Abstract
To report the efficacy and tolerability of lacosamide as an add-on treatment in patients with gliomas and uncontrolled seizures despite conventional antiepileptic drugs (AEDs). We conducted an observational study on 71 patients to describe patterns of response to lacosamide and the association between clinico-pathological factors and seizure control. We observed at 3, 6 and 9 months a seizure reduction ≥ 50% in 74.6, 76 and 86.2% of patients and a seizure freedom in 42.2, 43 and 50%, respectively. The median number of seizures in the 3 months before treatment was 13, and decreased to 3 between baseline and 6 months, and to 0.5 between 6 and 9 months. The best seizure response was observed at 3 months (62%). Sixty per cent of patients displayed the maximum seizure control with doses of lacosamide of 100–250 mg/day, while 21% needed doses up to 400 mg/day. Seizure reduction ≥ 50% and seizure freedom were higher in patients who received lacosamide as first add-on compared to those who received a later adjunctive therapy. A reduction ≥ 50% of seizures was observed in a proportion of patients with progressive disease on MRI. Age > 45 years (OR 0.11, 95% CI 0.02–0.63, p = 0.013) was a significant predictor of seizure freedom at 9 months on multivariate analysis. The study suggests that lacosamide, when added to any baseline AEDs, is effective in obtaining a high seizure reduction and seizure freedom regardless of the tumor activity and response to antineoplastic therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a common cause of morbidity in patients with brain tumors with a seizure frequency ranging from 60 to 90% in low grade gliomas (LGG) and from 30 to 50% in high grade gliomas (HGG) [1,2,3,4]. The management of seizures in these patients is complicated by tumor growth and drug interactions between antiepileptic drugs (AEDs) and antineoplastic treatments and steroids leading to an increased risk of side effects [5,6,7]. Moreover, a high percentage of seizures are pharmacoresistant [8, 9].
Evidence-based treatment guidelines are not available, and the optimal antiepileptic therapy in patients with brain tumors and epilepsy (BTRE) remains to be defined.
Limited data are available on the efficacy of AEDs in gliomas, with rates of seizure response ranging from 15 to 100% and seizure-freedom ranging from 20 to 100% following valproate, levetiracetam, topiramate or oxcarbazepine [10].
Lacosamide is a third generation AED with a novel mechanism of action of selectively enhancing slow inactivation of voltage-gated sodium channels [11, 12]. It was approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2008 as an add-on therapy in the treatment of partial-onset seizures in adult. Lacosamide has a favorable pharmacokinetic profile, including a lack of induction or inhibition of hepatic enzymes, low protein binding, rapid and complete oral absorption not affected by food intake, 13-hour-half-life that permits twice daily administration, and low potential for drug interactions. Such characteristics make lacosamide an interesting therapeutic option for patients with BTRE, especially for those who are undergoing antineoplastic treatments.
To date, there is paucity of data on the efficacy and safety of lacosamide in brain tumors [13, 14].
The aim of this single institution observational study was to evaluate the efficacy and tolerability of lacosamide as add-on treatment in a cohort of consecutive patients with gliomas and uncontrolled seizures despite conventional AEDs.
Methods
Patient selection
The inclusion criteria of the study were as follows: (1) age (≥ 18 years); (2) histologically verified supratentorial gliomas of grade II, III or IV according to WHO 2007; (3) uncontrolled seizures despite an appropriate treatment with AEDs in adequate doses and serum concentration or unacceptable adverse effects from previous AEDs; (4) at least one seizure in the 3 months preceding the start of lacosamide; (5) absence of ventricular or atrial arrhythmias; (6) signed informed consent.
The study was approved by the local Institutional Review Board.
Study design
Patients received an initial daily dose of lacosamide of 50 mg twice daily with increments of 50 mg every week until a maximum dose of 400 mg/day depending of seizure control and/or intolerable adverse events (AEs). Any change or dose increase of concomitant AEDs at baseline or during lacosamide treatment were not allowed.
Follow-up visits were scheduled at baseline, at week 2, and then monthly. At each visit information regarding type and frequency of seizures, and dose and side effects of lacosamide were recorded in patient diaries.
MRI with contrast enhancement was performed every 3 months.
Response of tumor following antineoplastic treatments was evaluated according to Response Assessment in Neuro-Oncology (RANO) criteria for high and low-grade gliomas [15, 16] (Supplementary Table 1). Patients not undergoing antineoplastic treatments during lacosamide were coded as stable disease.
Efficacy assessments
The primary efficacy endpoints were seizure reduction ≥ 50% versus baseline and seizure-freedom at 3, 6 and 9 months. Secondary endpoints of efficacy were latency and duration of best seizure response.
Safety assessment
Safety assessment included monitoring of all treatment-emergent AEs, treatment discontinuations, clinical laboratory tests results (chemistry, hematology, ECG and vital signs).
Side effects were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 [17].
Statistical analysis
Patient characteristics were described using percentage frequencies for categorical data, median [interquartile range, (IQR)] or mean with standard deviation (SD) for continuous data.
The comparison between baseline seizure frequency and that at 3, 6 and 9 months following lacosamide, was performed with the Chi square test.
We selected a priori the following factors potentially associated with seizure control: age, sex, tumor type, tumor location, tumor grade, extent of surgery, seizure type, seizures duration, seizure frequency, timing of lacosamide add-on, tumor status on MRI, and concurrent antineoplastic treatment. Crude and adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated using univariate and multivariate logistic regression models. Variables that showed an association in the univariate analysis and well known prognostic factors were included in the multivariate models.
All analyses were performed using Stata 13.
Results
Patient characteristics at baseline
Between January 2012 and June 2015, 71 patients were enrolled into the study and met the inclusion criteria: 58 patients (81.7%) completed the 9 months treatment of lacosamide, while 13 patients (18.3%) died before 9 months due to tumor progression. Clinical characteristics of the patients are summarized in Table 1.
A detailed description of seizures separately for low and high grade gliomas is reported in Supplementary Table 2.
Levetiracetam (84.5%) was the most frequently used concomitant AED, while few patients only (35.2%) were on EIAEDs (Supplementary Table 3).
Sixty patients (84.6%) started lacosamide because of uncontrolled seizures despite an adequate treatment with one or more AEDs, while in 11 patients (15.4%) lacosamide was introduced for adverse effects of the previous AEDs needing the interruption of therapy.
Fifty-four patients had seizures associated with a stable or responding tumor on MRI, while 46% had seizures associated with a progressive disease.
The median lacosamide dose was 200 mg (range 100–400 mg) at 3 and 6 months, and 250 mg (range 100–400 mg) at 9 months.
Efficacy
A seizure reduction ≥ 50% from baseline was achieved in 53/71 patients (74.6%, 95% CI 62.9–84.2) at 3 months from the start of lacosamide, in 50/65 patients (76.9%, 95% CI 64.8–86.5) at 6 months, and in 50/58 patients (86.2%, 95% CI 74.6–93.8) at 9 months. A seizure freedom was achieved in 30/71 patients (42.2%, 95% CI 30.6–54.6) at 3 months, in 28/65 (43.1%, 95% CI 30.8–56.0) at 6 months, and in 29/58 (50%, 95% CI 36.6–63.4) at 9 months. When considering the 58 patients only who completed the 9 months treatment with lacosamide the seizure reduction > 50% from baseline was observed in 43/58 patients (74.6%, 95% CI 60.9–84.7) and in 48/58 (82.7%, 95% CI 70.6–91.4) at 3 and 6 months, respectively. In the same population the seizure freedom was observed in 25/58 patients (43.1%, 95% CI 30.2–56.8) and in 26/58 (44.8%, 95% CI 31.7–58.5) at 3 and 6 months, respectively.
The best seizure response was observed at 3 months in 44/71(62%) patients, at 6 months in 13/65 (18.3%), and at 9 months in 2/58 (2.8%).
We observed a significant reduction of number of patients with both daily (from 10 to 1%) and weekly seizures (from 48 to 4%) with a parallel increase in the number of patients with only monthly seizures (from 42 to 45%) or seizure-free (half of patients) at 9 months (p < 0.001) (Fig. 1).
The best seizure response was obtained between 100 and 150 mg in 7/53 (13.2%) patients, 200–250 mg in 25/53 (47.2%), 300–350 mg in 10/53 (18.9%) and 400 mg in 11/53 (20.7%), with a median of 200 mg. In 15 patients achieving seizure freedom we were able to reduce the number of concomitant AEDs, and 1 patient remained on lacosamide monotherapy.
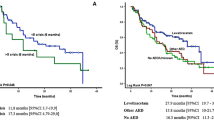
A seizure reduction ≥ 50% at 3, 6 and 9 months was achieved in 34/53 (64.1%), 36/50 (72.0%) and 32/50 (64.0%) of patients who received lacosamide as first add-on treatment, and in 19/53 (35.9%), 14/50 (28.0%) and 18/50 (36.0%) of patients who received lacosamide as a later adjunctive therapy. A seizure freedom at 3, 6 and 9 months was achieved in 20/30 (66.7%), 19/28 (67.9%) and 18/29 (62.1%) of patients who received lacosamide as a first-add on treatment, and in 10/30 (33.3%), 9/28 (32.1%) and 11/29 (37.9%) of patients who received lacosamide as a later adjunctive treatment.
A seizure reduction ≥ 50% at 3, 6 and 9 months was achieved in 19/53 (35.8%), 18/50 (36.0%), 17/50 (34.0%) of patients who received a traditional sodium channel blocker (SCB+), and in 34/53 (64.2%), 32/50 (64.0%) and 33/50 (66.0%) of patients who received a nontraditional sodium channel blocker (SCB). A seizure freedom at 3, 6 and 9 months was achieved in 11/30 (36.7%), 9/28 (32.1%) and 10/29 (34.5%) of patients receiving SCB + AEDs and in 19/30 (63.3%), 19/28 (67.9%) and 19/29 (65.5%) of patients receiving SCB-AEDs.
The relationships between the response to lacosamide in LGG and HGG and status of the tumor on MRI are reported in the Supplementary Tables 4 and 5. Notably, a number of patients with progressive disease had a reduction of seizures ≥ 50%, but none of them became seizure-free.
We next restricted the analysis to those patients, who either had not received any antineoplastic treatment before or during the therapy with lacosamide (11/21) or had received prior radiotherapy and/or chemotherapy with a minimum interval of 6 months (10/21). All these patients had received previous surgery more than 1 year before. Among LGGs a seizure reduction ≥ 50% was observed in 11/15 patients at 3 months, 13/15 at 6 months and 12/15 at 9 months, while among HGGs a seizure reduction ≥ 50% was observed in 3/6 patients at 3 months, 1/3 patients at 6 months, and 1/2 patients at 9 months. Among LGGs, seizure freedom was achieved in 5/15 patients at 3 months, and 6/15 at 6 and 9 months, while among HGGs seizure freedom was achieved in 2/6 patients at 3 months, 1/3 at 6 months and 1/2 at 9 months.
Six HGGs with status epilepticus refractory to either phenytoin or VPA received intravenous lacosamide. Following a daily dose of 400 mg/day, we observed a clinical and EEG remission within 24–72 h. Two out of six patients remained seizure-free at 9 months with a maintenance daily dose of lacosamide of 400 mg.
Tables 2 and 3 report the relationships between clinical factors and seizure control at 3 months following lacosamide. Univariate analysis shows that a seizure reduction ≥ 50% was more difficult to reach in older patients (OR 0.45, 95% CI 0.15–1.35), patients with astrocytoma (OR 0.54, 95% CI 0.17–1.74) and those who had a later add-on (OR 0.59, 95% CI 0.20–1.76). However, none of these correlations retained the statistical significance after multivariate analysis. A daily seizure frequency was a significant predictor of seizure freedom at 3 months both in univariate (OR 0.16, 95% CI 0.04–0.67, p = 0.012) and multivariate (OR 0.13, 95% CI 0.03–0.64, p = 0.012) analysis.
Tables 4 and 5 report the relationships between clinical factors and seizure control at 3 and 9 months following lacosamide in patients who completed the 9 month treatment (N = 58). Again, a daily seizure frequency was a significant predictor of seizure freedom at 9 months both in univariate (OR 0.18, 95% CI 0.04–0.77, p = 0.021) and multivariate (OR 0.07, 95% CI 0.01–0.55, p = 0.11) analysis. Moreover, weekly seizures (OR 0.13, 95% CI 0.02–0.65, p = 0.013) and age > 45 years (OR 0.11, 95% CI 0.02–0.63, p = 0.013) resulted significant predictors of seizure freedom at 9 months in the multivariate model.
Safety
Overall, lacosamide was well tolerated and most patients (87.3%) did not report any toxicity. We observed dizziness in 4 patients (5.7%, CTCAE grade III) leading to a discontinuation of the drug in 2, nausea/vomiting in 1 patient (1.4%, CTCAE grade II), fatigue in 1 patient (1.4%, CTCAE grade I) and palpitations in 1 patient (1.4%, CTCAE grade II). In two patients (2.8%) a discontinuation of the drug was necessary after 6 months due to a further increase of seizures or withdrawal of informed consent. We did not observe any difference in terms of tolerability between patients in whom lacosamide was added either to EIAEDs or non-EIAEDs, patients with HGG or LGG or patients on antineoplastic treatment or off-treatment.
Discussion
The management of seizures in patients with gliomas is challenging, as the choice of the most appropriate AED medication must take into account several aspects, including age, type of seizures, activity of the tumor and the potential interactions with chemotherapeutics and steroids [5, 18]. Moreover, the evaluation of the efficacy of AEDs must consider the use of antineoplastic treatments (surgery, radiotherapy, chemotherapy, targeted agents), that can favorably impact the seizure burden, thus being a sort of confounding factor [19]. Aside from a general agreement to prefer non-EIAEDs over EIAEDs, the choice of an AED is commonly based on the physician’s preference due to lack of firm-data from clinical studies. The most frequently used AED is levetiracetam, based on a good efficacy versus toxicity profile, leading to a seizure reduction ≥ 50% and a seizure freedom in 65–100% and 20–77% of patients, respectively, when used in add-on, and seizure freedom in 76–91% of patients when used in monotherapy [10]. Valproic acid, sometimes used for a potential concomitant antineoplastic efficacy as well, yields a seizure freedom around 60% in add-on, and 30.4–77.8% in monotherapy [10, 20].
Two retrospective studies only are available on the efficacy of lacosamide in brain tumors [13, 14]. The study by Maschio et al. [13] was a small case series of 14 patients with gliomas with a median duration of follow-up of 5.4 months: 35.7% of patients had a seizure reduction ≥ 50 and 42.9% additional patients were seizure-free. The larger retrospective study of Saria et al. [14] included 70 patients of whom 65 with gliomas with a median follow-up of 6.2 months. Overall, 54% of patients reported a decrease ≥ 50% of seizure frequency. However, neither seizure freedom nor the relationships with tumor status and antineoplastic treatments were evaluated. In our study we observed a seizure reduction ≥ 50% in 74.6, 76 and 86.2% of patients and a seizure freedom in 42.2, 43 and 50% at 3, 6 and 9 months. The values at 9 months appear slightly higher than those reported by the two aforementioned studies, and are in line with studies on lacosamide in non neoplastic patients with partial-onset seizures [11, 12].
The proportion of patients achieving both a seizure reduction ≥ 50% and seizure freedom increased from the 3-month interim visit to the final visit at 9 months. About 60% of patients displayed the maximum seizure control with relatively low doses (100–250 mg), while 21% of patients only needed doses up to 400 mg.
Seizure reduction ≥ 50% and seizure freedom were greater when lacosamide was added early in the treatment (i.e. after 1 prior AED only) compared to a later add-on. This observation is consistent with recent observational studies using lacosamide in a non-neoplastic epilepsy population [21,22,23]. Seizure reduction ≥ 50% and seizure freedom were greater in patients treated with a lacosamide SCB(−) combination compared to patients treated with lacosamide SCB(+) combination. This finding is similar to that observed in the LACO-EXP retrospective study [21], while in the non-interventional VITOBA study [22] seizure control did not differ between the two combinations. One could speculate that the lower efficacy of lacosamide in the SCB(+) group in our study is related to an interactions between EIADs, such as carbamazepine, oxcarbazepine or phenytoin, and chemotherapeutics and steroids, that weakened the efficacy of the antiepileptic regimen.
AED resistance is generally considered proportional to tumor grade [24, 25]. However, in this study both low and high grade glioma patients displayed a high seizure control following lacosamide.
Interestingly, some patients with progressive disease on MRI displayed a seizure reduction; however, we cannot rule out that this could be attributed at least in part to an increase in steroid medication more than a positive effect of lacosamide.
Antineoplastic treatments may favorably impact the frequency of seizures in gliomas [26,27,28,29,30], and this could have occurred in some patients of our series. Nonetheless, a proportion of patients, who never received or were off-treatment while on lacosamide, displayed a seizure control, that may be attributed to lacosamide alone.
Our study confirms that intravenous lacosamide is active and safe in managing patients with refractory status epilepticus [31, 32].
Most of the clinical factors that have been analyzed for a potential association with seizure control were not statistically significant in multivariate analyses, and this can be due, at least in part, to the relatively small sample size. Older age (> 45 years) was associated with a better chance of seizure freedom at 9 months, and a similar age effect has been reported in the VITOBA study [22]. As in the general population of patients with partial onset seizures [33, 34] lacosamide in this cohort of gliomas was well tolerated and we did not observe adverse events due to an interaction with antineoplastic treatments.
This study has several limitations. First, it lacks a control population, and the sample size is relatively small, Second, the patient population is quite heterogeneous in term of tumor types, location and treatments, thus rendering the conclusions from the different comparisons less reliable. However, to our knowledge, this is the first observational study analyzing in a population of patients with gliomas and medically intractable seizures the patterns of response to lacosamide as add-on treatment and the clinicopathological factors potentially associated with seizure response. Overall, lacosamide, when added to any baseline AEDs, is effective in obtaining a high seizure reduction and seizure freedom. This study reports some novel findings, i.e. that the efficacy of the drug is higher in older patients and when employed as first add-on, and may be independent of the tumor status and response to antineoplastic treatments.
References
van Breemen MS, Wilms EB, Vecht CJ (2007) Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 6:421–430. doi:10.1016/S1474-4422(07)70103-5
Rudà R, Trevisan E, Soffietti R (2010) Epilepsy and brain tumors. Curr Opin Oncol 22:611–620. doi:10.1097/CCO.0b013e32833de99d
Weller M, Stupp R, Wick W (2012) Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol 13:e375–382. doi:10.1016/S1470-2045(12)70266-8
Armstrong TS, Grant R, Gilbert MR et al (2016) Epilepsy in glioma patients: mechanisms, management, and impact of anticonvulsant therapy. Neuro Oncol 18:779–789. doi:10.1093/neuonc/nov269
Vecht CJ, Wagner GL, Wilms EB (2003) Treating seizures in patients with brain tumors: drug interactions between antiepileptic and chemotherapeutic agents. Semin Oncol 30:49–52
Rossetti AO, Stupp R (2010) Epilepsy in brain tumor patients. Curr Opin Neurol 23:603–609. doi:10.1097/WCO.0b013e32833e996c
Rudà R, Bello L, Duffau H et al (2012) Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol 14(Suppl 4):iv55–iv64 doi:10.1093/neuonc/nos199
Hildebrand J, Lecaille C, Perennes J et al (2005) Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology 65:212–215. doi:10.1212/01.wnl.0000168903.09277.8f
Rudà R, Soffietti R (2015) What is new in the management of epilepsy in gliomas? Curr Treat Options Neurol 17:351. doi:10.1007/s11940-015-0351-8
Vecht CJ, Kerkhof M, Duran-Pena A (2014) Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist 19:751–759. doi:10.1634/theoncologist.2014-0060
Beydoun A, D’Souza J, Hebert D et al (2009) Lacosamide: pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev Neurother 9:33–42. doi:10.1586/14737175.9.1.33
Scott LJ (2015) Lacosamide: a review in focal seizures in patients with epilepsy. Drugs 75:2143–2154. doi:10.1007/s40265-015-0514-7
Maschio M, Dinapoli L, Mingoia M et al (2011) Lacosamide as add-on in brain tumor-related epilepsy: preliminary report on efficacy and tolerability. J Neurol 258:2100–2104. doi:10.1007/s00415-011-6132-8
Saria MG, Corle C, Hu J et al (2013) Retrospective analysis of the tolerability and activity of lacosamide in patients with brain tumors: clinical article. J Neurosurg 118:1183–1187. doi:10.3171/2013.1.JNS12397
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
van den Bent MJ, Wefel JS, Schiff D et al (2011) Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12:583–593. doi:10.1016/S1470-2045(11)70057-2
Basch E, Iasonos A, McDonough T et al (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7:903–909. doi:10.1016/S1470-2045(06)70910-X
Jaeckle KA, Ballman K, Furth A et al (2009) Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology 73:1207–1213. doi:10.1212/WNL.0b013e3181bbfeca
Avila EK, Chamberlain M, Schiff D et al (2017) Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol 19:12–21. doi:10.1093/neuonc/now190
Kerkhof M, Dielemans JC, van Breemen MS et al (2013) Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol 15:961–967. doi:10.1093/neuonc/not057
Villanueva V, Lopez FJ, Serratosa JM et al (2013) Control of seizures in different stages of partial epilepsy: LACO-EXP, a Spanish retrospective study of lacosamide. Epilepsy Behav 29:349–356. doi:10.1016/j.yebeh.2013.07.024
Runge U, Arnold S, Brandt C et al (2015) A noninterventional study evaluating the effectiveness and safety of lacosamide added to monotherapy in patients with epilepsy with partial-onset seizures in daily clinical practice: the VITOBA study. Epilepsia 56:1921–1930. doi:10.1111/epi.13224
Zadeh WW, Escartin A, Byrnes W et al (2015) Efficacy and safety of lacosamide as first add-on or later adjunctive treatment for uncontrolled partial-onset seizures: a multicentre open-label trial. Seizure 31:72–79. doi:10.1016/j.seizure.2015.07.001
van Breemen MS, Rijsman RM, Taphoorn MJ et al (2009) Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol 256:1519–1526. doi:10.1007/s00415-009-5156-9
Rosati A, Tomassini A, Pollo B et al (2009) Epilepsy in cerebral glioma: timing of appearance and histological correlations. J Neurooncol 93:395–400. doi:10.1007/s11060-009-9796-5
Kaloshi G, Benouaich-Amiel A, Diakite F et al (2007) Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology 68:1831–1836. doi:10.1212/01.wnl.0000262034.26310.a2
Sherman JH, Moldovan K, Yeoh HK et al (2011) Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg 114:1617–1621. doi:10.3171/2010.12.JNS101602
Rudà R, Magliola U, Bertero L et al (2013) Seizure control following radiotherapy in patients with diffuse gliomas: a retrospective study. Neuro Oncol 15:1739–1749. doi:10.1093/neuonc/not109
Koekkoek JA, Dirven L, Heimans JJ et al (2016) Seizure reduction is a prognostic marker in low-grade glioma patients treated with temozolomide. J Neurooncol 126:347–354. doi:10.1007/s11060-015-1975-y
Koekkoek JA, Dirven L, Heimans JJ et al (2015) Seizure reduction in a low-grade glioma: more than a beneficial side effect of temozolomide. J Neurol Neurosurg Psychiatry 86:366–373. doi:10.1136/jnnp-2014-308136
Lang N, Lange M, Schmitt FC et al (2016) Intravenous lacosamide in clinical practice—results from an independent registry. Seizure 39:5–9. doi:10.1016/j.seizure.2016.01.008
Mnatsakanyan L, Chung JM, Tsimerinov EI et al (2012) Intravenous Lacosamide in refractory nonconvulsive status epilepticus. Seizure 21:198–201. doi:10.1016/j.seizure.2011.12.008
Biton V, Gil-Nagel A, Isojarvi J et al (2015) Safety and tolerability of lacosamide as adjunctive therapy for adults with partial-onset seizures: analysis of data pooled from three randomized, double-blind, placebo-controlled clinical trials. Epilepsy Behav 52:119–127. doi:10.1016/j.yebeh.2015.09.006
Steinhoff BJ, Eckhardt K, Doty P et al (2016) A long-term noninterventional safety study of adjunctive lacosamide therapy in patients with epilepsy and uncontrolled partial-onset seizures. Epilepsy Behav 58:35–43. doi:10.1016/j.yebeh.2016.02.041
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rudà, R., Pellerino, A., Franchino, F. et al. Lacosamide in patients with gliomas and uncontrolled seizures: results from an observational study. J Neurooncol 136, 105–114 (2018). https://doi.org/10.1007/s11060-017-2628-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2628-0