Abstract
Intraparenchymal epidermoid cysts (IECs) are rare lesions, thus the preoperative diagnosis and proper surgical management are still a challenge. We searched the database at our institution and performed a search of English literature in PubMed and Google Scholar. Keywords used were as follows: “intraparenchymal”; “intracerebral”; “intraaxial”; “epidermoid cyst”; “brainstem”; “cholesteatoma”; “pearly tumor”. Only cases that were true intraparenchymally located and contained adequate clinical information were included. Six cases of IECs were recorded at our institution. Total removal was achieved in all the six patients with good outcomes. 29 cases meeting the above criteria were found in the literature. Including ours, a total of 35 patients were analyzed. Females were more frequently affected (F/M ratio, 1.9:1). Most of them were located in the brainstem (42.9%) and temporal lobe (22.9%). While in children, all were located in the brainstem. 45.2% showed subtle peripheral enhancement on Magnetic Resonance Imaging (MRI), and all appeared hyperintense on Diffusion Weighted Imaging (DWI). In the subgroup of cerebral lobes and cerebellums, total resection was achieved in 89.5%, and they all showed good outcomes. While in the subgroup of brainstem, 46.7% (seven cases) underwent total resection and 50% (three cases) of them died postoperatively. MRI with DWI is helpful in the preoperative diagnosis. Total resection should be achieved for the IECs located in cerebral lobes and cerebellums, while subtotal resection is a wise and safe strategy for the IECs located in the brainstem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epidermoid cysts (ECs) are rare, congenital, slow-growing lesions that develop from ectodermal remnants during neuroembryogenesis [1,2,3]. They account for 0.2–1.8% of all intracranial tumors [4, 5]. Epidermoid cysts are commonly located in the cerebellopontine angle, juxtasellar areas, middle cranial fossa, diploe, and spinal canal [3, 6]. They characteristically spread along anatomical cleavage planes, progressively filling the subarachnoid spaces [2]. However, intraparenchymal epidermoid cysts (IECs) are extremely rare lesions. To the best of our knowledge, only 29 cases of true IECs have been previously reported [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. We present six new cases of IECs and discuss the presentation, radiographic features, preoperative differential diagnosis, and surgical management based on a review of the literature.
Materials and methods
The neurosurgical database at the Second Affiliated Hospital of Zhejiang University School of Medicine, was searched for all surgical cases of intracranial epidermoid cysts between January 2013 and December 2017. A retrospective chart review was then performed with research ethics board approval. The age, sex, image presentation, location, surgical management and follow-up were recorded. All patients underwent CT or MR imaging for diagnosis and surgical planning. Postoperative clinical information and neuroradiological presentation were evaluated.
Additionally, for the review, a search was conducted of the English literature in PubMed and Google Scholar for every case report, series, letter to the editor, original article and literature review related to intraparenchymal epidermoid cysts. In addition, the reference lists of the obtained articles and previous reviews were examined. Keywords used were as follows (single word or combination): “intraparenchymal”; “intracerebral”; “intraaxial”; “epidermoid cyst”; “brainstem”; “cholesteatoma”; “pearly tumor”. Only cases in the English language that were true intraparenchymally located and contained adequate clinical information pertinent to the analysis were included.
Results
Fifty-seven cases of pathologically confirmed intracranial epidermoid cysts were recorded between January 2013 and December 2017 at our institution. Six of them were intraparenchymally located, which were diagnosed as IECs (Table 1). They comprised two men and four women with an average age of 45 years (range 30–53 years). Headache was the most common presenting symptom, and was seen in four cases. Other symptoms and signs included seizure, hypoesthesia and cerebellar ataxia. In one patient (case 5), the lesion was found incidentally. The locations of the IECs varied. We found lesions in the frontal lobe in 2 cases, parietal lobe in 1 case, temporal lobe in 1 case, and cerebellum in 2 cases. Total removal was achieved in all the six patients with good outcome. At follow-up, there were no signs of recurrence clinically, so repeat MRIs were not performed.
The English medical literature in the PubMed and Google Scholar databases was reviewed, and 23 reports concerning 29 cases of true IECs meeting the above-mentioned criteria were included. The characteristics of the 29 cases were summarized in Tables 2 and 3. Including our six patients, a total of 35 cases were analyzed. The reported age range was between 1 and 70 years, with a median age of 30 years. Females were more frequently affected (F/M ratio, 1.9:1). Regarding the locations of IECs, 15 (42.9%) in the brainstem, 8 (22.9%) were in the temporal lobe, 6 (17.1%) in the cerebellum, 2 (5.7%) in the frontal lobe, 2 (5.7%) in the parietal lobe, 1 (2.8%) in the fronto-temporal lobe, and 1 (2.8%) in the thalamus. In children (age ≤ 14 years), all the IECs were located in the brainstem. On Magnetic Resonance Imaging (MRI), 80.0% appear homogeneous or inhomogeneous Cerebrospinal fluid (CSF)-like intensity (14 cases and 10 cases, respectively), 45.2% show subtle peripheral enhancement (14 cases), and 9.7% show slight peritumoral edea (3 cases). Diffusion Weighted Imaging (DWI) was performed in 17 patients, and all appeared hyperintense. Total resection was achieved in 62.9% of the patients (22 cases), and subtotal resection was achieved in 25.7% of the patients (9 cases). In three patients, aspiration was performed first, followed by total resection (2 cases) or subtotal resection (1 case) after recurrence. One patient was underwent partial resection. The follow-up period ranged from 1 month to 142 months (10 cases were not available and were excluded from analysis), 28% of the patients revealed recurrence of IECs (7 cases), and the total mortality rate was 12% (3 cases, 2 of them died after repetitive surgery for recurrence). In the subgroup of total resection, there was no recurrence. While in the subgroup of subtotal resection, the recurrence rate was 44.4% (4 cases), and the median time to recurrence was 11 months. All the three patients underwent aspiration revealed early recurrence of IECs, and the median time to recurrence was 3 months.
In the subgroup of cerebral lobes and cerebellums, total resection was achieved in 89.5% (17 cases), and subtotal resection was achieved in 10.5% (2 cases). All of them showed good outcome (neurological improvement or symptom free) without recurrence at follow-up. While in the subgroup of brainstem, total resection was achieved in 26.7% (4 cases), subtotal resection was achieved in 46.7% (7 cases), aspiration was achieved in 20.0% (3 cases), and partial resection was achieved in 6.7% (1 case). At follow-up, 7 cases developed tumor recurrence, and three of them converted to total resection. Finally, a total of 7 cases underwent total resection, and 50% (3 cases, follow-up of one patient was not available and was excluded from analysis) died after surgery.
Illustrate case presentation
A 43-year-old woman had suffered from headache for 1 year. On admission the neurological examination revealed left leg numbness. Her general status was good, and her past medical history was unremarkable.
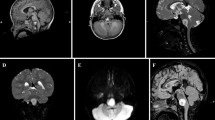
Magnetic resonance imaging showed an intra-axial right parietal lobe mass with a low T1 weighted images (T1WI) signal and a predominantly high T2 weighted images (T2WI) (Fig. 1a, b). There was no surrounding edema or mass effect. Gadolinium-enhanced T1WI demonstrated a slight enhancement of the tumor margin, similar to a cystic astrocytoma or glioma (Fig. 1c–e).
a An axial T1-weighted image showed a hypointense mass in the right parietal lobe. b An axial T2-weighted image showed a predominantly hyperintense mass without surrounding edema. c A contrast-enhanced axial image revealed a slight enhancement of the tumor margin. d A contrast-enhanced sagittal image revealed a slight enhancement of the tumor margin. e A contrast-enhanced coronal image revealed a slight enhancement of the tumor margin
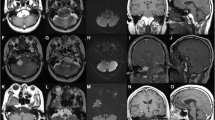
Upon review of the patient’s MRI characteristics, the preoperative diagnosis was low-grade glioma. The patient underwent a right parietal craniotomy. After opening the dura, no abnormalities were seen on the cortical surface. An avascular intra-axial mass was exposed through a posterior central gyrus corticotomy. The cystic lesion was filled with keratinized debris, no hair or obvious calcification or ossification was observed. The intraoperative findings revealed an epidermoid cyst. The contents and the capsule were totally removed.
Histologically, stratified squamous epithelium was found lining the cyst wall, and the cyst contained keratinized debris. No skin appendages were present. The final histopathological diagnosis confirmed epidermoid cyst (Fig. 2).
Postoperatively, the patient recovered well except for mild left leg numbness. The postoperative MRI confirmed tumor resection (Fig. 3a, b). At 1 year follow-up, the patient had no neurological complaints and there were no signs of recurrence.
Discussion
Intracranial epidermoid cysts arise between the third and fifth week of embryonic development from neuroectodermal epithelial remnants that remain after neural tube closure [7, 20, 24]. Epidermoid cysts are composed of an outer cystic capsule, a keratinized stratified squamous epithelial layer, and an inner cystic contents that include tissue debris, keratin, water, and solid cholesterol [3, 20]. ECs grow very slowly via progressive accumulation of desquamated cell debris from the cysts’ capsule [1, 3]. The vast majority of intracranial ECs occur in extraaxial space, while a few intraaxial located epidermoid cysts were reported in the literature, which were named “Intracerebral epidermoid cysts”, “Intraparenchymal epidermoid cysts”, or “Intra-axial epidermoid cysts” [3, 4, 8, 10, 17]. We reviewed 35 cases of this kind, including the reported cases in the English literature and six patients in our hospital, trying to figure out the accurate preoperative diagnosis, and proper surgical management of this rare disease.
The true incidence of IECs is unknown. They account for about 1.5% of intracranial epidermoid cysts in some reports [4, 6, 7]. However, in the present study, we found that IECs account for 10.5% of intracranial epidermoid cysts. It is much higher than that reported in the literature.
The pathogenesis of IECS is still controversial and many hypotheses have been suggested [7, 8]. Two main hypotheses have been propounded:(1) A subpopulation of cells originating from mesectodermal lines that give rise to skin may migrate and remain trapped within the primitive cerebral hemisphere during neural tube closure in the third to fifth weeks of embryogenesis. These trapped cells may develop and differentiate, resulting in an IEC [7, 8, 25]. (2) Proliferation of multipotential embryonic cells in the brain parenchyma [12]. The former hypothesis seems to better explain the occurrence of these rare IECs.
Clinical manifestations of the IECs are related to the volumes and locations of the tumors [10]. The mean age of presentation was significantly different between the subgroup of brainstem IECs and the subgroup of other locations. In the subgroup of brainstem, most of the cases occurred in children and the duration of symptoms was short, which was in contradiction to IECs occurring in other intracranial locations [10, 12, 24]. The common signs include epilepsy, hemiparesis, cranial nerve palsy, gait ataxia, and symptoms of increased intracranial pressure [6, 10]. Other notable signs included aseptic meningitis due to rupture or leakage of cyst contents spontaneously [24]. In the present study, we found that brainstem IECs preferentially afflict children, whereas other locations IECs preferentially present in adults. The possible reason may IECs growing in the brainstem present symptoms earlier than other locations.
Intraparenchymal epidermoid cysts possess specific imaging characteristics, and usually resemble other cystic lesions of the brain [12]. On computed tomography (CT) scan, IECs are usually homogeneously nonenhancing hypodense lesions without peritumoral edema [7, 16]. Sometimes, they may appear isodense or hyperdense because of the presence of lipid, protein, calcium and hemosiderin [11, 12, 24]. Ring enhancement may also be found in some cases [7, 11].
MRI remains the optimal imaging modality for the diagnosis of IECs. The MRI findings of IECs are basically as the same as those of extracerebral ECs. However, IECs is likely to have a mixed signal on MRI [8]. The signal intensity is variable and depends on the relative amount of lipid, cholesterol and keratin within the lesion [12]. On MRI, IECs usually appear hypointense on T1WI and hyperintense on T2WI [4]. They typically do not have peritumoral edema on T2WI and do not enhance upon contrast administration [6, 12]. While in some cases, contrast enhancement was observed peripherally [4, 8, 12, 13]. Takahashi et al. [13] described cyst wall enhancement in a 7-year-old girl with a brainstem epidermoid cyst. Two cases in our study appeared peripheral enhancement. In a review of the MRI appearance of IECs, signal heterogeneity was observed in 46.7% of cases, and peripheral enhancement was seen in up to 45.2% of cases.
Diffusion Weighted Imaging has emerged as the most helpful imaging sequence in diagnosing IECs [4]. On DWI, IECs were strongly hyperintense compared with cerebrospinal fluid and brain tissue. These characteristics facilitate the preoperative differentiation of IECs from other mimicking tumors [8]. Postoperatively, DWI can also be used to assess the extent of resection and to monitor recurrence [20]. IECs are usually hypointense on Apparent Diffusion Coefficient, and hyperintense on fluid-attenuated inversion recovery images (FLAIR) [1, 3, 4, 12]. Through review of the literature and our cases (Tables 1, 2, 3), DWI was performed in 17 patients, and all of them appear hyperintensity.
Accurate preoperative diagnosis of IECs can be very difficult due to the rarity and radiological similarities to other common intracerebral tumors [7]. However, increasing experience with MRI studies, especially with DWI, allow greater accuracy in the preoperative differential diagnosis [7, 19]. The common differential diagnosis includes dermoid cysts, arachnoid cysts, low-grade and cystic astrocytomas, oligodendroglioma, and inflammatory cysts [6,7,8]. IECs typically lack of peritumoral edema and do not significantly enhance upon contrast administration, which may be used to differentiate from the glioma and abscess [6, 12, 20]. Dermoid cysts are typically located along the midline and resemble fat, not CSF [8]. IECs are hyperintense on DWI, while astrocytomas, arachnoid cysts, oligodendroglioma and ganglioglioma are usually hypointense on DWI images [6, 8]. DWI, FLAIR, and constructive interference in steady state (CISS) sequences may also help to differentiate IECs from other cystic intraparenchymal lesions [6, 7]. IECs are usually hyperintense on FLAIR, hypointense on CISS, and hyperintense on DWI [6, 8, 26]. Since the DWI sequence plays an important role in the preoperative differential diagnosis, it should be suggested as a routine sequence in the investigation of intraparenchymal cystic lesions.
The optimal management of intraparenchymal epidermoid cysts is removal of cyst content with complete resection of capsule [1, 24]. However, radical removal of the tumor capsule can be very difficult because of the strong adherence of the tumor capsule to surrounding brain parenchyma, particularly in eloquent areas such as brainstem [1, 7]. In non-eloquent areas, although there may be no clear dissection plane between the capsule and the parenchyma, total excision of the capsule could be achieved without an obvious neurological deficit. In our case series, adherence of the capsule to the surrounding brain tissue was found in four patients, while sharp dissection was performed and total resection was achieved with good outcomes. In the eloquent areas such as the brainstem, small bits of capsule firmly adherent to the brainstem tissue can be left behind. Because complete resection of capsule may lead to disastrous complications [14, 21]. Fournier et al. [21] reported a patient who underwent three consecutive operations for tumor recurrence. The patient recovered well from the first two surgical procedures aimed at subtotal removal of the cyst capsule. However, he died in the third operation when total removal of the tumor capsule was performed. If eloquent tissue is involved, it is advisable to leave small portions of adherent tumor capsule to decrease postoperative neurologic deficits [6, 7]. As to the intrinsic brainstem epidermoid cysts, subtotal resection was recommended because the cyst usually adhered to the brainstem. Attempts to completely remove the capsule of IEC poses a surgical challenge with increased risks of morbidity and mortality [3]. Through analysis of the experiences reported in the literature (Tables 2, 3), we found three patients died after surgery. All of them with intrinsic brainstem ECs died after total resection.
Obviously the major concern following conservative resection is tumor recurrence. On reviewing the literature, we found a recurrence rate of 44.4% in patients underwent subtotal resection, and all the three patients underwent aspiration revealed early recurrence (Tables 2, 3). Although the IEC capsule that is left behind may increase recurrence risk, cases in the subgroup of subtotal resection showed better results with decreased morbidity and mortality than in the subgroup of total resection (Tables 1, 2, 3). What’s more, in some cases, the symptom-free interval before recurrence can be very long [4, 23]. Subtotal resection may also increase the risk of chemical meningitis, because the spillage of ECs contents into the surrounding subarachnoid space can cause an intense inflammatory reaction [2, 8]. Accurate preoperative diagnosis of IECs is important to prepare surgeons for the risk of chemical meningitis, and perioperative administration of corticosteroid and copious intraoperative irrigation of the surgical site with hydrocortisone has been shown to help prevent chemical meningitis and postoperative hydrocephalus [1, 2, 24].
Conclusion
In conclusion, IECs are extremely rare lesions. MRI imaging with DWI is very helpful in the preoperative diagnosis of the IECs. All the IECs in children are located in the brainstem. Total resection should be achieved for the IECs located in cerebral lobes and cerebellums, while subtotal resection is a wise and safe strategy with good outcomes for the IECs located in the brainstem.
References
Ahmed I, Auguste KI, Vachhrajani S, Dirks PB, Drake JM, Rutka JT (2009) Neurosurgical management of intracranial epidermoid tumors in children. Clinical article. J Neurosurg Pediatr 4:91–96
Mortini P, Bailo M, Spina A, Acerno S, Boari N, Gagliardi F (2016) Cyst-cisternal shunting for cystic multirecurrent brainstem epidermoid: case report and literature review. Acta Neurochir (Wien) 158:1197–1201
Shtaya A, Dabbous B, Fanou E, Bridges L, Hettige S (2017) Unusual intraparenchymal pontomedullary epidermoid cyst in a 2-year-old child: a case report and review of the literature. World Neurosurg 104:1046.e15–1046.e20
Aribandi M, Wilson NJ (2008) CT and MR imaging features of intracerebral epidermoid: a rare lesion. Br J Radiol 81:97–99
von Koch CS, Young G, Chin CT, Lawton MT (2002) Magnetic resonance imaging/spectroscopy of an intraaxial epidermoid: similarity to an abscess. Case illustration. J Neurosurg 97:492
Hanft SJ, Komotar RJ, Raper DM, Sisti MB, McKhann GM 2nd (2011) Epidermoid tumors of the temporal lobe as epileptogenic foci. J Clin Neurosci 18:1396–1399
Iaconetta G, Carvalho GA, Vorkapic P, Samii M (2001) Intracerebral epidermoid tumor: a case report and review of the literature. Surg Neurol 55:218–222
Hu XY, Hu CH, Fang XM, Cui L, Zhang QH (2008) Intraparenchymal epidermoid cysts in the brain: diagnostic value of MR diffusion-weighted imaging. Clin Radiol 63:813–818
Chandler WF, Farhat SM, Pauli FJ (1975) Intrathalamic epidermoid tumor. Case report. J Neurosurg 43:614–617
Yan PX, Yu CJ (2004) Minicraniotomy treatment of an intracerebral epidermoid cyst. Minim Invasive Neurosurg 47:245–248
Kaido T, Okazaki A, Kurokawa S, Tsukamoto M (2003) Pathogenesis of intraparenchymal epidermoid cyst in the brain: a case report and review of the literature. Surg Neurol 59:211–216
Gopalakrishnan CV, Dhakoji A, Nair S (2012) Epidermoid cyst of the brainstem in children: case-based update. J Child Neurol 27:105–112
Takahashi M, Paz Paredes A, Scavarda D, Lena G (2007) Brainstem epidermoid cyst in a child. Case report. Neurol Med Chir (Tokyo) 47:140–144
Leal O, Miles J (1978) Epidermoid cyst in the brain stem. Case report. J Neurosurg 48:811–813
Weaver EN Jr, Coulon RA Jr (1979) Excision of a brain-stem epidermoid cyst. Case report. J Neurosurg 51:254–257
Kansal R, Mahore A, Dange N (2012) Giant intramedullary epidermoid extending from the brain stem to the upper thoracic spinal cord. Turk Neurosurg 22:452–453
Sari A, Ozdemir O, Kosucu P, Ahmetoglu A (2005) Intra-axial epidermoid cysts of the brainstem. J Neuroradiol 32:283–284
Yoshizato K, Kai Y, Kuratsu J, Ushio Y (1996) Intramedullary epidermoid cyst in the brain stem: case report. Surg Neurol 45:537–540
Sener RN, Mechl M, Prokes B, Valek VA (2004) Epidermoid tumor of the pons. J Neuroradiol 31:225–226
Recinos PF, Roonprapunt C, Jallo GI (2006) Intrinsic brainstem epidermoid cyst. Case report and review of the literature. J Neurosurg 104:285–289
Fournier D, Mercier P, Menei P, Pouplard F, Rizk T, Guy G (1992) Recurrent intrinsic brain stem epidermoid cyst. Childs Nerv Syst 8:471–474
Mishra SS, Panigrahi S, Dhir MK, Pattajoshi AS (2014) Intrinsic brainstem white epidermoid cyst: an unusual case report. J Pediatr Neurosci 9:52–54
Caldarelli M, Colosimo C, Di Rocco C (2001) Intra-axial dermoid/epidermoid tumors of the brainstem in children. Surg Neurol 56:97–105
Patibandla MR, Yerramneni VK, Mudumba VS, Manisha N, Addagada GC (2016) Brainstem epidermoid cyst: an update. Asian J Neurosurg 11:194–200
Dutt SN, Mirza S, Chavda SV, Irving RM (2002) Radiologic differentiation of intracranial epidermoids from arachnoid cysts. Otol Neurotol 23:84–92
Dechambre S, Duprez T, Lecouvet F, Raftopoulos C, Gosnard G (1999) Diffusion-weighted MRI postoperative assessment of an epidermoid tumour in the cerebellopontine angle. Neuroradiology 41:829–831
Acknowledgements
The work was supported by the grant of the National Nature Science Foundation of China (Grant No. 81701153).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, J., Wang, C. & Liu, F. Intraparenchymal epidermoid cyst: proper surgical management may lead to satisfactory outcome. J Neurooncol 138, 591–599 (2018). https://doi.org/10.1007/s11060-018-2826-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2826-4