Abstract
Purpose
WHO grade II gliomas are uncommon in patients over the age of 60, and there is a lack in consensus regarding their management. We present molecular tumor characteristics as well as clinical outcomes in patients over the age of 60 undergoing surgical resection of a WHO grade II glioma.
Methods
After receiving IRB approval, patients were identified through the UCSF Brain Tumor Center. Pathologic diagnosis was completed using WHO 2016 grading criteria.
Results
Twenty-six patients with a mean age of 66 years met inclusion criteria with a median follow-up of 5.2 years. Diagnoses included diffuse astrocytoma IDH-mutant (19.2%), diffuse astrocytoma IDH-wildtype (26.9%), Oligodendroglioma IDH-mutant and 1p/19q-codeleted (50%), and a rare case of mixed oligoastrocytoma (3.9%). 66% of astrocytoma IDH-wildtype tumors possessed TERT mutation. Median extent of resection was 75.4%. Progression-free (PFS) and overall survival (OS) were 23.5 and 62.6 months, respectively. Shorter PFS was associated with the astrocytoma IDH-wildtype subtype despite similar extent of resection and adjuvant treatment rates compared to the other subtypes. OS did not differ between subtypes. Malignant transformation and death were associated with larger preoperative and residual tumor volume.
Conclusions
Older patients with diffuse gliomas may safely undergo aggressive treatment with surgical resection and adjuvant therapy. Elderly patients with low grade gliomas have worse clinical outcomes compared to their younger counterparts. This may be due to an increased frequency of diffuse astrocytoma IDH-wildtype tumors in this age group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low grade oligodendrogliomas and astrocytomas, grade II per World Health Organization (WHO) Classification of Central Nervous System Tumours, occur in a younger age group compared to WHO grade III and IV gliomas. The mean age of diagnosis for WHO grade II gliomas is in the late third or early fourth decade of life [1, 2]. However, only about 10–18% of low-grade gliomas occur in patients at least 60 years-of-age or older, and there is a lack of consensus regarding the optimal management strategy and molecular differences in older patients [2, 3].
The molecular profiles of these tumors have more recently been defined [2, 4], although the number of older patients within these cohorts are limited. The Cancer Genome Atlas Research Network performed a genome-wide analysis on 293 grade II/III gliomas and defined three separate glioma subgroups based on IDH, 1p/19q, and TP53 status. Within the cohort of grade II gliomas, there were 13 IDH-wildtype (9.5%), 48 IDH-mutant and 1p/19q-codeleted (35.3%), and 75 IDH-mutant and 1p/19q-intact (55.1%) tumors. However, only 14 patients had grade II tumors and were 60 years of age or older (2 IDH-wildtype, 2 IDH-mutant and 1p/19q-codeleted, and 4 IDH-mutant and 1p/19q-intact) [4]. Eckel-Passow et al. reported on 615 patients with grade II/III gliomas and defined 5 glioma subgroups based on IDH, 1p/19q and TERT status. Within the group of grade II tumors, there were 26 IDH-wildtype (8.5%), 111 IDH-mutant and 1p/19q-codeleted (36.1%), and 170 IDH-mutant and 1p19q-intact (55.4%) tumors. Interestingly, TERT promoter mutation-only tumors tended to occur more frequently in older patients with an average age of 59 with the second oldest subgroup being triple negative tumors [2]. While prior reports have examined outcomes in older patients with low grade gliomas, no prior reports to our knowledge have specifically examined such a cohort based on this newer molecular characterization.
Within the context of grade II glioma, older age often results in worse overall and progression free survival and more aggressive clinical behavior which may be related to a combination of patient, treatment, and tumor characteristics [5,6,7,8,9,10]. Older patients have been reported to have higher rates of neurological deficits on presentation, increased tumor burden and a higher incidence of contrast-enhancing features on imaging on retrospective analyses [11, 12]. Analysis of preoperative variables for overall survival has also demonstrated older age as a poor prognostic factor [7, 13]. Chang et al. reported on a pre-operative scoring system for low-grade gliomas in adults who were undergoing first-time resection using an institutional series of 281 patients. On multivariate analysis, eloquent location, KPS score, age > 50, and tumor diameter > 4 cm allowed for a 4-point scoring system predicting both OS and PFS with more points correlating with worse outcomes. Worse outcomes with age was believed to be secondary to a more malignant underlying biology as well as patient specific factors including natural life expectancy, higher rates of medical comorbidities, and increased vulnerability to illness post-operatively [7].
Currently the patient’s age plays a significant role in determining the adjuvant treatment course. Based on results of RTOG 9802, a phase 3 randomized trial comparing adjuvant radiation with combination chemoradiation in “high risk” patients with low-grade gliomas, adjuvant therapy with chemoradiation is recommended for all patients older than 40 years [14]. RTOG 9802 highlighted the aggressive nature of low grade gliomas in older patients and the clear need for and benefit of aggressive adjuvant therapy in this cohort. However, there still exists a hesitation in pursuing aggressive treatment strategies in older patients. Clinically, some providers advocate for aggressive upfront treatment while others recommend a more conservative approach [11, 15, 16]. Older patients with gliomas tend to undergo lower rates of treatment with surgical resection and radiotherapy compared to younger cohorts of patients due to concern for high rates of treatment-related complications [11, 12, 17]. National Comprehensive Cancer Network (NCCN) guidelines do not distinguish whether to offer maximal safe resection vs biopsy based on patient age. Additionally, if biopsy is pursued rather than resection, a low grade diagnosis in this age group may represent under-sampling of a high grade glioma. In this report, we sought to determine postsurgical morbidity within older patients with low grade gliomas associated with aggressive resection, the molecular features within this cohort, as well as more long-term clinical outcomes based on treatment and molecular features.
Methods
Patient selection, data collection, and defining molecular grouping and outcomes
After obtaining approval from the UCSF institutional IRB (Study Number 15-17826), a search query was performed through UCSF Brain Tumor Center to include patients with a primary histologic diagnosis of WHO grade II glioma at least 60 years-of-age at the time of initial surgery that had undergone surgical resection more than biopsy alone. Patients were excluded it they had prior chemotherapy, radiation treatment, or surgical resection. This query identified 26 patients diagnosed between 1997 and 2017. Patient and tumor characteristics in addition to perioperative outcomes were collected retrospectively from operative, radiology, pathology, and scanned documents available through the UCSF electronic medical record. An integrated diagnosis based on the 2016 WHO classification scheme was utilized. IDH mutation and 1p/19q-codeletion status was determined for all cases. The study neuropathologist (M.P.) reviewed all cases to confirm diagnostic classification and grading based on WHO 2016 criteria. Contrast enhancement of tumors was determined by both review of the radiology report as well as direct review of preoperative post-contrast MRI scans by the authors. Adjuvant therapy was defined as treatment that was received after surgery but before progression. Recommendation of adjuvant therapy was based on discussion within the UCSF Tumor Board or by the direction of the patient’s primary neuro-oncologist at UCSF and was based on pathologic diagnosis, age, patient preference, and availability of clinical trials. Progression-free survival (PFS) was defined as the time from the procedure date until the date of the first scan demonstrating unequivocal radiographic progression with confirmation based on the radiology report and clinic notes. Malignant progression was based on biopsy confirmation of a higher tumor grade or new enhancement on imaging with the consensus opinion of the UCSF tumor board that the findings represented progression to a higher grade rather than treatment effect.
Molecular analysis
Of the 26 surgical specimens, IDH mutation status as well as 1p/19q co-deletion status were available for all specimens and allowed for categorization of subtype based on the 2016 WHO classification [18]. IDH mutation status was assessed by immunohistochemistry (IHC) using primary antibody specific to IDH1R132H mutant protein (H09, Dianova GmbH, Hamburg, Germany) using standard techniques, and all cases with positive IDH1R132H stain were classified as IDH-mutant. In cases with negative IHC results, Sanger sequencing of IDH1 and IDH2 genes covering exon 4 regions was performed and tumors were classified as mutant or wildtype accordingly. ATRX alterations were assessed by IHC (HPA001906, Sigma Aldrich, St. Louis, MO) using standard techniques, and loss of nuclear staining in the majority of the tumor cells in the presence of an internal positive control was interpreted as loss of ATRX expression, suggestive of ATRX alterations. TP53 alterations were assessed by IHC (DO-7, Dako, Agilent, Santa Clara, CA) using standard techniques, and strong nuclear staining in more than 50% of tumor nuclei was interpreted as positive, and these cases were classified as TP53-mutant. A subset of cases was assessed by Sanger sequencing of TP53 gene covering exons 4 through 8 and tumors were classified as mutant or wildtype according to sequencing results. Chromosome 1p and 19q deletions were separately assessed by fluorescence in situ hybridization using Vysis 1p36/19q13 Dual color Probe kit (Abbott Laboratories. Abbott Park, IL). Upon counting at least 50 non-overlapping cells, a 1p36:1q25 (or 19q13:19p13) ratio of 0.8 or less was accepted as deleted if > 15% of cells have an at least 2:1 ratio of target:reference probe. Cases with both 1p and 19q deletion were classified as 1p/19q-codeleted. TERT promoter mutation was assessed either by a single- or a 2-step PCR and Sanger sequencing of a 244 base-pair segment of TERT promoter region spanning the C228T and C250T mutations, and the cases were classified as TERT-mutant or TERT-wildtype accordingly.
Volumetric analysis and extent of resection quantification
Pre-operative and post-operative tumor volumes were quantified by using BrainLab Smartbrush software (Brainlab, Munich, Germany). Manual segmentation was performed with region-of-interest analysis based on fluid-attenuated inversion-recovery (FLAIR) sequences from pre- and post-operative MRI scans to quantify tumor volume. Extent of Resection (EOR) was calculated as: (pre-operative tumor volume − post-operative tumor volume)/pre-operative tumor volume × 100%. Manual segmentations were performed by one operator (R.M.) with tumor volumetrics verified for accuracy after an initial training period (S.H.). Knowledge of clinical outcomes was withheld from participants involved in tumor volumetrics and perioperative outcomes data collection. Pre-operative MRI scans were obtained within 24 h prior to resection, and post-operative scans were all obtained within 48 h post-resection. All patients in the cohort had available preoperative and postoperative MRI scans for analysis. To ensure that post-operative FLAIR signal was not surgically induced edema or ischemia, FLAIR pre- and post-operative MRIs were carefully compared alongside DWI sequences prior to including each region in the volume segmentation. Post-contrast T1 weighted images were not used for EOR analysis because for many patients with enhancement, this was diffuse and patchy, making volumetric analysis unreliable. For the analysis, GTR, STR, and partial resection were defined as 100%, 70–99%, and < 70%, respectively.
Statistical analysis
Descriptive statistics were used to define the patient cohort, tumor locations and characteristics, treatment details, EOR, and neurological outcomes. One-way analysis of variance (ANOVA), χ2 test, and Pearson linear correlation coefficient were performed for univariate analysis. Log-rank test was performed to assess differences in PFS and OS and included patients with at least 30 days of follow-up. The level of significance was 0.05 for all analyses. Statistical analysis was performed using JMP Pro 13 (SAS Institute, Cary, NC).
Results
Patient and tumor characteristics
Demographics and tumor characteristics are listed in Table 1. The mean age at the time of surgery was 66.2 years (range 60.3–76.7) with a median follow-up of 5.2 years (range 0.4–12.4 years). The majority of tumor involved the frontal lobe (38.5%) and were left-sided (57.7%). Pathologic diagnoses based on the updated 4th edition of WHO classification included five cases of diffuse astrocytoma, IDH-mutant, seven cases of diffuse astrocytoma, IDH-wildtype, and 15 cases of oligodendroglioma, IDH-mutant and 1p/19q-codeleted. There was one case designated as a mixed oligoastrocytoma. For this case, histology demonstrated distinct regions of oligodendroglioma and astrocytoma morphology. The oligodendroglioma-like component showed IDH1 R132H mutation, 1p/19q-codeletion, retained ATRX expression and negative p53 staining, and astrocytoma-like component demonstrated IDH1 R132H mutation, loss of ATRX expression, intact 1p/19q and wildtype TERT promoter. Further molecular characterization of the diffuse astrocytoma, IDH-wildtype tumors demonstrated that 66.7% harbored a TERT promoter mutation (Table 2). There were five cases (19.2%) with enhancement on preoperative imaging, which was not significantly associated with any particular subtype (see Table 2). Mean preoperative tumor volume was found to be 58.8 cm3.
Surgical treatment characteristics and adjuvant therapies
Treatment characteristics of the cohort are summarized in Table 3. As many of these lesions were located adjacent to eloquent cortex, 14 cases (53.8%) utilized either language or motor mapping. The median extent of resection (EOR) was 75.4% with 8 patients (30.8%) undergoing a GTR. The majority of patients underwent adjuvant treatment postoperatively (53.9%) and after evidence of progression (75%).
Perioperative outcomes
Perioperative outcomes are summarized in Table 3. In terms of complications (including persistent neurological deficit by 90 days), one patient developed a subdural hematoma requiring evacuation and five patients developed persistent neurological deficits. Neurological deficits included two cases of persistent mild speech hesitancy, one case of single extremity weakness (4/5 proximal and 1/5 distal strength), one case of combined facial droop and upper extremity weakness (4/5 strength), and one case of a persistent superior quadrantanopsia.
Long-term clinical outcomes
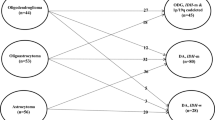
Long-term outcomes are summarized in Table 3. Median censored PFS and OS were found to be 23.5 and 62.6 months, respectively. Nine cases (34.6%) on follow-up demonstrated malignant transformation. Table 2 depicts treatment characteristics and differences in clinical outcomes between the glioma molecular subtypes. Although EOR and adjuvant therapy did not significantly differ between the three molecular subtypes, diffuse astrocytoma IDH-wildtype cases were associated with a significantly shorter PFS (Fig. 1a; Table 2). However, molecular subtype did not appear to significantly impact overall survival (Fig. 1b; Table 2). Univariate analysis of other patient, tumor, and treatment features associated with PFS and OS did not reveal any significant associations (Table 4). Larger preoperative tumor size and a greater amount of residual tumor on postoperative scans were associated with more frequent malignant progression and death (Table 5). Contrast enhancement was not associated with a difference in PFS, OS, or malignant transformation.
Discussion
The United States population is aging, and therefore, the management of elderly patients with gliomas involves numerous considerations including the expected longevity of these patients. In 2014, life expectancy in the US at age 65 and 75 was 19.3 and 12.2 years, respectively [19]. As the life expectancy of patients age 60–69 and 70–79 with low-grade gliomas were found to be about 6 and 1.3 years respectively, further therapeutic intervention has the potential to improve both overall survival and quality of life [20]. Differences already exist in the management of older and younger patients with malignant gliomas. Kaloshi et al. compared treatment and outcomes data between 62 patients at least 60 years-of-age and 704 patients < 60 years-of-age with low-grade gliomas. The authors found that in elderly patients time-to-treatment was shorter (1.2 months), chemotherapy was more often used as first line treatment (60%), and tumor resection was done at a lower rate with only 27% undergoing partial or total resection compared to 61% in the younger cohort [11]. This difference in treatment paradigm may come from prior reports demonstrating increased risk of perioperative complications and worse outcomes in older patients with malignant gliomas [7, 14]. However, patients in this age group with glioblastoma often undergo aggressive resection with acceptable perioperative outcomes, and thus, it seems counterintuitive that such an intervention would be withheld for older patients in which there is still much to be gained in terms of life expectancy.
There are a limited number of reports that have examined outcomes specifically in older patients with low-grade glioma, and most of these studies did not include a cohort of patients undergoing aggressive surgical intervention as were included in the current cohort. Schomas et al. examined outcomes in 32 patients with low-grade gliomas who were at least 55 years of age. While a greater extent of resection did not correlate with improved outcome, only two patients underwent gross or near gross total resection, precluding meaningful statistical analysis. A greater percentage of patients also received post-operative radiotherapy (72%) with 97% receiving no chemotherapy. The only factor found to be associated with shorter PFS was enhancement on CT [12]. Chi and Batchelor reported on 16 patients with low-grade gliomas who were at least 55 years of age with 7 patients undergoing biopsy and 9 undergoing at least a partial resection. Ten patients were given adjuvant treatment with various regimens of radiation, temozolomide, or PCV. Longer PFS correlated with a histologic diagnosis of oligodendroglioma and a MIB-1 index of 5% or higher [21]. In a separate report, Pouratian et al. analyzed the outcomes of 20 patients at least 60 years of age with low-grade gliomas. 75% of patients were treated with biopsy alone with only one patient undergoing gross total resection. Interestingly, multivariate analysis demonstrated that, even within the cohort of older individuals, younger age was associated with better outcomes [20]. Interestingly, these studies demonstrate differing distributions of histological diagnoses with Pouratian et al., Kaloshi et al., and Chi and Batchelor suggesting that oligodendrogliomas occur more frequently [11, 20, 21] while Schomas et al. found astrocytoma to be the most common diagnosis in the older population [12].
Results from this report provide additional insights into molecular features and treatment outcomes for older patients with low grade gliomas. First, we demonstrate that extensive resection may still be achieved in most cases with acceptable rates of long-term neurological morbidity. The complication rates encountered are similar to those described in larger cohorts of patients with low grade glioma inclusive of all age groups [13]. Although the treatment paradigm varied during the study period, the majority of patients received adjuvant therapy after resection. However, 38.5% of patients did not undergo adjuvant therapy, in most cases due to patient preference. The most frequent diagnosis in the cohort was oligodendroglioma which is in line with some but not all prior reports in older patients [11, 12, 20, 21]. We report a median PFS of 23.5 months (1.9 years), similar to prior reports, and a median OS of 62.6 months (5.2 years), longer than prior reports in a similar age group. This could be in part due to more extensive resection as most patients in prior studies focusing on this older age group underwent biopsy alone with adjuvant therapy [11, 12, 20].
However, these PFS and OS times are still shorter when compared to younger cohorts of low-grade glioma patients [11]. For example, in a phase III clinical trial comparing treatment with PCV after radiation compared to radiation alone in patient with grade II gliomas who had undergone surgical resection or biopsy. Median PFS and OS in the radiation only control arm were 4.0 and 7.8 years, respectively, higher than that noted in our study [14]. Similarly, prior data from our group in a surgical cohort of low grade glioma patients demonstrated a median PFS of 5.5 years [22]. Time to malignant transformation also appears to be shorter for older patients. Murphy et al. identified the incidence of malignant transformation to be 21% in a cohort of 599 patients with low-grade glioma in which the average age was 36 years. Median time to malignant transformation was 56.4 months, and on multivariate analysis, older age, extent of resection, and male sex were significant predictors of malignant transformation [23].
The reason for the prognostic differences based on age in patients with low grade glioma is not well understood. The majority of astrocytomas in our cohort were IDH-wildtype (approximately 27% of the total cohort), which has been previously shown to portend similar overall survival to a diagnosis of glioblastoma [1]. Furthermore, two-thirds of IDH-wildtype astrocytomas also harbored TERT promoter mutation which portends an even worse prognosis within this low grade glioma subtype, and current recommendation includes reporting of these cases as “with molecular features of glioblastoma, WHO grade IV” [1, 24]. Although we did not observe a difference in overall survival between molecular subtypes in the current study, this may be due to small sample size rather than a lack of prognostic utility of these markers in older patients. Another reason for the age-associated prognostic difference may be in part due to worse functional status at presentation. KPS scores in older patients with low-grade gliomas were previously found to be lower than their younger counterparts [11], and lower preoperative KPS is known to be a poor prognosticator [7]. Finally, although the median EOR was 75.4% in this cohort, this is still lower than previously reported in a younger cohort of patients [22]. As greater EOR is associated with longer PFS and OS in the context of low grade glioma, this may be another key factor associated with the differences in outcomes observed between older and younger patient cohorts [25, 26].
Given the retrospective nature of this study, there are expected limitations in the interpretation of the results. As several patients elected to transition care to non-affiliated institutions, there were two patients lost-to-follow-up. However, to the best of our knowledge this was due to patients selecting to have care closer to home rather than due to the aggressiveness of their disease per se. Additionally, the cohort included for analysis is relatively small, despite being at a referral center, which reflects the low frequency of WHO grade II tumors in this age group. Finally, although all patients had undergone at least a partial resection, we cannot completely rule-out under-sampling of a higher-grade lesion.
Conclusion
This study demonstrates that older patients with diffuse gliomas may safely undergo aggressive treatment with surgical resection and adjuvant therapy. Shorter PFS was associated with a diagnosis of diffuse astrocytoma IDH-wildtype which occurred with a higher frequency than has been previously reported in younger cohorts. Differences in OS based on the glioma subtype were not observed in this cohort. Despite aggressive treatment, PFS and OS were shorter than in prior reports examining outcomes in younger patients with grade II glioma. Decreased residual tumor volume was associated with lower rates of malignant transformation, and thus, maximum safe resection should be recommended for these tumors regardless of age. Further population-based studies are needed to examine the frequency of low grade molecular subtypes within this age group. Additional studies are also needed to determine the impact of specific treatment regimens in older patients with low grade gliomas to understand the poorer clinical outcomes observed.
References
Pekmezci M, Rice T, Molinaro AM et al (2017) Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol. https://doi.org/10.1007/s00401-017-1690-1
Eckel-Passow JE, Lachance DH, Molinaro AM et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508. https://doi.org/10.1056/NEJMoa1407279
Rasmussen BK, Hansen S, Laursen RJ et al (2017) Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I–IV in the the Danish Neuro-Oncology Registry. J Neurooncol. https://doi.org/10.1007/s11060-017-2607-5
Network CGAR, Brat DJ, Verhaak RGW et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498. https://doi.org/10.1056/NEJMoa1402121
Lote K, Egeland T, Hager B et al (1997) Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol 15:3129–3140. https://doi.org/10.1200/JCO.1997.15.9.3129
Okamoto Y, Di Patre PL, Burkhard C et al (2004) Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol 108:49–56. https://doi.org/10.1007/s00401-004-0861-z
Chang EF, Smith JS, Chang SM et al (2008) Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg 109:817–824. https://doi.org/10.3171/JNS/2008/109/11/0817
Davis FG, Freels S, Grutsch J et al (1998) Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973-1991. J Neurosurg 88:1–10. https://doi.org/10.3171/jns.1998.88.1.0001
Pignatti F, Van den Bent M, Curran D et al (2002) Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20:2076–2084. https://doi.org/10.1200/JCO.2002.08.121
Shaw E, Arusell R, Scheithauer B et al (2002) Prospective randomized trial of low-versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group Study. J Clin Oncol 20:2267–2276. https://doi.org/10.1200/JCO.2002.09.126
Kaloshi G, Psimaras D, Mokhtari K et al (2009) Supratentorial low-grade gliomas in older patients. Neurology 73:2093–2098. https://doi.org/10.1212/WNL.0b013e3181c6781e
Schomas DA, Laack NN, Brown PD (2009) Low-grade gliomas in older patients: long-term follow-up from Mayo Clinic. Cancer 115:3969–3978. https://doi.org/10.1002/cncr.24444
Capelle L, Fontaine D, Mandonnet E et al (2013) Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases. J Neurosurg 118:1157–1168. https://doi.org/10.3171/2013.1.JNS121
Buckner JC, Shaw EG, Pugh SL et al (2016) Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med 374:1344–1355. https://doi.org/10.1056/NEJMoa1500925
Berger MS, Deliganis AV, Dobbins J, Keles GE (1994) The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer 74:1784–1791. https://doi.org/10.1002/1097-0142(19940915)74:6%3C1784::AID-CNCR2820740622%3E3.0.CO;2-D
Keles GE, Lamborn KR, Berger MS (2001) Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg 95:735–745. https://doi.org/10.3171/jns.2001.95.5.0735
Iwamoto FM, Reiner AS, Nayak L et al (2009) Prognosis and patterns of care in elderly patients with glioma. Cancer 115:5534–5540. https://doi.org/10.1002/cncr.24612
International Agency for Research on Cancer (2016) WHO Classification of Tumours of the Central Nervous System, 4th edn. World Health Organization, Geneva
National Center for Health Statistics (2017) Health, United States, 2016. Hyattsville
Pouratian N, Mut M, Jagannathan J et al (2008) Low-grade gliomas in older patients: a retrospective analysis of prognostic factors. J Neurooncol 90:341–350. https://doi.org/10.1007/s11060-008-9669-3
Chi A, Batchelor T (2007) Low-grade gliomas in older patients: a malignant tumor? Neuro Oncol 9:545
Smith JS, Chang EF, Lamborn KR et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345. https://doi.org/10.1200/JCO.2007.13.9337
Murphy ES, Leyrer CM, Parsons M et al (2018) Risk factors for malignant transformation of low-grade glioma. Int J Radiat Oncol Biol Phys 100:965–971. https://doi.org/10.1016/j.ijrobp.2017.12.258
Brat DJ, Aldape K, Colman H et al (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. https://doi.org/10.1007/s00401-018-1913-0
Hervey-Jumper SL, Li J, Osorio JA et al (2016) Surgical assessment of the insula. Part 2: validation of the Berger-Sanai zone classification system for predicting extent of glioma resection. J Neurosurg. https://doi.org/10.3171/2015.4.JNS1521
Sanai N, Polley M-Y, Berger MS (2010) Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. https://doi.org/10.3171/2009.6.JNS0952
Acknowledgements
We would like to thank Jing Li for completing the search of the institutional database for patients qualifying for this study.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the experimental design, analyses, interpretation of the data and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morshed, R.A., Han, S.J., Hervey-Jumper, S.L. et al. Molecular features and clinical outcomes in surgically treated low-grade diffuse gliomas in patients over the age of 60. J Neurooncol 141, 383–391 (2019). https://doi.org/10.1007/s11060-018-03044-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03044-4