5 months (p = 0.006). Whole brain radiation therapy (WBRT) in combination with immunotherapy performed worse than immunotherapy alone, median OS of 7.7 versus 11.5 months (p = 0.0255).
Conclusions
For melanoma patients requiring WBRT, immunotherapy alone may be reasonable in asymptomatic patients. For those eligible for SRS, combination therapy may provide better outcomes. Results of ongoing prospective studies will help provide guidance regarding the use of radioimmunotherapy in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Malignant melanoma arises from aberrant melanocytes and has a propensity to metastasize to intracranial sites. Given the poor penetration of the blood–brain-barrier by chemotherapeutic agents, local therapy has historically been the mainstay of treatment for intracranial metastatic disease. Local treatments include surgical resection and/or radiotherapy with either whole brain radiation therapy (WBRT) or stereotactic radiosurgery (SRS) [1]. However, with the advent of immunotherapy and emerging evidence suggesting intracranial penetration and tumor response in up to 50–60% of patients, the need for local intracranial therapy is being called into question [2, 3]. Anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibody therapy was first shown to improve survival in 2010 [4] and was shortly after tested in combination with an antibody against the programmed cell death 1 (PD-1) receptor and shown to even more drastically improve outcomes [5]. Despite these advances, the elusive diagnostic characteristics of melanoma present a challenge for early diagnosis and patients continue to present with metastatic disease, particularly to the brain, leaving to question the role of combination radiotherapy with immunotherapy in management. As such, we sought to use the National Cancer Database (NCDB) to examine the utility and benefit of different forms of radiotherapy in newly diagnosed melanoma patients with metastases to the brain on presentation.
Methods
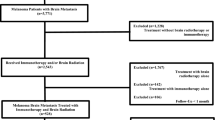
We queried the NCDB from 2010 to 2015 for patients with cutaneous melanoma with brain metastasis at the time of diagnosis whom underwent treatment with immunotherapy with or without intracranial radiotherapy. Figure 1 is a CONSORT diagram outlining all inclusion and exclusion criteria. The NCDB is overseen by the American College of Surgeons and the Commission on Cancer and encompasses an estimated 70% of annual newly diagnosed cancer cases in the United States. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. Given its retrospective nature and de-identified dataset, this study was exempt from institutional review board approval.
Within the NCDB, race was categorized as Caucasian, African American, or other. The Charlson/Deyo comorbidity index was also recorded and quantified the degree of comorbidities [6]. Socioeconomic data in the patients’ residence census tract were divided into quartiles based upon the percentage of persons with less than a high school education and median household income. Facility type was grouped according to the Commission on Cancer accreditation category. Location was described based on data provided by the US Department of Agriculture Economic Research Service. Insurance status is documented in the NCDB as it appears on the admission page. Radiotherapy is recorded as modality, dose and location. Chemotherapy and immunotherapy are recorded as given in the NCDB but specific agents and number of cycles are not.
Statistical methodology utilized in an NCDB study has been reported previously [7]. Data were analyzed using Medcalc Version 18 (Ostend, Belgium). Summary statistics are presented for discrete variables. Clinicopathologic and treatment related variables were first tabulated and a multivariable logistic regression was then performed to identify predictors of receiving immunotherapy alone. A Cox proportional hazards model (forward method) was used for multivariable survival analysis [8]. Adjusted hazard ratios and 95% confidence intervals are reported, using an alpha level of 0.05 to indicate statistical significance.
Propensity score-adjusted survival analysis was used to account for indication bias due to lack of randomization between the use of immunotherapy alone versus in combination with WBRT or SRS [9]. Multivariable logistic regression was used with all baseline variables to calculate a propensity score indicative of conditional probability of receiving immunotherapy alone. The propensity model included all baseline characteristics listed in Table 1. Using that propensity score as an exact match, 141 matches were generated. These matched pairs were then used within a Kaplan–Meier analysis to compare rates of overall survival (OS) across the entire cohort but also by stage group [10]. Overall survival was calculated in months from time of diagnosis to date of last contact or death which is the standard way this data is recorded in the NCDB. A minimum survival cutoff of 1 month was employed to account for immortal time bias.
Results
Using the above eligibility criteria, we identified 670 patients who received immunotherapy ± intracranial radiotherapy. Table 1 contains full details of the study group. The majority of patients (68.8%) had extracranial disease and were not treated with chemotherapy (87.0%). The median patient age was 62 (range, 20–90). A total of 528 patients (78.8%) received a combination of immunotherapy and intracranial radiotherapy while 142 patients (21.2%) were treated with immunotherapy alone. Of all patients receiving intracranial radiotherapy in addition to immunotherapy, 273 received WBRT and 255 received SRS. The only predictor for treatment with immunotherapy alone was a low comorbidity score of 1 (Table 2).
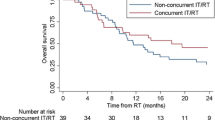
Across the entire cohort, median OS for all patients was 11.0 months (Fig. 2). On multivariable analysis, extracranial disease at the time of diagnosis, as well as, treatment at a community cancer program compared to an academic/research program was associated with worse overall survival (Table 3). Propensity score was generated via multivariable logistic regression as described in the methods. Using an exact match on the generated propensity score resulted in 141 pairs of patients. On this propensity matched subset, treatment with SRS in addition to immunotherapy was associated with improved survival compared to immunotherapy alone, with a median OS of 19.0 versus 11.5 months (p = 0.006) (Fig. 2b). However, WBRT in combination with immunotherapy was associated with worse OS compared to immunotherapy alone [median OS of 7.7 vs. 11.5 months (p = 0.0255)] (Fig. 2c).
a Overall survival of entire population broken down into combination therapy versus immunotherapy alone. (IO = immunotherapy/immuno oncology).The median OS was 10.8 months (95% CI 7.8–14.8) versus 11.5 months (95% CI 8.0–14.5) for combination therapy and immunotherapy alone, respectively (p = 0.57). b Overall survival of SRS and immunotherapy combination therapy versus imunotherapy alone. (IO = immunotherapy/immuno oncology).The median OS was 19.0 months (95% CI 12.3–28.6) and 11.5 months (95% CI 8.0–14.5) for SRS and immunotherapy and immunotherapy alone, respectively (p = 0.0061). c Overall survival of WBRT and immunotherapy combination therapy versus immunotherapy alone. (IO = immunotherapy/immuno oncology).The median OS was 7.7 months (95% CI 6.4–9.8) and 11.5 months (95% CI 8.0–14.5) for WBRT and immunotherapy and immunotherapy alone, respectively (p = 0.0255)
Discussion
Keeping in mind the limitations of studies such as this, the results presented here appear to suggest that in select patients with a diagnosis of metastatic melanoma having de novo intracranial metastasis, exclusion of intracranial radiation may be reasonable in certain clinical scenarios. As shown, in patients with presumably more limited intracranial disease who are candidates for SRS, this appears to improve outcomes when used in combination with immunotherapy. This is likely affected by number of lesions present (data not recorded in the NCDB) and the higher dose of radiation delivered to overcome the relative radioresistance of melanoma [11,12,13]. In addition, one hypothesis generating notion is that perhaps those higher doses of radiation are generating an immunogenic or abscopal effect, leading to better outcome. This concept is currently being explored across numerous clinical trials [14]. On the other hand, for patients with an extensive intracranial disease burden in which WBRT would typically be recommended, exclusion of radiotherapy may be justified in the proper context (i.e. asymptomatic patient with small non-hemorrhagic metastasis). It is important to note, however, that patients with a lower comorbidity score were more likely to be treated with immunotherapy alone, likely affecting the results seen here.
Historically, WBRT was the standard of care for melanoma brain metastasis. One retrospective study indicated omission of WBRT was associated with increased rates of distant intracranial disease progression and decreased overall survival in patients with multiple brain metastasis [15]. The role of WBRT began to diminish after SRS was shown to be non-inferior in patients with up to 3 brain metastasis while providing a higher biologic dose and less long term toxicity [16]. Furthermore, an ongoing phase 3 trial enrolling patients with 4 to 10 intracranial metastasis, a population previously reserved for WBRT, is evaluating the utility of SRS alone with promise of quality of life and survival preservation [17]. A similar NRT/CCTG trial is currently recruiting patients with 5–15 brain metastases and randomizing them to SRS or WBRT, with the primary endpoint of OS; results of which will help shape the evolving standard of care [18]. Intriguingly, the addition of WBRT to SRS was also compared in the upfront setting for brain metastasis and found to have no significant clinical benefit in terms of overall survival and progression free survival when compared to SRS alone, but it is important to note that this was not limited to a population of melanoma patients [19]. However, this finding was exhibited in a melanoma specific population in a phase 3 clinical trial comparing adjuvant WBRT after local treatment of one to three intracranial brain metastases that showed no clinical benefit in terms distant metastatic occurrence, survival or preservation of performance status compared to those treated with local control alone [20].
Immunotherapy has recently been shown to have drastic improvements in survival in patients with metastatic melanoma, even in the setting of intracranial metastasis [2, 21, 22]. With these changes, the utility of radiotherapy in combination with immunotherapy has been called into question. Lanier et al. recently published results suggesting improvement in overall survival (15.9 months vs. 6.1 months) and decrease in neurologic death with the use of immunotherapy after initial SRS in patients presenting with brain metastasis [23]. Similarly, nivolumab in combination with SRS was shown to improve intracranial control of melanoma as well as progression free survival, although 15% of patients experienced radiation-induced necrosis; a result higher than expected compared to historical controls [24]. However, to put that finding in context, it is important to note that immunotherapy trials have shown adverse event rates in the 50–60% range [2].
While ongoing trials continue to evaluate the efficacy of different forms of radiation in combination with immunotherapy, the role of WBRT is seemingly on the decline but continues to be used in patients with high volume intracranial disease. Our data suggests that omission of WBRT does not shorten overall survival in the setting of immunotherapy. This study also coincides with those previously described that SRS (when feasible) in combination with immunotherapy is the superior approach [23, 24]. Additionally, we should mention that a similar topic has been explored using the NCDB, looking at patients from a shorter time frame (2011–2013) with brain metastases managed with radiation with or without immunotherapy [25]. That study showed an expected increase in immunotherapy use, as well as an increase in overall survival for patients treated with immunotherapy and SRS compared to SRS alone.
As is typical with NCDB analyses, interpretation is limited by the data provided in the NCDB due to its retrospective nature and what is an inherent, often heavy, selection bias. Compounding this, the NCDB lacks information on toxicity, symptomatology at baseline, patterns of failure, actual systemic therapeutic agent(s) selection and dosing/number of cycles completed, all of which play an important role in management and outcome. Additionally, specifics regarding the immunotherapeutic agent are not coded within the NCDB, neither are the number and/or volume of intracranial metastatic disease. Finally, performance status and other intangibles such as social support system and follow up reliability are factors clinicians consider in treatment decision making but are also absent from the NCDB. Despite these shortcomings, our study is the largest known analysis examining patterns of care and outcomes in patients with metastatic melanoma receiving combination IO and intracranial radiotherapy.
Conclusions
For melanoma patients contraindicated to SRS and otherwise appropriate for WBRT, immunotherapy alone may be a potential therapeutic option in asymptomatic patients. For patients with more limited intracranial disease that are eligible for SRS, combination therapy appears to provide better outcomes. Results of ongoing prospective studies will continue to provide more guidance in the combination of immunotherapy and radiation for this patient population.
References
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Tawbi HA, Forsyth PA, Algazi A et al (2018) Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 379:722–730
Long GV, Atkinson V, Lo S et al (2018) Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 19:672–681
Hodi FS, O'Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Postow MA, Chesney J, Pavlick AC et al (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–2017
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619
Stokes WA, Bronsert MR, Meguid RA et al (2018) Post-treatment mortality after surgery and stereotactic body radiotherapy for early-stage non-small-cell lung cancer. J Clin Oncol 36:642–651
Cox DR (1972) Regression models and life- tables. J R Stat Soc 34:187–220
D'Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17:2265–2281
Meier ELKP (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Doss LL, Memula N (1982) The radioresponsiveness of melanoma. Int J Radiat Oncol Biol Phys 8:1131–1134
Espenel S, Vallard A, Rancoule C et al (2017) Melanoma: last call for radiotherapy. Crit Rev Oncol Hematol 110:13–19
Fertil B, Malaise EP (1985) Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys 11:1699–1707
Mohamad O, de Leon AD, Schroeder S et al (2018) Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy. Oncoimmunology. 7:e1440168
Dyer MA, Arvold ND, Chen YH et al (2014) The role of whole brain radiation therapy in the management of melanoma brain metastases. Radiat Oncol 9:143
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141
Zindler JD, Bruynzeel AME, Eekers DBP, Hurkmans CW, Swinnen A, Lambin P (2017) Whole brain radiotherapy versus stereotactic radiosurgery for 4–10 brain metastases: a phase III randomised multicentre trial. BMC Cancer 17:500
Stereotactic Radiosurgery Compared with hippocampal-avoidant whole brain radiotherapy (HA-WBRT) plus memantine for 5–15 brain metastases. https://ClinicalTrials.gov/show/NCT03550391. Accessed 11 Oct 2019
Soon YY, Tham IW, Lim KH, Koh WY, Lu JJ (2014) Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev 2014:CD009454
Hong AM, Fogarty GB, Dolven-Jacobsen K et al (2019) Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: a multicenter, randomized phase III trial. J Clin Oncol 37:3132–3141
Goldberg SB, Gettinger SN, Mahajan A et al (2016) Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17:976–983
Margolin K, Ernstoff MS, Hamid O et al (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13:459–465
Lanier CM, Hughes R, Ahmed T et al (2019) Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neurooncol Pract 6:402–409
Minniti G, Anzellini D, Reverberi C et al (2019) Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer 7:102
Gabani P, Fischer-Valuck BW, Johanns TM et al (2018) Stereotactic radiosurgery and immunotherapy in melanoma brain metastases: patterns of care and treatment outcomes. Radiother Oncol 128:266–273
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors involved have nothing to disclose and no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
White, R.J., Abel, S., Horne, Z.D. et al. Melanoma brain metastases: is it time to eliminate radiotherapy?. J Neurooncol 149, 27–33 (2020). https://doi.org/10.1007/s11060-020-03485-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03485-w