Abstract
Background
For several types of cancer, biological differences and outcome disparities have been documented in adolescents/young adults (AYAs, 15–39 years old) versus children. This study compared clinicopathological features and survival between younger AYAs and children with low-grade glioma (LGG), a common brain tumor among AYAs.
Methods
This was a secondary analysis of Children’s Oncology Group legacy study CCG-9891/POG-9130, which enrolled participants 0–21 years of age with newly-diagnosed LGG treated with surgery alone. For analysis, participants were categorized as children (0–14 years old) or early AYAs (eAYAs, 15–21 years old) and compared on demographics, clinical presentation, tumor characteristics, surgical outcomes, progression-free survival (PFS) and overall survival (OS).
Results
Among 468 children and 50 eAYAs, more eAYAs presented with seizures (34.0% vs. 19.2%; p = 0.015), without other significant differences in clinicopathological features. 5-year PFS rates for children and eAYA were 80.2% (95% confidence interval [95% CI], 76.1–83.7) and 83.0% (95% CI 68.8–91.1), respectively; 5-year OS rates were 97.3% (95% CI 95.2–98.5) and 95.4% (95% CI 82.7–98.8), respectively. Multivariable analysis including all participants showed presence of residual tumor to be an independent predictor of PFS (< 1.5 cm3, hazard ratio [HR] 5.93 [95% CI 3.45–10.18]) and (≥ 1.5 cm3, HR 8.38 [95% CI 4.75–14.79]) (p < 0.001), while midline-chiasmatic location (HR 9.69 [95% CI 3.05–30.75], p < 0.001) and non-pilocytic astrocytoma histology (HR 6.77 [95% CI 2.35–19.49], p < 0.001) were independent predictors of OS.
Conclusion
Unlike several other cancers, LGG has similar presenting features and survival for both eAYAs and children. This support continuing a unified treatment approach and enrollment of eAYAs in pediatric clinical trials for LGGs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adolescents and young adults (AYA, 15–39 years of age) are a population recognized by the United States (US) National Cancer Institute as facing critical, age-related challenges in cancer care and research compared with children and older adults [1]. Inferior survival for AYAs compared with children has been reported for several cancer types, including acute lymphoblastic and myeloid leukemia, soft tissue sarcoma, osteosarcoma, and Ewing sarcoma [2,3,4,5]. Additionally, excess frequency and severity of treatment-related toxicity has been documented among AYAs [6, 7]. Although these disparities remain poorly understood, they are indicators of potential differences in cancer and host biology, receipt of appropriate therapy, and health behaviors among AYAs. Knowledge about these disparities is important for improving clinical care and informing additional research in this at-risk population [8, 9].
Low-grade glioma (LGG) constitutes a heterogeneous group of tumors that occurs in all age groups. LGG has a peak incidence between the second and fourth decades of life and is one of the most common brain tumors among older adolescents [10]. However, little is known about the biology and clinical behavior of LGG among AYAs. In young children versus older adults, LGGs exhibit contrasting biological and clinical features [9]. Most pediatric LGG are characterized by BRAF and other RAS/RAF pathway alterations, are unlikely to transform to high-grade glioma, and are associated with excellent long-term overall survival (OS) [11]. In contrast, RAS/RAF pathway alterations are rare in adult LGG, which is typified by IDH1/2 and ATRX mutations/alterations, a high incidence of malignant transformation, and a more guarded prognosis [9, 12, 13]. Whether LGG among AYAs has more in common with pediatric or adult LGG phenotype, or whether it is a third biological entity distinct from both, has not been established [14]. This question is of pressing clinical importance, as the therapeutic approaches for adult and pediatric LGGs are divergent, including the choice between chemotherapy and radiation therapy. Radiation therapy, in particular, is commonly used in adults but only rarely in children with LGG [10, 11].
The Children’s Oncology Group (COG) legacy study CCG9891/POG9130 was a prospective observational cohort study of patients aged 0–21 years with newly-diagnosed LGG treated only with surgical resection. This study design afforded a unique opportunity to explore potential differences in the biological behavior of LGG across the age spectrum of infancy to young adulthood. The primary aim of this secondary analysis was to compare the clinicopathological features and survival of early adolescents and young adults (eAYA, 15–21 years old) and children (0–14 years old) who participated in the original clinical trial.
Methods
CCG-9891/POG-9130
CCG-9891/POG-9130 was a prospective cohort study conducted by the Children’s Cancer Group (CCG) and Pediatric Oncology Group (POG) from October 1991 to August 1996 at participating sites in the US and Canada. The study design has been previously described [15]. Key eligibility criteria included age 0–21 years, newly-diagnosed and pathologically-confirmed LGG (WHO grade I or II), and treatment consisting only of surgical resection followed by observation. Eligible patients were enrolled after tumor biopsy/resection and followed until they met off-study criteria (entry onto another CCG/POG therapeutic study, confirmation of being lost to follow-up, or death). Participants could have tumors in any intracranial location except intrinsic brainstem tumors (medulla, pons, midbrain) or isolated optic nerve tumors without involvement of the chiasm.
Exclusion criteria included neuro-ocular-cutaneous syndromes other than neurofibromatosis, multiple sites of disease within the central nervous system (CNS), other major progressive illness, other cancers, or having been previously treated for cancer. Gross total resection was defined as absence of residual tumor by operative report and postoperative imaging. Those with residual tumor were further classified as having < 1.5 cm3 (near-total resection) or ≥ 1.5 cm3 (subtotal resection) of residual tumor.
Current study
The current study is a retrospective analysis of the CCG9891/POG9130 dataset. All participants eligible for the primary study were included in this analysis. For the purposes of this study, participants were categorized as children (age 0–14 years) or eAYA (age 15–21 years) at the time of study entry. The following parameters were compared: sex, race/ethnicity, histopathological features, tumor location, seizure at presentation, level of consciousness at presentation, extent of resection, surgical complication, progression-free survival (PFS) and OS.
Statistical methods
Pearson Chi square test was performed to compare the proportional frequency of clinicopathological features between children and eAYAs. The primary endpoints for analysis of treatment efficacy were PFS and OS, as defined in the original protocol [15]. PFS was measured from the time of enrollment to first disease relapse or progression, death from any cause, or until last follow-up if no progression occurred, whichever occurred first. OS was measured from the time of enrollment to date of death. Patients not experiencing progression of disease or death were censored at the time of last follow-up. Nonparametric PFS and OS survival distributions were estimated by using the Kaplan–Meier method [16]. Point estimates of PFS and OS were reported with 95% confidence intervals (95% CI). The equality of survival functions between eAYAs and children were assessed by the log-rank test.
Associations between risk of progression/death and prognostic factors were assessed using multivariable Cox regression analysis. Based on published results of the multivariable analysis from the primary study (CCG9891/POG9130), an a priori model was used to generate a Cox regression model, with the age variable modified to be < 15 versus 15–21 years, consistent with the aims of this secondary analysis. This is in contrast with the original analysis, which used < 5 and ≥ 5 as the age variable [15]. Results of this analysis were validated using a backward selection multivariable model, where univariate Cox regression was used to estimate the main effect of each potential prognostic factor. Variables with p value < 0.2 from univariate models were considered for inclusion in the multivariable model. Backwards selection with likelihood ratio test was used to generate a Cox regression model with a threshold p value of 0.05 for inclusion in the final model (Supplemental Table 2). A variation of this approach was also used where the age variable < 15 versus 15–21 years was preferentially kept in the model until the final stage.
For all statistical tests, all p values were two-sided with < 0.05 considered significant. All statistical computations were performed using SAS version 9.4 and STATA version 14.
Results
Participant characteristics
A total of 518 participants were assessed (468 children and 50 eAYAs). Participant characteristics are summarized in Table 1. Between the two age groups, there were no differences by sex, race/ethnicity, pathological subtype, tumor location, extent of resection, preoperative level of consciousness, or occurrence of surgical complications. However, seizures at presentation were significantly more common among eAYAs than children (17/50, 34% vs. 90/468, 19%; p = 0.015).
Survival
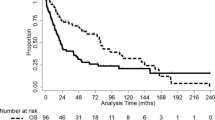
Median follow-up period for participants who were progression-free was 7.4 years. In total, there were 104 progressions (95 children, 9 eAYA) and 16 deaths (13 children, 3 eAYA). As shown in Fig. 1a, b, there was no difference in PFS or OS between the two age groups. When the groups were further stratified by age (0–4, 5–9, 10–14, and 15–21 years), there was still no significant difference in PFS or OS (Fig. 1c, d). Among participants with pilocytic astrocytoma (PA) histology (n = 358 children and 36 eAYAs), there was no difference by age group in PFS and OS (log rank p = 0.27 and 0.64, respectively). The 5-year PFS for children and eAYA was 80.2% (95% CI 76.1–83.7) and 83.0% (95% CI 68.8–91.1), respectively; while the 5-year OS for the groups was 97.3% (95% CI 95.2–98.5) and 95.4% (95% CI 82.7–98.8), respectively.
Results of Cox regression analysis on PFS for all participants combined are summarized in Table 2 and Supplemental Table 1. In univariate analysis, race/ethnicity, history of seizures, level of consciousness at presentation and presence/absence of surgical complications were not associated with PFS. In multivariable analysis, residual tumor was the only independent predictor of PFS. Patients with residual tumor < 1.5 cm3 had almost sixfold, and those with ≥ 1.5 cm3 over eightfold, greater likelihood of disease progression than patients who had no visible tumor on post-operative imaging (Table 2).
Results of Cox regression analysis on OS for all participants combined are summarized in Table 2 and Supplemental Table 1. In univariate analysis, non-White participants had poorer OS when compared to White participants. History of seizures, level of consciousness at presentation and presence/absence of surgical complications were not associated with OS. In multivariable analysis, pathology type and tumor location were independent prognostic factors for OS. In the adjusted analysis, patients with non-PA patients were 6.77 times more likely to die than those with PA. Patients with midline-chiasmatic patients were 9.69 times more likely to die than those with non-midline-chiasmatic tumors (Table 2).
Discussion
In this comparative analysis of children and eAYAs with LGG, we found no significant differences in clinicopathological features or outcomes. To our knowledge, this is the first study of LGG to compare the clinical presentation, tumor location, extent of resection, histopathological characteristics, and survival between these two age groups. The possibility of age-related differences for LGG is an important question because outcome disparities affecting AYAs have been documented for several forms of cancer [2,3,4,5]. Our finding that this is not true for LGG is clinically significant because it supports continued enrollment of eAYAs in pediatric clinical trials for LGGs.
Of approximately 70,000 AYAs diagnosed with cancer annually in the United States, approximately 13,000 have a brain tumor [17, 18]. According to the Central Brain Tumor Registry of the United States (CBTRUS), between 2009 and 2013 a total of 16,653 and 6869 CNS tumors were diagnosed among children aged 0–14 and eAYA aged 15–19 years, respectively [19]. Yet, there remains a paucity of published studies regarding these eAYAs. Here, we explored potential age-related differences between pediatric patients and eAYA patients with LGGs. Of the several variables examined in our study, seizures occurring more commonly amongst eAYAs was the only significant difference. Both tumor location and tumor type are known to influence risk for seizures. Tumors involving the cerebral rather than cerebellar hemispheres, especially the temporal, frontal and parietal lobes, are more associated with seizures. Glioneuronal tumors such as gangliogliomas have also been associated with increased seizure frequency compared to glial-type tumors [20]. In this study, the eAYA group had a higher proportion of tumors that originated from the cerebral hemispheres (36.0% vs. 22.9%) and/or were gangliogliomas (12.0% vs. 7.1%). Even though these differences did not reach statistical significance due to the relatively small sample, the combination may explain the higher incidence of seizures among eAYAs. Importantly, we found no other age-related differences in tumor location, histopathological characteristics, or survival.
When these patients were analyzed in aggregate, certain outcome predictors emerged. As reported in the primary study, tumors in the midline and chiasmatic region, non-PA tumors and the presence of residual tumor were associated with worse PFS and OS on univariate analysis [15]. As part of this secondary analysis, we expanded univariate analysis to include race/ethnicity, history of seizures, level of consciousness at presentation and presence/absence of surgical complications. While most of these factors did not significantly affect PFS or OS, interestingly, race/ethnicity significantly affected OS. Here, non-White race was significantly associated with poorer OS on univariate analysis, but not multivariable analysis. This finding is likely to be multifactorial, where impaired access to care and lower socioeconomic status could be relevant, as both have been associated with more advanced-stage disease in children with CNS tumors, non-CNS solid tumors and melanoma [21,22,23,24]. Future epidemiology studies of LGG with larger and more diverse samples should investigate this potential association more definitively.
Multivariable analysis using the AYA age variable (< 15 vs. 15–21 years) was consistent with results of the primary study previously published by Wisoff et al. (which used age variable < 5 vs. ≥ 5), showing extent of resection to be an independent predictor of PFS. This finding is consistent with previous reports in both children and adults, and one that supports gross total resection as the primary therapeutic goal for pediatric LGGs, when this can be achieved with acceptable functional outcome [15, 25, 26]. Multivariable analysis also showed tumor in the midline and chiasmatic region to be independent predictors of OS. These findings suggest that while the lower resectability of midline and chiasmatic tumors likely contributes to worse PFS, the extent of resectability alone does not completely account for the difference in OS outcomes in these tumors [27, 28]. Lastly, PA histopathology was found to be an independent predictor of OS, consistent with the known biological behaviors of these tumors [11].
Interestingly, eAYA were underrepresented in this study, as evidenced by the almost 10:1 ratio between children and eAYA who participated. Based on the CBTRUS data and incidence of LGGs, this ratio should be closer to 5:1 [19]. This discrepancy exemplifies the pernicious problem of low AYA participation in cancer clinical trials, which likely results from a complex combination of factors operating at the national-, site-, provider-, and patient/family-levels [29]. As our understanding of the biology and treatment of pediatric CNS tumors continues to evolve through molecular testing and development of targeted therapy, poor participation of AYA in clinical trials and resulting lack of biologic specimens may prove to be a significant obstacle in advancing the management of LGGs and other brain tumors in AYAs.
This secondary analysis of legacy COG study has several strengths with some limitations. An important strength is the prospective observational cohort design that followed patients after surgical resection alone, without the addition of adjuvant therapy such as chemotherapy or radiation therapy that could alter outcomes. This provided a unique opportunity to characterize the natural history of LGG across a spectrum of ages. Other notable strengths of this study include the relatively large sample in aggregate, good generalizability of a multicenter cooperative group trial, relatively long follow-up period, and central pathology review of all cases. Limitations of this study include the fact that CCG9891/POG9130 was completed over 20 years ago. Advances in surgical and laboratory techniques could alter diagnostic classification and outcomes. In particular, given that molecular analysis is now widely performed for risk stratification and therapeutic decision making, we would have ideally analyzed all tumor tissues for specific genetic aberrations [30]. However, tumor tissues were not collected as part of the study and therefore unavailable for analysis. Additionally, it is noteworthy that the majority of participants in both age groups were diagnosed with PA, which could be due to the fact that PA has a higher incidence compared to other types of pediatric LGGs and because patients who received only surgical resection were considered evaluable for the study; this may have influenced the survival analysis.
Lastly, because AYAs aged 22–39 are not included in this analysis, our results cannot be readily generalized to that older age group. This would be an important study to replicate among older AYAs because some studies suggest that a significant proportion of older AYA may have adult-type LGGs. One example is seen with diffuse astrocytoma (WHO grade II), which frequently bears an IDH mutation in adults. Interestingly, almost half of the patients diagnosed with IDH-mutant diffuse astrocytoma are between 22 and 39 years of age. In contrast, the IDH mutation is rare in pediatric LGGs (age 0–21), reported to range from 0 to 17% [31, 32]. OS rates for patients aged 22–39 with IDH-mutant diffuse astrocytoma are similar to patients over 39 years of age [33]. This supports the notion that some older AYA have adult-type LGGs and are likely better treated on adult-focused therapeutic protocols. This also emphasizes the importance of further research of biological specimens and molecular genetics, as these studies will be imperative to fully characterize the biological differences between LGGs in the pediatric, AYA and older adult populations.
References
Adolescent and Young Adult Progress Review Group: National Cancer Institute and LiveStrong Young Adult Alliance (2016) Closing the gap: research and care imperatives for adolescents and young adults with cancer. National Institute of Health, Bethesda (NIH Publication 06-6067)
Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, Larson RA, Nachman J, Children’s Cancer Group, Cancer and Leukemia Group B studies (2008) What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood 112:1646–1654. https://doi.org/10.1182/blood-2008-01-130237
Janeway KA, Barkauskas DA, Krailo MD, Meyers PA, Schwartz CL, Ebb DH, Seibel NL, Grier HE, Gorlick R, Marina N (2012) Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children’s Oncology Group. Cancer 118:4597–4605. https://doi.org/10.1002/cncr.27414
Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A (2009) Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol 27:3391–3397. https://doi.org/10.1200/JCO.2008.19.7483
Albritton K, Krailo M, Marina NM (2012) Older age as an independent prognostic factor in Ewing sarcoma treated on Children’s Oncology Group protocols. In: Poster presentation at connective tissue oncology society 2012 annual meeting
Gupta AA, Anderson JR, Pappo AS, Spunt SL, Dasgupta R, Indelicato DJ, Hawkins DS (2012) Patterns of chemotherapy-induced toxicities in younger children and adolescents with rhabdomyosarcoma: a report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Cancer 118:1130–1137. https://doi.org/10.1002/cncr.26358
Gupta AA, Chi YY, Anderson JR, Lyden E, Weigel B, Arndt C, Meyer WH, Rosenberg A, Hawkins DS (2017) Patterns of chemotherapy-induced toxicities and outcome in children and adolescents with metastatic rhabdomyosarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.26479
Barr RD, Ferrari A, Ries L, Whelan J, Bleyer WA (2016) Cancer in adolescents and young adults: a narrative review of the current status and a view of the future. JAMA Pediatr 170:495–501. https://doi.org/10.1001/jamapediatrics.2015.4689
Zapotocky M, Ramaswamy V, Lassaletta A, Bouffet E (2018) Adolescents and young adults with brain tumors in the context of molecular advances in neuro-oncology. Pediatr Blood Cancer 65 https://doi.org/10.1002/pbc.26861
Grier JT, Batchelor T (2006) Low-grade gliomas in adults. Oncologist 11:681–693. https://doi.org/10.1634/theoncologist.11-6-681
Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Marcus KJ, Guo D, Ullrich NJ, Robison NJ, Chi SN, Beroukhim R, Kieran MW, Manley PE (2014) Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer 61:1173–1179. https://doi.org/10.1002/pbc.24958
Bergthold G, Bandopadhayay P, Bi WL, Ramkissoon L, Stiles C, Segal RA, Beroukhim R, Ligon KL, Grill J, Kieran MW (2014) Pediatric low-grade gliomas: how modern biology reshapes the clinical field. Biochim Biophys Acta 1845:294–307. https://doi.org/10.1016/j.bbcan.2014.02.004
Venneti S, Huse JT (2015) The evolving molecular genetics of low-grade glioma. Adv Anat Pathol 22:94–101. https://doi.org/10.1097/PAP.0000000000000049
Nishikawa R (2010) Pediatric and adult gliomas: how different are they? Neuro Oncol 12:1203–1204. https://doi.org/10.1093/neuonc/noq175
Wisoff JH, Sanford RA, Heier LA, Sposto R, Burger PC, Yates AJ, Holmes EJ, Kun LE (2011) Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery 68:1548–1554. https://doi.org/10.1227/NEU.0b013e318214a66e (discussion 1554–1545)
Kaplan EL, Meier P (1958) Nonparametric-estimation from incomplete observations. J Am Stat Assoc 53:457–481. https://doi.org/10.2307/2281868
Johnson DR, Brown PD, Galanis E, Hammack JE (2012) Pilocytic astrocytoma survival in adults: analysis of the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. J Neurooncol 108:187–193. https://doi.org/10.1007/s11060-012-0829-0
Roth ME, O’Mara AM, Seibel NL, Dickens DS, Langevin AM, Pollock BH, Freyer DR (2016) Low enrollment of adolescents and young adults onto cancer trials: insights from the community clinical oncology program. J Oncol Pract 12:e388–e395. https://doi.org/10.1200/JOP.2015.009084
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol 18:v1–v75. https://doi.org/10.1093/neuonc/now207
Ruda R, Bello L, Duffau H, Soffietti R (2012) Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol 14(Suppl 4):iv55–iv64. https://doi.org/10.1093/neuonc/nos199
Austin MT, Hamilton E, Zebda D, Nguyen H, Eberth JM, Chang Y, Elting LS, Sandberg DI (2016) Health disparities and impact on outcomes in children with primary central nervous system solid tumors. J Neurosurg Pediatr 18:585–593. https://doi.org/10.3171/2016.5.PEDS15704
Austin MT, Nguyen H, Eberth JM, Chang Y, Heczey A, Hughes DP, Lally KP, Elting LS (2015) Health disparities are important determinants of outcome for children with solid tumor malignancies. J Pediatr Surg 50:161–166. https://doi.org/10.1016/j.jpedsurg.2014.10.037
Hamilton EC, Nguyen HT, Chang YC, Eberth JM, Cormier J, Elting LS, Austin MT (2016) Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr 175:182–187. https://doi.org/10.1016/j.jpeds.2016.04.068
Kish JK, Yu M, Percy-Laurry A, Altekruse SF (2014) Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Natl Cancer Inst Monogr 2014:236–243. https://doi.org/10.1093/jncimonographs/lgu020
McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, Olivi A, Brem H, Quinones-Hinojosa A (2008) Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 63:700–707. https://doi.org/10.1227/01.NEU.0000325729.41085.73 (author reply 707–708)
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345. https://doi.org/10.1200/JCO.2007.13.9337
Stokland T, Liu JF, Ironside JW, Ellison DW, Taylor R, Robinson KJ, Picton SV, Walker DA (2010) A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro Oncol 12:1257–1268. https://doi.org/10.1093/neuonc/noq092
Fouladi M, Wallace D, Langston JW, Mulhern R, Rose SR, Gajjar A, Sanford RA, Merchant TE, Jenkins JJ, Kun LE, Heideman RL (2003) Survival and functional outcome of children with hypothalamic/chiasmatic tumors. Cancer 97:1084–1092. https://doi.org/10.1002/cncr.11119
Freyer DR, Seibel NL (2015) The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep 3:137–145. https://doi.org/10.1007/s40124-015-0075-y
Liu KW, Pajtler KW, Worst BC, Pfister SM, Wechsler-Reya RJ (2017) Molecular mechanisms and therapeutic targets in pediatric brain tumors. Sci Signal. https://doi.org/10.1126/scisignal.aaf7593
Lassaletta A, Zapotocky M, Bouffet E, Hawkins C, Tabori U (2016) An integrative molecular and genomic analysis of pediatric hemispheric low-grade gliomas: an update. Childs Nerv Syst 32:1789–1797. https://doi.org/10.1007/s00381-016-3163-6
Ryall S, Tabori U, Hawkins C (2017) A comprehensive review of paediatric low-grade diffuse glioma: pathology, molecular genetics and treatment. Brain Tumor Pathol 34:51–61. https://doi.org/10.1007/s10014-017-0282-z
Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, Sahm F, Koelsche C, Korshunov A, Olar A, Hartmann C, Reijneveld JC, Wesseling P, Unterberg A, Platten M, Wick W, Herold-Mende C, Aldape K, von Deimling A (2015) IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol 129:867–873. https://doi.org/10.1007/s00401-015-1438-8
Funding
Research reported in this manuscript was supported by the Children’s Oncology Group and the National Clinical Trials Network (NCTN) of the National Institute of Health under award number U10CA098543, U10CA098413, U10CA180886 and U10CA180899. This study was also funded in part by the Aflac Foundation (ASM, DRF) and the COG Award MA-O: FP000113087_SUB18_01, sub award AYA WO: FP00016518_SUB03_01 (GD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCTN or NIH.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Margol, A.S., Yeo, K.K., Xia, C. et al. A comparative analysis of clinicopathological features and survival among early adolescents/young adults and children with low-grade glioma: a report from the Children’s Oncology Group. J Neurooncol 140, 575–582 (2018). https://doi.org/10.1007/s11060-018-2983-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2983-5