Abstract
Introduction
Surgical resection of high-grade brainstem gliomas is challenging and treatment mostly involves radiation and chemotherapy. In this study, we utilized registry data to determine prognostic features and impact of chemotherapy and radiation on overall survival.
Methods
The National Cancer Database was queried from 2006 to 2015 for adult cases with histologically confirmed high-grade brainstem glioma. Covariates including patient demographics, comorbidities, tumor characteristics and treatment parameters were captured. Multivariable Cox proportional hazards regression was performed to identify predictors of survival.
Results
A total of 422 patients were analyzed. Most patients (66.6%) underwent postoperative radiation with chemotherapy, 9.2% underwent radiation alone, while the remaining had no postoperative treatment (24.2%). Overall median survival was 9.8 months (95% CI 8.8–12). Survival was longer (p < 0.001) in the radiation + chemotherapy group (median: 14.2 months, 95% CI 11.7–17.1) compared to radiation alone (median: 5.7 months, 95% CI 3.7–12) and no postoperative treatment (median: 1.8 months, 95% CI 1.4–4) groups. In multivariable analysis, increasing age was associated with worse survival (HR: 1.87, 95% CI 1.47–2.37, p < 0.001), whereas radiation + chemotherapy was associated with lower mortality compared to radiation alone (HR: 0.67, 95% CI 0.46–0.98, p = 0.038). In subgroup analysis, postoperative chemotherapy with radiation was associated with significant survival benefit compared to radiation alone for grade IV (HR: 0.46, 95% CI 0.28–0.76, p = 0.003), but not for grade III tumors (HR: 0.87, 95% CI 0.48–1.58, p = 0.65).
Conclusion
Analysis from a national registry illustrated the effectiveness of radiation with chemotherapy for adult patients with high-grade brainstem gliomas, particularly grade IV. Further research should identify specific patient profiles and molecular subgroups that are more likely to benefit from multimodality therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In contrast to their pediatric counterpart, brainstem gliomas are relatively rare central nervous system (CNS) tumors in adults, comprising 1.5–2% of all brain tumors [1, 2]. Infiltrative gliomas represent the most common pathologic subtype, accounting for 45–50% of all lesions [3,4,5,6]. Among them, a World Health Organization (WHO) low grade (grade II) histology is observed in most cases (70%), in contrast to pediatric patients where grade IV gliomas account for 50–60% of all cases [6, 7]. Median survival has been reported to range from 7 years in low-grade tumors to 1 year for higher-grade lesions [3]. Brainstem gliomas may also carry significant morbidity secondary to involvement of the lower cranial nerves, cerebellar peduncles and upper spinal cord.
Compared to infiltrative low grade brainstem gliomas, high grade lesions appear in older adults (age > 40) [3, 4]. Prior studies have identified relevant clinical and radiological predictors of overall survival for high-grade adult brainstem gliomas, including duration of symptoms, contrast enhancement and IDH mutation status [8,9,10]. However, the impact of available treatment modalities on prognosis is less clear. The scope of surgical treatment is limited to stereotactic biopsy, and shunt placement in certain cases with hydrocephalus [9]. The main modality of treatment is radiation; however high-grade brainstem gliomas have been suggested to be highly radio-resistant with only a minority of patients showing clinical improvement [3]. It has been previously proposed that chemoradiation might confer survival benefit compared to patients receiving radiation alone; however, present data are mostly limited to small retrospective case series and are insufficient to draw firm conclusions [8]. To address this knowledge gap, we analyzed data from a national cancer registry in order to determine the impact of chemotherapy and radiation on overall survival of high-grade brainstem gliomas.
Materials and methods
Data source
The National Cancer Database (NCDB) is one of the largest cancer registries in the United States, established in 1989, currently capturing 70% of all newly diagnosed malignancies in the US annually and containing almost 34 million cases from over 1500 hospitals [11]. Data are collected from selected health registries accredited by the American Cancer Society and the Commission on Cancer of the American College of Surgeons [12]. It was developed mainly for surveillance and quality improvement in cancer care and captures a large number of cancer cases with de-identified data. It can be used to identify high risk groups, to study cancer care over time, patterns of care, and related patient outcomes [11, 13]. Data reporting to NCDB has a high degree of standardization; information is abstracted from the patient charts by Certified Tumor Registrars who undergo specialized training for the registry operations. If data is missing, the registrars can reach out to physicians to complete the patient record [14]. The NCDB Participant User File data is de-identified and therefore exempt from Institutional Review Board approval. Furthermore, the American College of Surgeons has executed a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals.
Inclusion and exclusion criteria
The NCDB registry was queried for all adult patients (i.e. age ≥ 18) with a histologically-confirmed diagnosis of high-grade brainstem glioma (grade III or IV) between 2006 and 2015. Cases were identified using the International Classification of Disease for Oncology, 3rd edition (ICD-O-3) pathology codes [9401, 9440, 9441, 9442, 9451 (Supplemental Table 1)] in combination with the topology code designating tumor location in the brainstem (C71.7). Diffuse intrinsic gliomas were out of the scope of this study. The codes have been previously shown to be accurate in capturing primary high-grade brain tumors [15]. For this study, patients who were deemed too sick to receive surgery (n = 12), radiation (n = 5) or chemotherapy (n = 13), as well as those who received palliative treatment (n = 27) or for whom information about treatment was missing (n = 13), were excluded from the analysis.
Primary outcome
The primary outcome measure was duration of survival at last follow-up, defined as the timeframe from time of diagnosis until death or censoring due to loss to follow-up or administrative limitations.
Covariates
Data regarding the following variables were also collected: (i) patient demographics: age, sex, race, Charlson-Comorbidity Index (CCI; 0, 1, 2, 3+), zip-code household income (stratified into four categories: < $38,000, $38,000–$47,999, $48,000–$62,999 and > $63,000), insurance status and distance between residence and the treatment facility, (ii) tumor characteristics: size in mm (defined as the largest tumor diameter on imaging, typically based on contrast enhancement) and histology; (iii) hospital characteristics: type of facility type based on designation from the Commission on Cancer [community cancer programs, comprehensive community cancer programs, academic/research facilities and integrative network cancer care programs (definitions provided in Supplemental Table 2)] and U.S. census region of reporting facility; (iii) treatment parameters: receipt of radiation + chemotherapy, radiation dosage, single- vs multiple-agent chemotherapy, days from diagnosis to starting treatment. The number of patients who received chemotherapy alone was extremely small (n = 5) precluding meaningful analysis, and were therefore excluded from the final analysis.
Statistical analysis
Descriptive statistics (medians with interquartile ranges for continuous variables; frequencies with proportions for categorical variables) are presented. Outcome was examined in an as-treated fashion. Cox proportional hazards regression models were constructed in order to evaluate the effect of treatment on overall survival adjusting for age, sex, race, CCI, insurance type and tumor size. Kaplan–Meier survival curves for different treatment groups were constructed and compared using the log-rank test. Assumptions of proportional hazards were evaluated by examining the Schoenfeld residuals and log–log plots of survival against time. When significant interactions were found, those interaction terms were included in the final model at time-dependent covariates. Collinearity among all independent variables was evaluated with the variance inflation factor. Coefficient for age was calculated using the interquartile range effect (i.e. change in risk of death for 75th vs 25th percentile), which allows for modeling nonlinear relationship of the predictor with the outcome of interest.
The interaction term between tumor grade and treatment received was statistically significant (p = 0.038). Therefore, we performed subgroup analysis in order to investigate the differential effect of radiation + chemotherapy within grade III and grade IV tumors. Finally, missing variables were imputed using multiple imputation from the rms package, which employs a combination of additive regression, bootstrapping and predictive mean matching [16]. A total of ten imputed datasets were created and imputation-specific coefficients were subsequently pooled to produce a single result. Given anaplastic oligodendrogliomas have distinctly different molecular phenotype from astrocytomas, analysis was repeated excluding those cases; however, results were found to be similar and therefore not shown.
Statistical analysis was performed using R Statistical Computing software version 3.1.2 (Vienna, Austria; https://www.R-project.org/). p values < 0.05 were considered statistically significant.
Results
Baseline characteristics
A total of 422 patients met criteria and were included in the analysis (Table 1). Median age was 51 years (IQR: 36–62.5) and 60% were males (n = 254). The majority of patients were Whites (n = 352, 85%) and were covered by private insurance (n = 260, 62%). Approximately 9% of patients were Hispanics (n = 35). The most common category of median zip-code household income was ≥ $63,000 (n = 141, 34%), followed by $48,000–$62,999 (n = 128, 30%). In terms of overall health status, most patients did not have other Charlson Index comorbidities (n = 317, 75%). Academic center comprised the most frequent facility type (n = 178, 60%) and the most common facility region was the South (n = 116, 39%), followed by the Midwest (n = 72, 24%). Median distance travelled was 18 miles (IQR: 7–53), while median follow-up was 9.3 months (IQR: 3.6–21).
The overwhelming majority of patients were diagnosed with glioblastoma (n = 261, 62%) or anaplastic astrocytoma (n = 152, 36%) (Table 2). Median tumor size was 27 mm (IQR: 21–37). With regard to treatment received, most patients underwent both radiation + chemotherapy (n = 281, 66%), whereas 24% of patients (n = 102) did not receive either (Table 2). Finally, thirty-nine patients (9%) underwent radiation alone. The most common chemotherapeutic regimen consisted of a single-agent (n = 253, 91%). Median time to radiation or chemotherapy was 26 (IQR: 17–40) and 27 (IQR: 18–43) days, respectively. Median radiation dosage was 54 Gy (IQR: 45–58).
Radiation with chemotherapy vs radiation alone
Significant differences were observed between the three cohorts in regards to age, with patients receiving radiation + chemotherapy (median: 47 years, IQR [35–60]) or radiation alone (median: 49 years, IQR [4–62]) being younger compared to those that did not receive any postoperative treatment (median: 61 years, [QR: 46–71]). Significant differences were also observed with regard to insurance status (overall p < 0.001), CCI (overall p = 0.007) and distance travelled (overall p = 0.048). The distribution of tumor histology was also significantly different between the three cohorts (overall p < 0.001); glioblastoma was found to be more frequent in radiation + chemotherapy (62%), whereas anaplastic astrocytoma was more common in the radiation alone group (51%).
Survival analysis
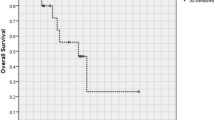
Median overall survival was 9.8 months (95% CI 8.8–12). We observed significant difference between the three groups (p < 0.001); survival was significantly longer in the radiation + chemotherapy cohort (median: 14.2 months, 95% CI 11.7–17.1), followed by radiation alone (median: 5.7 months, 95% CI 3.7–12) and no postoperative treatment (median: 1.8 months, 95% CI 1.4–4). Survival curves for the different treatment groups are shown in Fig. 1a. Furthermore, patients with grade IV tumors had significantly shorter survival (median: 8.9 months, 95% CI 7.7–10.2) compared to those with grade III tumors (median: 14.2 months, 95% CI 10.3–18.8) (Supplemental Fig. 1). No difference was observed with regard to chemotherapeutic regimen employed (single: 13.7 [95% CI 11.2–17.5] vs multiple 14.2 [95% CI 10.5–21.9], p = 0.39) (Supplemental Fig. 2).
In multivariable analysis (Table 3), increasing age (HR 1.87, 95% CI 1.47–2.37, p < 0.001) was associated with worse survival, whereas radiation + chemotherapy (HR 0.67, 95% CI 0.46–0.98, p = 0.038) was associated with lower hazards of death compared to radiation alone. Sex, insurance, Charlson Comorbidity Index and tumor size were found to have no significant effect on mortality.
Subgroup analysis by tumor grade
Similar survival patterns were noted within grade III and grade IV cases. In grade IV group, patients undergoing radiation + chemotherapy lived significantly longer (median: 12.4, 95% CI 9.6–15.8) compared to those that received radiation alone (median: 4.6 months, 95% CI 3.6–12.0) or no treatment (median: 1.4 months, 95% CI 1.1–2.5) (p < 0.001) (Fig. 1b). Within grade III tumors, patients undergoing radiation + chemotherapy also lived significantly longer (median: 17.3, 95% CI 13.4–25.1) compared to radiation alone (median: 5.9 months; 95% CI 3.2–40.9) and no postoperative treatment (median: 4.3, 95% CI 2.4–29.7) (p = 0.01) (Fig. 1c). In multivariable proportional hazards analysis, the effect of postoperative chemotherapy with radiation remained significant for grade IV (HR 0.46, 95% CI 0.28–0.76, p = 0.003), but not for grade III tumors (HR 0.87, 95% CI 0.48–1.58, p = 0.65) (Table 3). However, the hazard ratio of no treatment versus radiation alone was non-significant (HR 1.25, 95% CI 0.82–1.89, p = 0.30).
Discussion
In the present analysis, we sought to investigate the role of chemotherapy and radiation in adult patients with histologically-confirmed high-grade brainstem gliomas. Overall, we found that age and type of treatment received were significant predictors of overall survival. However, we found that the impact of treatment was different for grade III and grade IV tumors. Among grade IV tumors, radiation + chemotherapy was associated with significant survival benefit compared to radiation alone, while radiation by itself did not improve survival as compared to no treatment. Among grade III tumors, radiation conferred a significant survival benefit compared to no treatment on adjusted analysis.
We also did not have sufficient evidence to indicate if the addition of chemotherapy to radiation improves survival for grade III tumors. The unadjusted analysis showed longer median survival (17.3 months vs 5.9 months), however the adjusted hazard ratio for mortality was not significant (HR 0.87, 95% CI 0.48–1.58, p = 0.65). This may be have been observed due to the limited number of patients with grade III tumors receiving radiation alone compared to those undergoing chemoradiation. Interim findings from the CATNON trial have indicated that addition of temozolomide to radiation significantly improved 5-year survival in grade 3 astrocytomas, even in 1p/19q non co-deleted tumors which have lower chemosensitivity [17]. However, we did not have information on the specific chemotherapeutic agent used for patients included in our cohort, although standard practice would suggest most cases receive temozolomide.
Our findings are similar to a retrospective single-institutional series of adult brainstem gliomas (n = 143) by Theeler et al. [8] The authors showed that patients with brainstem glioblastomas (grade IV, n = 28) treated with the Stupp regimen (concurrent radiation and chemotherapy followed by adjuvant temozolomide) had significantly longer survival (median OS: 23 months) as compared to those who were treated with radiation alone (median OS: 4 months). However, only an unadjusted analysis was performed and the study was limited by a small sample size. The authors also observed a significantly longer survival for patients in their series, as compared to our analysis, both for grade III (median OS: 21 vs 14 months) and grade IV tumors (median OS: 14.8 vs 8.9 months). This discrepancy may be attributed to younger median age (36 vs 50 years) and facility type in that study (i.e. single integrated network cancer program). Most patients in the series (n = 118) also had field radiotherapy (median dose: 54 Gy) included as part of their treatment regimen, including all cases where a biopsy was not performed (n = 42) and diagnosis of brainstem glioma was made only radiographically. In another single-arm retrospective study of 15 patients with recurrent “low-grade” adult brainstem gliomas, treatment with temozolomide at the time of tumor progression resulted in radiographic responses in 40% of patients with median PFS and OS of 9.5 and 14.4 months respectively [9]. Limited evidence in the form of small case series and case reports have also pointed to clinical and radiographic improvement following antiangiogenic therapy such as bevacizumab or apatinib [18, 19]. This may suggest that both temozolomide and bevacizumab may be beneficial in carefully selected adult patients with brainstem gliomas.
The differential effect of chemotherapy and radiation in grade III versus grade IV adult brainstem gliomas is also important to consider from a surgical management standpoint. It suggests that a biopsy should be attempted whenever feasible, to ascertain the histological grade and set treatment expectations. MRI alone is suggested to have a low diagnostic accuracy for differentiating between high and low-grade brainstem lesions [20]. With improvement in imaging techniques, guided stereotactic brainstem biopsies have become relatively safe with low perioperative morbidity (1.4–2.5%) and rare mortality (0.6%) [21, 22].
Our analysis also suggested that increasing age at diagnosis and higher histological grade were associated with shorter overall survival. These results are in tandem with prior analyses investigating prognostic markers of overall survival in brainstem gliomas [8,9,10, 23, 24]. Effect of age is important to note, since patients receiving radiation + chemotherapy were likely to be younger and this may have improved survival in this group. Multivariable analysis adjusting for age still suggested that the survival benefit was significant for grade IV tumors. Tumor size and Charlson Comorbidity Index did not correlate with overall survival. These results might be due to inconsistency in what was measured—T2 lesion vs enhancing lesion, unmeasured confounding in tumor characteristics carrying prognostic importance, such as radiological features (contrast enhancement, infiltrating vs focal) and relative involvement of brainstem structures (pons vs midbrain vs medulla). We did not observe significant differences with regard to racial or insurance status; nevertheless, investigating the impact of socioeconomic differences on survival of patients with high-grade brain tumors is extremely important and we therefore encourage continued research in future studies.
Strengths and limitations
The present analysis represents the largest study to date investigating markers of overall survival in high-grade adult brainstem gliomas. National databases allow pooling of a large number of cases which may not be otherwise feasible in single-institutional studies. However, there are limitations as well. First, the NCDB only captures data from CoC-accredited hospitals, so the results are not population-based. Whether centralization of care is associated with improved outcomes for this challenging pathology also remains to be elucidated in future studies. Second, there may be residual confounding as the data lacks granularity on important clinical information, such as specific radiation and chemotherapeutic regimens used. Information about recurrence is also not available in the database, thereby precluding analysis of progression-free survival, limiting any inferences about salvage therapies such as bevacizumab. Third, we could not adjust for other important radiological features which might be relevant for survival analysis-such as contrast enhancement and infiltrating versus focal nature of the lesion. Fourth, there were significant amount of missing data for the Karnofsky performance scale and extent of resection with surgery [24]. The latter might be less important, as surgical treatment of brainstem gliomas almost universally involves a stereotactic biopsy or subtotal resection, given the significant associated surgical morbidity of attempted gross total resection [25]. That said, some brainstem gliomas may be classified as such based on extension of a primarily cerebellar lesion into the brainstem—something we are unable to evaluate in this data. Fifth, there was no information of molecular markers such as MGMT and IDH mutations, although it has been suggested that IDH mutations are very rare in brainstem gliomas (6–8%) [8, 26]. Whether there is a modifying effect of these markers on response to treatment remains to be clarified in future studies. Sixth, there is always a risk for grade misclassification based on sampling approach and adequacy. Finally, despite shorter survival with radiation than chemoradiation in grade 4 lesions, the no-treatment group did demonstrate a tail of long-term survivors. Further evaluation will be needed to ascertain whether this finding results from an unforeseen confounder or represents a biologically meaningful observation. In multivariable analysis, patients that received no treatment were also observed to have slightly lower hazard of death compared to radiation alone, albeit statistically non statistically significant. This could potentially be attributed to the relatively small number of patients with grade IV tumors receiving radiation alone (n = 21), thereby decreasing statistical power to detect survival benefit in favor of radiation.
Conclusions
The findings of the present analysis illustrate the effectiveness of radiation with chemotherapy for adult patients with high-grade brainstem gliomas, particularly WHO grade IV. Further research should aim on identifying specific patient profiles and molecular subgroups in this specific location that are more likely to benefit from multimodality therapy.
References
White HH (1963) Brain stem tumors occurring in adults. Neurology 13:292–300
Eisele SC, Reardon DA (2016) Adult brainstem gliomas. Cancer 122:2799–2809
Guillamo JS, Monjour A, Taillandier L et al (2001) Brainstem gliomas in adults: prognostic factors and classification. Brain 124:2528–2539
Landolfi JC, Thaler HT, DeAngelis LM (1998) Adult brainstem gliomas. Neurology 51:1136–1139
Schumacher M, Schulte-Mönting J, Stoeter P et al (2007) Magnetic resonance imaging compared with biopsy in the diagnosis of brainstem diseases of childhood: a multicenter review. J Neurosurg 106:111–119
Rineer J, Schreiber D, Choi K, Rotman M (2010) Characterization and outcomes of infratentorial malignant glioma: a population-based study using the Surveillance Epidemiology and End-Results database. Radiother Oncol 95:321–326
Salmaggi A, Fariselli L, Milanesi I et al (2008) Natural history and management of brainstem gliomas in adults. J Neurol 255:171–177
Theeler BJ, Ellezam B, Melguizo-Gavilanes I et al (2015) Adult brainstem gliomas: correlation of clinical and molecular features. J Neurol Sci 353:92–97
Reyes-Botero G, Laigle-Donadey F, Mokhtari K et al (2014) Temozolomide after radiotherapy in recurrent “low grade” diffuse brainstem glioma in adults. J Neurooncol 120:581–586
Ueoka DI, Nogueira J, Campos JC et al (2009) Brainstem gliomas–retrospective analysis of 86 patients. J Neurol Sci 281:20–23
Mohanty S, Bilimoria KY (2014) Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol 109:629–630
Commission on Cancer. In: American College of Surgeons. https://www.facs.org/quality-programs/cancer/coc. Accessed 25 Jan 2018
Brown DA, Himes BT, Kerezoudis P et al (2018) Insurance correlates with improved access to care and outcome among glioblastoma patients. Neuro Oncol 20:1374–1382
Bilimoria KY, Stewart AK, Winchester DP, Ko CY (2008) The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15:683–690
Castillo MS, Davis FG, Surawicz T et al (2004) Consistency of primary brain tumor diagnoses and codes in cancer surveillance systems. Neuroepidemiology 23:85–93
Harrell F (2016) Regression modeling stratigies. R package version 4.4-2
van den Bent MJ, Baumert B, Erridge SC et al (2017) Interim results from the CATNON trial (EORTC study 26053–22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet 390:1645–1653
Yu D, Han G, Liu H et al (2019) Treatment of adult brainstem glioma with combined antiangiogenic therapy: a case report and literature review. Onco Targets Ther 12:1333–1339
Moriya S, Ohba S, Adachi K et al (2018) A retrospective study of bevacizumab for treatment of brainstem glioma with malignant features. J Clin Neurosci 47:228–233
Rachinger W, Grau S, Holtmannspötter M et al (2009) Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry 80:1134–1139
Rajshekhar V, Chandy MJ (1995) Computerized tomography-guided stereotactic surgery for brainstem masses: a risk-benefit analysis in 71 patients. J Neurosurg 82:976–981
Hamisch C, Kickingereder P, Fischer M et al (2017) Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: a systematic review and meta-analysis of 735 cases. J Neurosurg Pediatr 20:261–268
Kesari S, Kim RS, Markos V et al (2008) Prognostic factors in adult brainstem gliomas: a multicenter, retrospective analysis of 101 cases. J Neurooncol 88:175–183
Reithmeier T, Kuzeawu A, Hentschel B et al (2014) Retrospective analysis of 104 histologically proven adult brainstem gliomas: clinical symptoms, therapeutic approaches and prognostic factors. BMC Cancer 14:115
Reyes-Botero G, Mokhtari K, Martin-Duverneuil N et al (2012) Adult brainstem gliomas. Oncologist 17:388–397
Ellezam B, Theeler BJ, Walbert T et al (2012) Low rate of R132H IDH1 mutation in infratentorial and spinal cord grade II and III diffuse gliomas. Acta Neuropathol 124:449–451
Funding
None.
Author information
Authors and Affiliations
Contributions
PK: Conceptualization and design, data collection, analysis and drafting of manuscript. AG: Conceptualization and design, drafting of manuscript. VML: reviewing and revising original draft. MAA: reviewing and revising original draft. MB: reviewing and revising original draft. SK: study supervision, reviewing and revising original draft. TB: study supervision, reviewing and revising original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2019_3374_MOESM3_ESM.tiff
Electronic supplementary Figure 2—Kaplan-Meier Survival curves for different chemotherapy groupsacross grade III and IV tumors (TIFF 66 kb)
Rights and permissions
About this article
Cite this article
Kerezoudis, P., Goyal, A., Lu, V.M. et al. The role of radiation and chemotherapy in adult patients with high-grade brainstem gliomas: results from the National Cancer Database. J Neurooncol 146, 303–310 (2020). https://doi.org/10.1007/s11060-019-03374-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03374-x