Abstract
Background
Standard glioblastoma therapy is long-lasting. Among second-line therapy, choices could be bevacizumab and nitrosoureas depending on National Agencies approval. There is no consensus on 3rd line therapy or clinical trials specifically designed for this setting.
Methods
We reviewed our institutional database on all consecutive patients who received 3rd line therapy for glioblastoma.
Results
Data on 168 out of 1337 (12.6%) glioblastoma patients who underwent 3rd line therapy treatment were collected. Third line treatments were bevacizumab or chemotherapy (nitrosourea, temozolomide or carboplatin plus etoposide). Median progression free survival was 2.9 months and median survival time was 6.6 months from the start of 3rd line therapy. Bevacizumab significantly improved progression-free survival (4.7 vs. 2.6 months, p = .020) and survival from 3rd line start (8.0 vs. 6.0 months, p = .014) in respect to chemotherapy. Toxicity of grade ≥ 3 occurred in 13.7% of patients. In multivariate analysis, survival in 3rd line treatment depends on MGMT methylation (p = .006) and treatment with Bevacizumab (p = .011).
Conclusions
Third line therapy in selected glioblastoma patients may be feasible and well tolerated. Bevacizumab improved outcome in 3rd line in respect to chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Glioblastoma (GBM) is the most aggressive primary brain tumor given its survival of < 15 months from diagnosis and < 10% patients alive at 5 years. It is also the most common intracranial neoplasm since it represents 15% of them and 45–50% of all primary brain tumors.
The standard of care for newly diagnosed GBM is total surgical resection followed by radiotherapy (RT) plus concomitant and adjuvant temozolomide (TMZ) [1].
There is no univocal consensus on approach at recurrence. If total resection could be achieved, second surgery might be considered [2]. Possible alternatives for medical treatment include bevacizumab (Beva) alone or in combination with other chemotherapy agents [3,4,5,6] in USA, nitrosoureas (NU) such as fotemustine (FTM) or lomustine (CCNU) [7, 8] in Europe, and TMZ rechallenge in selected patients [9, 10].
Studies in literature report 21–62% of GBM patients getting access to 3rd line therapy after a second recurrence [6, 11,12,13,14]. In this setting, there is no defined chemotherapy treatment and no clinical trial assessed the issue of which would be an appropriate approach.
Bevacizumab in recurrent GBM proved to prolong progression free survival, but did not improve overall survival when compared to NUs [5, 6, 15].
We present a study on patients who received 3rd line therapy for recurrent GBM with focus on the role of Beva in this setting, in order to define this population and explore possible suggestions for 3rd line therapy.
Methods
Patients and methods
We analyzed data from our Institutional data warehouse on all consecutive patients who received 3rd line therapy between 2005 and 2016 at the Medical Oncology Department of Bellaria Hospital in Bologna—Italy.
Inclusion criteria were age > 18 years; histology proven glioblastoma; adjuvant treatment with RT plus concomitant and adjuvant TMZ [1]; two further chemotherapy lines.
Histopathology features including O6-methylguanin-DNA-methyltransferase (MGMT) promoter methylation status and clinical data regarding therapy, toxicity and survival were collected and reviewed at the time of the analysis.
The MGMT methylation status had been determined by methylation-specific polymerase chain reaction with primers specific for either methylated or the modified unmethylated DNA [16].
Disease assessment had been conducted by magnetic resonance imaging (MRI) with gadolinium contrast mean and T1-weighted with contrast mean and fluid attenuation inversion recovery (FLAIR) sequences were evaluated. Disease progression was defined according MacDonald criteria [17] by expert neuroradiologists and oncologists. Disease evaluation was reviewed according to RANO—HGG criteria (Response Assessment in Neuro-Oncology—high-grade gliomas) [18] in patients treated with Beva or other antiangiogenic drug.
Local Ethic Committee approved the purposes of files review and data collection in GBM patients with approval number CE09113.
Statistical analysis
Data are reported as median, range and frequencies. Kruskal–Wallis, Fisher’s exact and Pearson’s Chi-squared tests were used. Survival data (median survival times with 95% confidence interval) were computed by Kaplan–Meier procedure and were analyzed by log-rank test and Forward Stepwise Multivariate Cox proportional hazards model. The hazard ratios (HRs) were computed together with their 95% CIs.
Gender, age, extent of surgical resection, MGMT promoter methylation and 3rd line therapy were considered in multivariate analysis.
Primary end-points were progression-free survival and survival time in 3rd line. Survival was calculated from the start of 3rd line therapy to death from any cause (OS3). Progression free survival was calculated as the time between 3rd line treatment start and disease progression or death from any cause (PFS3).
We also evaluated progression-free survival to first (PFS1) and second line (PFS2) and overall survival (OS) from diagnosis. PFS1 was calculated from diagnosis to relapse/progression, PFS2 from the start of second-line to relapse/progression and OS from diagnosis to death from any cause.
The SPSS (Version 13.0 for Windows; SPSS Inc., IL, USA) was used as a statistical package. Two-tailed p-values < 0.05 were considered significant.
Results
Patients demographics and characteristics
One-hundred-sixty-eight patients matched inclusion criteria and were included in the analysis, 115 were male (68.5%) and 53 were female (31.5%) with an M:F ratio of 2.17:1. Demographics baseline data are summarized in Table 1.
Median age was 51 years (range 20–72). Median follow-up time was 105.1 months from diagnosis and 77.4 months from the start of 3rd line therapy. Overall, median OS from diagnosis was 30.3 months.
All patients had diagnosis of GBM based on pathology report and received adjuvant standard of care [1].
The MGMT methylation status was available for 130 patients (77.4%): MGMT was methylated in 65 specimens (mMGMT, 50%) and unmethylated in 65 (nmMGMT, 50%). MGMT status was not available in 38 cases (22.6%): in 33 of them was not performed (19.6%) because of insufficient material, while in 5 of them was not assessable (3%).
Surgery resection was performed in 156 patients (92.9%): total resection was achieved in 63 patients (37.5%), partial resection in 93 (55.4%). Biopsy was performed in 12 patients (7.1%).
Thirty-two out of 168 patients received Beva (Beva group, 19%) and 136 a chemotherapy regimen (Chemo group, 81%): 69 NU (41.1%), 36 TMZ (21.4%) and 31 CBDCA-VP (18.5%).
All patients in the Beva group received the drug at 10 mg/kg dose every 2 weeks, alone (n = 23) or in combination with chemotherapy: irinotecan (n = 5), lomustine (n = 6).
The Beva and the Chemo group were not statistically different in terms of age (51 vs. 50 years, p = .31), gender (65.6 vs. 69.1% male, p = .68), type of surgery (p for heterogeneity = .88), mMGMT rate (37.5 vs. 52.8%, p = .26), PFS1 (11.2 vs. 11.5 months, p = .30) and PFS2 (3.8 vs. 3.6 months, p = .16), respectively, as shown in Table 2.
Progression-free survival
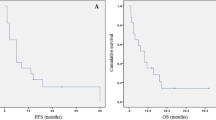
Overall, median PFS3 was 2.9 months: in the Beva and Chemo group, PFS3 was 4.7 and 2.6 months, respectively (p = .020) (Fig. 1).
Patients with mMGMT and nmMGMT showed similar PFS3 (3.5 vs. 2.6 months, respectively; p = .108).
In the Beva group MGMT methylation status was available for 24/32 patients (75%; 9 MGMT methylated). PFS3 was 4.6 months (95% CI 0.4–8.7) and 4.6 months (95% CI 2.2–7.1) in the MGMT methylated and unmethylated group, respectively (p = .516).
At univariate analysis, Beva was associated with reduced risk for progression.
Beva (HR 0.518, 95% CI 0.311–0.864, p = .012) and mMGMT (HR 0.659, 95% CI 0.455–0.957, p = .028) were positive prognostic factors at multivariate analysis.
Overall survival
OS3 was 6.6 months: 8.0 and 6.0 months in the Beva and Chemo group, respectively (p = .014) (Fig. 2).
Patients with mMGMT had better outcome than those with nmMGMT: OS3 was 8.3 vs. 5.6 months (p = .025), respectively.
OS3 was 8.7 months (95% CI 4.7–12.7) and 8.0 months (95% CI 5.6–10.5) in the MGMT methylated and unmethylated, respectively (p = .730).
As showed in univariate and multivariate analyses, a reduced risk for death was associated with mMGMT (HR 0.58; 95% CI 0.39–0.85, p = .006) and 3rd line therapy with Beva (HR 0.48, 95% CI 0.28–0.84, p = .011).
Neither age nor extension of surgery affected survival from diagnosis or from 3rd line treatment start.
Toxicity
Overall, 23 patients (13.7%) experienced grade > 2 toxicity according to Common Terminology Criteria for Adverse Events v4.0 (CTCAE).
In the Beva group, 5 out of 32 patients (15.6%) had G > 2 adverse events: two hypertension, one hyperpyrexia, one pneumonia and one cerebral ischemia (grade 5).
In the chemo group, 18 out of 136 patients (13.2%) experienced G > 2 adverse events: seven hematologic toxicity (five thrombocytopenia, one lymphocytopenia and one neutropenia), four increase in liver enzymes, three deep vein thrombosis, one creatinine increase, one hyperpyrexia, one intracranial hemorrhage (grade 5) and one bowel perforation (grade 5).
Discussion
The analysis of our database suggests that 3rd line treatment in recurrent GBM is feasible for a select group of patients with favorable prognosis. Our study suggested that 3rd line therapy with Beva was associated with longer PFS3 and OS3 than chemotherapy, i.e. a NU, TMZ or CBDCA-VP. Methylated MGMT promoter was a positive prognostic factor for OS, OS3 and PFS3.
The proportion of patients who get access to 3rd line or further treatment is reported to be between 21 and 62%, as found in retrospective and prospective studies in literature [6, 11,12,13,14, 19]. However, this proportion seems overestimated because of the poor prognosis of GBM. In the same period covered by our study, in fact, 1337 consecutive GBM patients underwent standard radiotherapy and concomitant and adjuvant TMZ at our Institution. Thus, 168 patients receiving 3rd line therapy included in this study out of 1552 GBM diagnosis and subsequent standard of care set the proportion to 12.6% only.
The 12.6% of patients receiving 3rd line chemotherapy after second GBM recurrence were young (median age 51 years), had methylation of MGMT (50%) and had at least undergone partial resection (55.4% partial and 37.5% total resections).
Third line GBM treatment has not been specifically studied and all available data derive from retrospective or phase II trials on recurrent GBM, which included small proportions of patients treated beyond second line.
Nitrosoureas are the standard therapy for recurrent GBM, especially where Beva is not approved or available, or after Beva. Nitrosoureas are the standard control arm in studies on recurrent GBM and showed 1.0–3.45 median PFS and 8.0–8.7 median OS [5, 6, 15].
TMZ rechallenge has been explored in prospective and retrospective studies, reporting median PFS ranging from 2 to 9.5 months and OS from 6 to 22 months [7, 11, 14, 20,21,22,23].
Combination regimens with carboplatin plus etoposide had also been used in recurrent GBM [24,25,26], obtaining a median PFS of 2.5–3 months and OS of 3.3–9.0 months.
In the Chemo group (CCNU, FTM, TMZ or CBDCA-VP) we obtained a median PFS3 of 2.6 months and a OS3 of 6.0 months.
Beva has been studied in second or 3rd line treatment in recurrent GBM both as single agent or in combination with other drugs, such as irinotecan or nitrosoureas with reported median PFS of 3.1–6.0 months and survival time of 6.4–9.2 months [3, 4, 12, 27,28,29,30].
Piccioni et al. evaluated 88 patients treated with bevacizumab at second recurrence with median PFS and OS of 4.2 and 9.3 months, respectively [12]. In a recent retrospective analysis, 36 out of 62 patients (58%) received Beva at 3rd line. Overall, PFS and OS were 3.5 and 7.5 months, respectively [30].
Similarly, in the phase II non-randomized study by Kreisl et al., 23 out of 48 patients (48%) received bevacizumab after three or more prior chemotherapies: median PFS was 4.0 months and OS was 7.75 months [3].
In the “BRAIN” randomized phase II trial, about 19–20% of the 167 patients randomized to bevacizumab alone or in combination with irinotecan, were at their second progression. Median PFS were 3.1 and 5.6 months and median survival time 9.2 and 7.0 months, respectively. No significant difference was observed in the “BRAIN” trial if Beva was administered at 2nd or 3rd line: PFS was 4.4 and 5.5 months and OS was 9.1 and 8.7 in Beva single agent or in combination, respectively [4].
The phase II part of the EORTC study 26101 reported data on 82 patients treated with 3rd line Beva after CCNU and 77 treated with 3rd line Beva plus CCNU after Beva: median survival was 6.3 and 5.1 months, respectively [31].
In 3rd line treatment, we obtained similar results: 4.7 months median PFS3 and 8.0 months OS3, which were significantly longer than those obtained by patients treated with chemotherapy in the same setting (2.6 months p = .020 and 6.0 months p = .014, respectively).
As shown in Table 2, we compared the two groups in order to highlight discrepancies which should have affected survival times throughout disease history. The absence of significant differences lead us to think, within the limits of a retrospective analysis, that the two groups are homogenous for known or unknown factors affecting the clinical course before exposure to bevacizumab.
In our series, regardless of therapy, patients who received 3rd line treatment had 7.0 months median OS3 and 3.0 months median PFS3.
Better benefit was seen in mMGMT patients (OS3 8.3 vs. 5.6 months, p = .025; OS 38.1 vs. 21.0 months, p = .002). The MGMT promoter methylation is a well-known prognostic factor in GBM, and confirmed its prognostic value in our series throughout the whole GBM disease history (HR 0.48 for OS), after second progression as well (HR 0.58 for OS3 and 0.66 for PFS3). Nevertheless, in the Beva group no differences were observed in terms of PFS3 or OS3 in MGMT methylated or unmethylated patients.
From a clinical point of view, proposing or not a 3rd line therapy should not depend on age since it did not affect outcome in our series. Third line therapy should be offered especially, but not only, to MGMT methylated patients, because they are more likely to benefit from it, and Beva could be chosen if patient had not received it previously and according to comorbidities, due to the advantage in progression-free and survival time.
Limitations of our study mainly derive from its retrospective nature and design. Collecting data from patients who received 3rd line therapy (168/1337, 12.6%) introduced a selection bias by inclusion of a favorable population on the prognostic side. Features that resemble it were the long overall survival (30.3 months), the relatively young age (51 years) and enriched mMGMT population, which accounted for half of the patients with known MGMT status.
Nevertheless, a prospective trial addressing the issue of 3rd line therapy in GBM to overcome these limitations would be difficult to lead.
In conclusion, our study shows that 3rd line therapy in recurrent GBM patients may be feasible and well tolerated. In this setting, treatment should be offered especially in methylated MGMT patients, and bevacizumab, if feasible, should be proposed due to the advantage in terms of OS3 and PFS3 showed over chemotherapy.
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Brandes AA, Danieli D, Zunarelli E et al (2017) Role of MGMT methylation status at time of diagnosis and recurrence for patients with glioblastoma: clinical implications. Oncologist 22:432–437. https://doi.org/10.1634/theoncologist.2016-0254
Kreisl TN, Kim L, Moore K et al (2008) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745. https://doi.org/10.1200/JCO.2008.16.3055
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. https://doi.org/10.1200/JCO.2008.19.8721
Taal W, Oosterkamp HM, Walenkamp AME et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953. https://doi.org/10.1016/S1470-2045(14)70314-6
Brandes AA, Finocchiaro G, Zagonel V et al (2016) AVAREG: a phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro Oncol 18:1304–1312. https://doi.org/10.1093/neuonc/now035
Wick A, Pascher C, Wick W et al (2009) Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol 256:734–741. https://doi.org/10.1007/s00415-009-5006-9
Brandes AA, Bartolotti M, Tosoni A, Franceschi E (2016) Nitrosoureas in the management of malignant gliomas. Curr Neurol Neurosci Rep 16:13. https://doi.org/10.1007/s11910-015-0611-8
Wick A, Felsberg J, Steinbach JP et al (2007) Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 25:3357–3361. https://doi.org/10.1200/JCO.2007.10.7722
Brandes AA, Tosoni A, Cavallo G et al (2006) Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO). Br J Cancer 95:1155–1160. https://doi.org/10.1038/sj.bjc.6603376
Taal W, Segers-van Rijn JMW, Kros JM et al (2012) Dose dense 1 week on/1 week off temozolomide in recurrent glioma: a retrospective study. J Neurooncol 108:195–200. https://doi.org/10.1007/s11060-012-0832-5
Piccioni DE, Selfridge J, Mody RR et al (2014) Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro Oncol. https://doi.org/10.1093/neuonc/nou028
Wick W, Puduvalli VK, Chamberlain MC et al (2010) Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28:1168–1174. https://doi.org/10.1200/JCO.2009.23.2595
Omuro A, Chan TA, Abrey LE et al (2013) Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol 15:242–250. https://doi.org/10.1093/neuonc/nos295
Wick W, Brandes A, Gorlia T et al (2015) Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 Trial. Neuro Oncol 17:v1.5–v1. https://doi.org/10.1093/neuonc/nov306
Esteller M, Hamilton SR, Burger PC et al (1999) Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia 1. Cancer Res 59:793–797
MacDonald D, Cascino T, Schold SJ, Cairncross J (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Chang SM, Van Den Bent MJ et al (2017) Response assessment in neuro-oncology clinical trials. J Clin Oncol 35:2439–2449. https://doi.org/10.1200/JCO.2017.72.7511
Wiestler B, Radbruch A, Osswald M et al (2014) Towards optimizing the sequence of bevacizumab and nitrosoureas in recurrent malignant glioma. J Neurooncol 117:85–92. https://doi.org/10.1007/s11060-013-1356-3
Franceschi E, Omuro AMP, Lassman AB et al (2005) Salvage temozolomide for prior temozolomide responders. Cancer. https://doi.org/10.1002/cncr.21564
Brada M, Stenning S, Gabe R et al (2010) Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol 28:4601–4608. https://doi.org/10.1200/JCO.2009.27.1932
Perry JR, Bélanger K, Mason WP et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28:2051–2057. https://doi.org/10.1200/JCO.2009.26.5520
Weller M, Tabatabai G, Kastner B et al (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res 21:2057–2064. https://doi.org/10.1158/1078-0432.CCR-14-2737
Franceschi E, Cavallo G, Scopece L et al (2004) Phase II trial of carboplatin and etoposide for patients with recurrent high-grade glioma. Br J Cancer 91:1038. https://doi.org/10.1038/sj.bjc.6602105
Tonder M, Weller M, Eisele G, Roth P (2015) Carboplatin and etoposide in heavily pretreated patients with progressive high-grade glioma. Chemotherapy 60:375–378. https://doi.org/10.1159/000440678
Brandes AA, Basso U, Vastola F et al (2003) Carboplatin and teniposide as third-line chemotherapy in patients with recurrent oligodendroglioma or oligoastrocytoma: a phase II study. Ann Oncol Off J Eur Soc Med Oncol 14:1727–1731
Poulsen HS, Grunnet K, Sorensen M et al (2009) Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol 48:52–58. https://doi.org/10.1080/02841860802537924
Gil MJ, de las Peñas R, Reynés G et al (2012) Bevacizumab plus irinotecan in recurrent malignant glioma shows high overall survival in a multicenter retrospective pooled series of the Spanish Neuro-Oncology Research Group (GEINO). Anticancer Drugs 23:659–665. https://doi.org/10.1097/CAD.0b013e3283534d3e
Schaub C, Schäfer N, Mack F et al (2016) The earlier the better? Bevacizumab in the treatment of recurrent MGMT-non-methylated glioblastoma. J Cancer Res Clin Oncol 142:1825–1829. https://doi.org/10.1007/s00432-016-2187-3
Wenger KJ, Wagner M, You S-J et al (2017) Bevacizumab as a last-line treatment for glioblastoma following failure of radiotherapy, temozolomide and lomustine. Oncol Lett 14:1141–1146. https://doi.org/10.3892/ol.2017.6251
Wick W, Stupp R, Gorlia T et al (2016) Phase II part of EORTC study 26101: the sequence of bevacizumab and lomustine in patients with first recurrence of a glioblastoma. J Clin Oncol. https://doi.org/10.1200/JCO.2016.34.15_suppl.2019
Acknowledgements
We thank patients and their families. Authors declare no conflict of interests. No funding or financial support was received for the realization of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franceschi, E., Lamberti, G., Paccapelo, A. et al. Third-line therapy in recurrent glioblastoma: is it another chance for bevacizumab?. J Neurooncol 139, 383–388 (2018). https://doi.org/10.1007/s11060-018-2873-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2873-x