Abstract
Recurrence rates of meningiomas have been widely reported in the literature, but it remains challenging for clinicians to predict recurrence rate depending on treatment, patient demographics and tumor characteristics. To address these needs, we performed a systematic analysis of the literature to determine the recurrence rate ranges of meningiomas following surgery or radiation. Our search yielded 13 studies that met all criteria for inclusion, allowing us to include 1539 patients in the assessment. Recurrence rates ranged from 0.00 to 2.36 per 100-person-years for WHO grade I meningiomas; and from 7.35 to 11.46 per 100-person-years for WHO grade II meningiomas. Our findings suggest that (1) reported recurrence rates are variable and complicated by the heterogeneity of study populations; (2) as expected, WHO grade II meningiomas generally have a higher recurrence rate than WHO grade I, when controlling for time of diagnosis (by employing person-years); and (3) there is a need for more rigorous reporting of recurrence rates, WHO grade, and Simpson grading for individual patients in order to determine a robust mean of recurrence across WHO grades.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningiomas are one of the most common brain tumors in adults, representing between 20–35% of primary brain tumors [1, 2]. With advances in treatment over the past 30 years, meningiomas have become a chronic disease, and it is crucial to consider the risk of meningioma recurrence and symptomatic progression when deciding on treatment [1, 3]. One method of predicting recurrence is to stratify risk based on WHO grade, as tumor grade has been demonstrated to predict the risk of recurrence following treatment [1, 4]. Regardless, reports regarding the risk of recurrence and symptomatic progression of meningiomas following surgical resection vary greatly, depending on population selection, population size, and the imaging modality used to evaluate for recurrence/progression [5, 6]. To address this variability, we used an organized approach to systematically analyze the literature in order to determine the recurrence rates of intracranial meningiomas in adult patients (> 18 years of age) as stratified by WHO grade following first-time treatment.
Methods
Search strategy and selection criteria
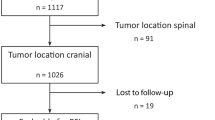
We performed a systematic analysis to identify studies that reported recurrence rate by WHO grade following surgery or radiation therapy. References for were identified through Ovid MEDLINE and EMBASE from January 1st, 1977 to November 23rd, 2016, within the magnetic resonance imaging (MRI) era. Two independent searches were conducted by V.L. and S.D. and the results compared for duplicate articles. The search terms used were: meningioma; grade; recurrence; progression; return; reappear; regrow, repeat. Search history with limitations is available in Supplementary Materials A1. The articles were screened and full text reviewed independently by V.L. and S.D and results compared. Only articles in English were included. Articles were chosen for full review if the abstract corroborated report of recurrence rate data. Selected abstracts were then reviewed to confirm reporting of recurrence rate data stratified by WHO grade in adult patients. Inclusion criteria included: (1) randomized control trials or observational studies with at least one cohort involving at least 20 adults (≥ 18 years of age); (2) assessment for postoperative recurrence using MRI in all patients; and (3) stratification of some measurement of recurrence by WHO grade. Studies were excluded if the reported patients: (1) received chemotherapy; (2) had a history of recurrent meningiomas at baseline; or (3) had a history of extra-cranial meningioma. Thirteen studies were selected for inclusion in the final analysis (Fig. 1).
We chose the above inclusion criteria with the goal of limiting bias due to variations in study design. Studies with a small sample size suffer from low statistical power, inflated effect size estimates, low probability of finding true effects, and low positive predictive value [7]. In order to avoid using studies with small sample size but also to avoid excluding too many studies, we decided a priori on a cut-off for studies reporting < 20 patients. To limit bias from different imaging modalities, we ensured that MRI was used as the main imaging modality for recurrence assessment. CT has difficulty distinguishing between tumor recurrence and radiation-induced adverse effects due to similarities, such as (1) diffuse hypo-intensity of the white matter extending into and compressing the overlying cortex likely caused by edema, (2) enhancing focal areas of lucency suggesting necrosis, (3) irregular and/or extensive contrast enhancement, and (4) mass effect necrosis [8]. Since radiation therapy may be a part of treatment, CT remains limited by its relatively low power to differentiate between treatment necrosis and tumor recurrence [8]. The articles were reviewed to determine the level of evidence using the criteria designated by the Oxford Centre for Evidence-Based Medicine (available in Supplementary Materials A2) [9]. No randomized control trials were identified. Five level two studies and eight level four studies were identified and included in the analysis.
Quality and risk of bias
Quality and risk of bias of the studies were assessed using the Newcastle–Ottawa Scale (NOS) for cohort studies (available in Supplementary Materials A3) [10, 11]. NOS evaluates for quality based on the selection of patients, comparability of cohorts, and outcomes; studies that achieved 6 or more stars were considered to be of high quality [11].
Data extraction and analysis
The data was parsed to retrieve the following: WHO grade, mean/median age, sample size, gender distribution, tumor location, intervention, Simpson grade, recurrence rate, postoperative symptoms, and follow-up period. Symptomatic progression was defined as the development of new symptoms or any worsening of existing symptoms.
For comparison, when recurrence was provided as progression/recurrence-free survival (PFS), the value was converted to recurrence rate using the formula: \(recurrence\;rate=1 - PFS\). Recurrence rate per person year was calculated, when possible, based on the equation:
Studies that did not report a mean time to recurrence could not be used to calculate recurrence rate per 100-person-years. Linear and exponential regression models were performed using Microsoft Excel, in order to demonstrate the relationship between PFS and time.
Results
Description of studies
The search yielded 1953 unique abstracts, of which 280 studies were selected for full text review. Thirteen studies met all criteria for inclusion (Fig. 1) [5, 12,13,14,15,16,17,18,19,20,21,22,23]. The studies used for the analysis were published between 2010 and 2016 and included data accrued in five different countries: US (8); China (2); Germany (1); Poland (1); and UK (1).
The patient populations identified from these sources were heterogeneous. In total, 1539 patients were assessed for recurrence, with a preponderance of female patients (442 men: 1123 women). The cohort included 1293 WHO grade I meningiomas, 242 WHO Grade II meningioms, and four WHO grade III meningiomas. Patient demographics are listed in Table 1. In terms of intervention, 1432 patients received surgery alone, 131 patients received some form of surgery and radiation therapy, and two patients received radiation therapy alone. Follow-up ranged from 1 to 204 months.
The NOS quality assessment of the five cohort studies is available is available in Supplementary Materials A4 [12, 16, 19,20,21]. All cohort studies were high quality evidence based on the NOS scale [11].
Recurrence rates and follow-up for WHO grade
Recurrence rates ranged from 0% at 5 years to 22.5% over a mean of 26.2 months for WHO grade I meningiomas;[17, 21] and from 15% at 2 years (2-year PFS = 85%) to 37% over a period of greater than 10 years for WHO grade II meningiomas [18, 23]. The search identified only four patients with a WHO grade III meningioma and as a result, they were not included in the recurrence rate analysis. Alternatively, the recurrence rate ranged from 0.00 to 2.36 per 100-person-years for WHO grade I meningiomas;[5, 15] and from 7.35 to 11.46 per 100-person-years for WHO grade II meningiomas [12, 14]. Recurrence rates per 100-person-years are listed in Table 2.
The recurrence rate of WHO grade I meningiomas treated by surgery alone ranged from 0% (at 32.5 months follow up) to 22.5 (mean recurrence of 26.2 months); [5, 17] and was 37% for WHO grade II (mean recurrence > 10 years) [23]. WHO grade II meningioma treated with surgical intervention followed by radiation therapy had a recurrence rate 17% at an unspecified time or a PFS of 85% at 2 years or 62% at 3 years [18].
Analysis of the Level 2 data alone revealed that WHO grade I meningioma recurrence ranged from 0% at 5 years to 21% over 5 years [12, 21]. Using Level 2 data alone, the WHO grade II meningioma recurrence rate was 29.6% over a mean period of 31 months [12].
Symptomatic progression
Preoperative symptoms and postoperative symptoms are displayed in Supplementary Materials A5. Symptomatic progression is available in Table 3. Only five studies reported postoperative symptomatic progression [5, 13, 15, 22, 23]. Following treatment for WHO grade I tumors, 67.5% of patients experienced general improvement, compared to 16.7% who experienced general worsening [5, 22]. Symptomatic progression following surgery included cranial nerve III palsy (7.4%); general cranial nerve deficits not specified (5.6%); development of hydrocephalus (5.6%); worsening of headaches (5.0%); and hemianopia (5.0%) [5, 15, 22].
Recurrence rate over time
Only three studies provided PFS rates at different points in time [12, 14, 18]. Supplementary Materials A6 demonstrates the PFS points for the three aforementioned studies with their coefficient of determination. Due to the limited number of data points, a meaningful statistical significance was unable to be calculated.
Post-hoc analysis of Simpson grade and recurrence rate
Eight studies provided stratification of recurrence rates by WHO grade and Simpson grade and the results are available in Table 4. For WHO grade I meningiomas, recurrence rate for Simpson grade I, II, III, IV and V ranged from 0 to 21, 0–33, 12–40, 18–40, and 0% respectively [13,14,15,16, 19,20,21]. For WHO grade II meningiomas, recurrence rates for Simpson grade I–II were 36.6% and grade III–IV were 63.6% from 1 study [23]. There were only three studies that had at least 20 patients in a Simpson grade cohort and provided a mean time until recurrence to calculate recurrence rate per 100-person-years [13,14,15]. Amongst these WHO grade I studies, the recurrence rate per 100-person-years for Simpson grade I was 0.00 to 0.72 and for WHO grade II was 1.06 [13, 15].
Discussion
Summary of evidence
We performed a systematic analysis of the literature to determine risk of meningioma recurrence following surgical resection or radiotherapy, as stratified by WHO grade and determined by postoperative MR imaging. There were only four WHO grade III meningioma patients identified in our analysis and as a result, WHO grade III meningioma recurrence was not analyzed [5, 17]. Survival rates for WHO grade III meningiomas have been reported to be as low as 8.3% at 5 years, making it difficult to collect long term recurrence rate data on this population, which likely contributed to the low number of patients captured by our search [24]. Overall, our findings suggest that (1) reported recurrence rates are variable and complicated by the heterogeneity of study populations; (2) as expected, WHO grade II meningiomas generally have a higher recurrence rate than WHO grade I, when controlling for time of diagnosis (by employing person-years); and (3) there is a need for more rigorous reporting of recurrence rates, WHO grade, and Simpson grading for individual patients in order to determine a robust mean of recurrence that factors different qualities that influence recurrence rate.
Our analysis identified a cohort reflecting the real world distribution of meningioma WHO grades [1]. In our analysis, 84.0% of the identified meningiomas were WHO grade I, 15.7% WHO grade II, and 0.3% WHO grade III, which closely reflects real world distribution of > 80, 5–15, and 1–3% for WHO grade I, II, and III meningiomas respectively [1]. Since all the studies included were published from 2010 to 2016, the definition for WHO grade would reflect the WHO Classification of Brain Tumors 2007 definitions, which removes the limitation of differing WHO grade definitions over time from our analysis [25].
Study implications
Our study’s importance lies in its ability to provide clinicians with an appreciation of how recurrence rates have ranged by WHO grade in the literature. The minimum and maximum recurrence rates allows for an estimate of expected recurrence and can be acknowledged as a potential range of recurrence when planning future studies or considering treatment options.
Characteristics from our analysis that have been predicted to increase the risk of tumor recurrence include the presence of any atypical features; MIB-1 index ≥ 3%; and low ADC on preoperative MRI [19, 21]. Koutourousiou and colleagues identified four main factors influencing the risk of tumor recurrence following surgical resection: (1) incomplete tumor resection; (2) histological grade; (3) length of the postoperative follow-up period; and (4) mode and quality of the assessment for tumor recurrence [13]. Considering the various factors that influence meningioma recurrence, we attribute the variability in recurrence rates found in our analysis to the heterogeneity of the populations we studied. Many studies reported on patients with varying tumor grade who had different forms of treatment. For example, Marciscano and colleagues reported on 71 WHO grade I meningiomas (67 patients received surgery alone, three patients received surgery and radiation therapy, and one patient received surgery alone). The Simpson grade varied from grade I to IV. Overall, they found a recurrence rate of 11.1% over a median of 25.6 months [19]. In comparison, Ma and colleagues reported on 40 lateral trigone WHO grade I meningiomas that had a 0% recurrence following 32.1 months follow up [5]. Controlling for WHO grade, we were unable to determine if differences in recurrence rates was due to variability in time of follow-up, difference in Simpson grade, or the addition of radiation therapy. None of the studies provided specific demographic or treatment information per patient. As a result, pooling of the data was not possible and the only alternative to presentation of recurrence was using ranges. Overall, the heterogeneity of the patient population may be responsible for the variability in recurrence rates.
One of the factors that influences recurrence for any condition is follow-up time. The longer the follow-up, the greater the increase in recurrences by allowing for more time for recurrence. In order to control for follow-up time, we employed a recurrence per 100-person-year system that factored in the mean time of recurrence. As expected, the results demonstrate that WHO grade II meninigomas had a greater recurrence per 100-person-years than WHO grade I meningioms [5, 12, 14].
Our search identified five studies that reported on postoperative symptoms [5, 13, 15, 22, 23]. Following treatment the majority of patients reported neurologic improvement [5, 22]. This has been previously demonstrated in the literature as a number of studies have reported improvement in quality of life following meningioma resection and improved capabilities of performing usual activities [26, 27]. The most common symptoms/signs following surgery were cranial nerve deficits, however this finding might be the result of bias, as Liu et al. were the only identified group that reported on postoperative cranial nerve deficits [15]. More studies reported on postoperative headache outcomes, with more patients reporting improvement of headaches in comparison to worsening [5, 13, 23]. Previous studies have also demonstrated reduction in pain and anxiety following meningioma surgery [26, 27].
The literature supports that meningiomas can fall into one of three growth categories: no growth, linear growth, or exponential growth [2, 28, 29]. We attempted to calculate the relationship between PFS and time. Due to the limited number of data points (studies at most included recurrence rates at 3 points in time) it was not possible to calculate a meaningful statistical significance to determine whether recurrence reflected a linear or exponential model. As a substitute, coefficients of determinations were calculated using Microsoft Excel and broadly compared in order to demonstrate whether a linear or exponential model best fit the data. The coefficient of determination of WHO grade I meningiomas was r2 = 0.986 for an exponential model and r2 = 0.988 for a linear model which suggests that recurrence could fit either an exponential or linear model in Nowak et al. study [12]. The coefficient of determination of WHO grade II menigniomas were more varied in our analysis and it is unclear whether recurrence followed a linear or exponential model. Although growth rate patterns have been demonstrated to follow one of three aforementioned categories, in order to elucidate recurrence rate patterns future studies need to include more time interval points at which recurrence rates are assess [2, 28, 29].
Simpson grade for resection has been demonstrated to influence the recurrence rate of meningiomas [30]. We attempted to perform a post-hoc analysis to investigate the relationship between Simpson grade and WHO grade with recurrence. Only three studies had cohorts with at least 20 patient, but from our analysis WHO grade I Simpson grade I had a lower recurrence rate per 100-person-years when compared to WHO grade I Simpson grade II [13,14,15]. These results reflect the literature where a higher Simpson grade is correlated with increased rates of recurrence [31]. In order to determine level of significance and perform a systematic analysis, more studies need to provide stratification of recurrence rates by WHO grade and by Simpson grading.
Limitations
Our analysis has multiple limitations. Of the 13 included studies, only five were cohort studies (Level 2 evidence), and the remaining eight studies were case series (Level 3 or 4). As both cohort and case series are unable to determine causation, our analysis did not enable us to determine causality for differences in recurrence rates following intervention, but focused instead on the observational changes of recurrence based on WHO grade [32]. Due to the lack of reporting of recurrence rates for individual patients, variability in reporting of recurrence rate, and a lack of reporting of time until recurrence, it was not possible to pool the recurrence rates for each WHO grade to compare to see whether there was a meaningful statistical significance between recurrence rate. This represents a major limitation and as a result we were unable to account for significance or draw conclusions, but instead focus on ranges of recurrence rates in the literature, describe noticeable patterns, and provide suggestions for future research.
The heterogeneity in the study populations also made comparison difficult. Heterogeneity of demographics is useful for generalizability of results, but the heterogeneity in intervention type (i.e. different Simpson grading; inclusion of radiation therapy; radiation therapy dosage) made it difficult to compare results [33].
Finally, there were limitations from the search and gathering of data. There is a possibility that some studies may have been missed, though we attempted to minimize this limitation through use of a comprehensive search strategy, as outlined in the “Methods”.
Conclusion
Our analysis reveals a need for more rigorous study of meningioma cure and control following surgical therapy. In order to allow for a more rigorous study of recurrence rates by WHO grade, future studies should consider stratifying recurrence rates by WHO grade and Simpson grade and including recurrence rates at several points in time so that clinicians can determine whether a linear, exponential or other model best reflects the recurrence rates of menigniomas. Future studies should be prospective in nature to account for the most recent definitions based on the WHO grade classification of brain tumors. If possible, demographics and outcomes for each patient should be included in the study to allow for combining data points to accrue the most robust mean of recurrence.
References
Saraf S, McCarthy BJ, Villano JL (2011) Update on meningiomas. Oncologist 16:1604–1613. https://doi.org/10.1634/theoncologist.2011-0193
Wong RH, Wong AK, Vick N, Farhat HI (2013) Natural history of multiple meningiomas. Surg Neurol Int 4:71. https://doi.org/10.4103/2152-7806.112617
Miao Y, Lu X, Qiu Y, Jiang J, Lin Y (2010) A multivariate analysis of prognostic factors for health-related quality of life in patients with surgically managed meningioma. J Clin Neurosci 17:446–449. https://doi.org/10.1016/j.jocn.2009.07.111
Walcott BP, Nahed BV, Brastianos PK, Loeffler JS (2013) Radiation treatment for WHO Grade II and III meningiomas. Front Oncol 3:227. https://doi.org/10.3389/fonc.2013.00227
Ma J, Cheng L, Wang G, Lin S (2014) Surgical management of meningioma of the trigone area of the lateral ventricle. World Neurosurg 82:757–769. https://doi.org/10.1016/j.wneu.2014.05.026
Palma L, Celli P, Franco C, Cervoni L, Cantore G (1997) Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. Neurosurg Focus 2:e3
Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376. https://doi.org/10.1038/nrn3475
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol 15:515–534. https://doi.org/10.1093/neuonc/nos307
DeVries JG, Berlet GC (2010) Understanding levels of evidence for scientific communication. Foot Ankle Spec 3:205–209. https://doi.org/10.1177/1938640010375184
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2007) Coding manual for cohort studies. Newcastle–Ottawa Scale. Hospital Research Institute Ottawa. http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf. Accessed 03 Sept 2017
Hu WH, Zhang C, Zhang K, Meng FG, Chen N, Zhang JG (2013) Selective amygdalohippocampectomy versus anterior temporal lobectomy in the management of mesial temporal lobe epilepsy: a meta-analysis of comparative studies. J Neurosurg 119:1089–1097. https://doi.org/10.3171/2013.8.JNS121854
Nowak A, Dziedzic T, Krych P, Czernicki T, Kunert P, Marchel A (2015) Benign versus atypical meningiomas: risk factors predicting recurrence. Neurol Neurochir Pol 49:1–10. https://doi.org/10.1016/j.pjnns.2014.11.003
Koutourousiou M, Fernandez-Miranda JC, Stefko ST, Wang EW, Snyderman CH, Gardner PA (2014) Endoscopic endonasal surgery for suprasellar meningiomas: experience with 75 patients. J Neurosurg 120:1326–1339. https://doi.org/10.3171/2014.2.jns13767
Lee KD, DePowell JJ, Air EL, Dwivedi AK, Kendler A, McPherson CM (2013) Atypical meningiomas: is postoperative radiotherapy indicated? Neurosurg Focus 35:E15. https://doi.org/10.3171/2013.9.FOCUS13325
Liu Z, Wang C, Wang H, Wang Y, Li JY, Liu Y (2013) Clinical characteristics and treatment of angiomatous meningiomas: a report of 27 cases. Int J Clin Exp Pathol 6:695–702
Sughrue ME, Kane AJ, Shangari G, Rutkowski MJ, McDermott MW, Berger MS, Parsa AT (2010) The relevance of Simpson Grade I and II resection in modern neurosurgical treatment of World Health Organization Grade I meningiomas. J Neurosurg 113:1029–1035. https://doi.org/10.3171/2010.3.JNS091971
Bloss HG, Proescholdt MA, Mayer C, Schreyer AG, Brawanski A (2010) Growth pattern analysis of sphenoid wing meningiomas. Acta Neurochir (Wien) 152:99–103. https://doi.org/10.1007/s00701-009-0556-2 (discussion 103)
Press RH, Prabhu RS, Appin CL, Brat DJ, Shu HK, Hadjipanayis C, Olson JJ, Oyesiku NM, Curran WJ, Crocker I (2014) Outcomes and patterns of failure for grade 2 meningioma treated with reduced-margin intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 88:1004–1010. https://doi.org/10.1016/j.ijrobp.2013.12.037
Marciscano AE, Stemmer-Rachamimov AO, Niemierko A, Larvie M, Curry WT, Barker FG 2nd, Martuza RL, McGuone D, Oh KS, Loeffler JS, Shih HA (2016) Benign meningiomas (WHO Grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. J Neurosurg 124: 106–114 https://doi.org/10.3171/2015.1.JNS142228
Gallagher MJ, Jenkinson MD, Brodbelt AR, Mills SJ, Chavredakis E (2016) WHO grade 1 meningioma recurrence: are location and Simpson grade still relevant? Clin Neurol Neurosurg 141:117–121. https://doi.org/10.1016/j.clineuro.2016.01.006
Hwang WL, Marciscano AE, Niemierko A, Kim DW, Stemmer-Rachamimov AO, Curry WT, Barker FG 2nd, Martuza RL, Loeffler JS, Oh KS, Shih HA, Larvie M (2016) Imaging and extent of surgical resection predict risk of meningioma recurrence better than WHO histopathological grade. Neuro Oncol 18: 863–872. https://doi.org/10.1093/neuonc/nov285
Nanda A, Konar SK, Maiti TK, Bir SC, Guthikonda B (2016) Stratification of predictive factors to assess resectability and surgical outcome in clinoidal meningioma. Clin Neurol Neurosurg 142:31–37. https://doi.org/10.1016/j.clineuro.2016.01.005
Nanda A BS, Konar S, Maiti T, Kalakoti P, Jacobsohn JA, Guthikonda B (2016) Outcome of resection of WHO Grade II meningioma and correlation of pathological and radiological predictive factors for recurrence. J Clin Neurosci 31:112–121
Violaris K, Katsarides V, Karakyriou M, Sakellariou P (2013) Surgical outcome of treating Grades II and III meningiomas: a report of 32 cases. Neurosci J. https://doi.org/10.1155/2013/706481
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Jakola AS, Gulati M, Gulati S, Solheim O (2012) The influence of surgery on quality of life in patients with intracranial meningiomas: a prospective study. J Neurooncol 110:137–144. https://doi.org/10.1007/s11060-012-0947-8
Kalkanis SN, Quinones-Hinojosa A, Buzney E, Ribaudo HJ, Black PM (2000) Quality of life following surgery for intracranial meningiomas at Brigham and Women’s Hospital: a study of 164 patients using a modification of the functional assessment of cancer therapy-brain questionnaire. J Neurooncol 48:233–241
Chamoun R, Krisht KM, Couldwell WT (2011) Incidental meningiomas. Neurosurg Focus 31:E19. https://doi.org/10.3171/2011.9.focus11220
Nakasu S, Fukami T, Nakajima M, Watanabe K, Ichikawa M, Matsuda M (2005) Growth pattern changes of meningiomas: long-term analysis. Neurosurgery 56:946–955 (discussion 946–955)
İldan F, Erman T, Göçer A, Tuna M, Bağdatoğlu H, Çetinalp E, Burgut R (2007) Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: a multivariate analysis in the midterm follow-up. Skull Base 17:157–171. https://doi.org/10.1055/s-2007-970554
Voss KM, Spille DC, Sauerland C, Suero Molina E, Brokinkel C, Paulus W, Stummer W, Holling M, Jeibmann A, Brokinkel B (2017) The Simpson grading in meningioma surgery: does the tumor location influence the prognostic value? J Neurooncol 133:641–651. https://doi.org/10.1007/s11060-017-2481-1
Esene IN, Ngu J, El Zoghby M, Solaroglu I, Sikod AM, Kotb A, Dechambenoit G, El Husseiny H (2014) Case series and descriptive cohort studies in neurosurgery: the confusion and solution. Childs Nerv Syst 30:1321–1332. https://doi.org/10.1007/s00381-014-2460-1
Kravitz RL, Duan N, Braslow J (2004) Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q 82:661–687. https://doi.org/10.1111/j.0887-378X.2004.00327.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lam Shin Cheung, V., Kim, A., Sahgal, A. et al. Meningioma recurrence rates following treatment: a systematic analysis. J Neurooncol 136, 351–361 (2018). https://doi.org/10.1007/s11060-017-2659-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2659-6