Abstract
Primary central nervous system tumors (PCNST) are rare tumors responsible for high mortality and morbidity. Their epidemiology is poorly known, and clinical data are scarcely analyzed at a national level. In this study, we aimed at providing descriptive epidemiological data and incidence rates for all histological subtypes of PCNST according to the WHO classification. We conducted a nationwide population-based study of all newly diagnosed and histologically confirmed PCNST in France, between 2006 and 2011. A total of 57,816 patients were included: male 46.4%, median age at diagnosis 56 years old (range 0–99). For all newly diagnosed PCNST with histological confirmation the crude incidence rate was 15.5/105 per 100,000 person-years. To enable international comparisons, standardized rates were calculated: 14.1/105 (population of reference: USA), 14.5/105 (population of reference: Europe), and 12.0/105 (population of reference: world). 23.4% of samples were cryopreserved. Resection was performed in 79.1% of cases. Results are detailed (incidence rate, sex ratio, median age at diagnosis, number of cryopreserved samples, and type of surgery) for each of the 143 histological subtypes of PCNST, including all rare tumors. For example, incidence rates (population of reference: USA) were 0.018/105 for anaplastic gangliogliomas, 0.054/105 for malignant meningiomas, and 0.036/105 for hemangiopericytomas. Our study is the first to describe incidence rates and epidemiological data for all histological subtypes of PCNST, including rare tumors, at a national level. Its methodology ensures the exhaustiveness of the data collection for histologically-proven cases. Histological population-based studies have many perspectives in the field of clinical epidemiology and research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system tumors (PCNST) are defined as all primary tumors located within the central nervous system (CNS) including the envelopes of the CNS and the origin of the nerves [1–3]. They show a malignant, benign or uncertain evolution. PCNST represent a heterogeneous group of tumors, with 143 histological subtypes according to the World Health organization (WHO) classification [1–3]. They have different causes, prognostic factors and treatments. The incidence rate (IR) of all PCNST ranges from 17.6/105 to 22.0/105 in American [4] and European studies [5–7]. However, considering the high number of histological subtypes, nearly each subtype may be considered as a rare tumor. These tumors are a major public health issue as they are responsible for high morbidity [8] and mortality [4, 8].
Epidemiological data on PCNST are poor, as the declaration is not mandatory in many countries. Moreover, brain tumor registration at the worldwide level is often limited to malignant tumors, with the exclusion of non-malignant or borderline tumors [9]. The difficulties for tumor registries are numerous. An exhaustive recording of all cases is very time-consuming as it requires collecting data from a high number of sources: death certificates, data from pathologists, radiologists, neurologists and neurosurgeons [6]. For these reasons, except in countries where declaration is mandatory, tumors with no histological diagnosis are particularly challenging to collect.
There are few national registries. Among them, the Central Brain Tumor Registry of the United States (CBTRUS) provides extensive data on PCNST, classified according to a histology-grouping scheme (without detail of all the WHO subtypes) [4]. The Austrian Brain Tumor Registry [5] and the registries from Scandinavia [10–12] and England [13] also report descriptive epidemiological data at a national level. Some specific registries [14–16] and regional registries (in Spain, Italy, France…) [6, 17, 18] also provide information. However, the registries that collect all histological subtypes described by the WHO 2007 classification do not report results on all subtypes as they use various histology-grouping schemes [4]. Moreover, clinical and biological data, despite their usefulness (in particular for rare tumors), are rarely investigated.
Our study primary objective was to provide descriptive epidemiological data and the IRs of all histological subtypes of PCNST at the national level, including all rare subtypes of PCNST for which a histological registry is crucial. Even though our data is based on the 2007 WHO classification [1, 2], and not on the recently published 2016 classification [3], it will serve as a reference for future epidemiological studies, as the incidence rates of all subtypes of PCNST described by the 2007 classification have not yet been reported.
Materials and methods
Settings
The French Brain Tumor Database (FBTDB) is a national histological database of all PCNST in France. It is based on a network of all neurosurgeons, pathologists and neuro-oncologists involved in PCNST, in collaboration with all the societies focused on PCNST. Its methodology has previously been published [19–21]. The FBTDB’s main objective is to prospectively record all histologically-proven cases of PCNST diagnosed in France. Data are collected from two sources. First, a data sheet, that is available in all operating rooms where neurosurgery is performed, is filled by both the neurosurgeon and the pathologist. Second, listings of all cases analyzed by each pathology department are collected annually, to ensure the exhaustiveness of the collection [22].
Design
We conducted a nationwide population-based study of newly diagnosed and histologically confirmed PCNST in Metropolitan France between 2006 and 2011.
Patients
All patients (no age limit) with a histologically-proven PCNST diagnosed between 2006, January 1st and 2011, December 31st in metropolitan France were included (metropolitan France includes mainland France and nearby islands in the Atlantic Ocean and Mediterranean Sea, and excludes overseas territories). Tumors were described using the 2000 WHO classification until 2006 [23], then the 2007 WHO classification [1, 2]. Note: pituitary tumors do not belong to the PCNST according to the WHO classification [1], but are included in a few PCNST registries such as the CBTRUS, and were included in this study.
Exclusion criteria were as follows: secondary tumors of the SNC (metastases), and duplicate records for recurrent disease (in that case, data from the first surgery was recorded if within the inclusion period).
Main measures
The IR, sex ratio, median age at diagnosis, number of cryopreserved samples, and type of surgery (resection or biopsy) were provided for each histological subtype according to the ICD-O-3 (International Classification of Diseases for Oncology) PCNST classification [1–3, 23] and to the Systematized Nomenclature of Medicine (SNOMED) codes from Louis et al. (the list of the included codes is provided in Table 1) [2].
In order to reduce the number of cases with an imprecise histology, in particular gliomas labeled as “not otherwise specified”, an analysis of the pathological report by an expert pathologist (V.R.) was performed for cases of diffuse gliomas with unclear grading according to the WHO classification.
Statistical analysis
Crude and age-standardized IRs per 100,000 person-years were computed for each histological subtype of PCNST. Crude IR is the total number of new cases divided by the corresponding population and multiplied by 100,000. To enable international comparisons, standardized IRs based on a direct standardization model were also calculated with Europe, USA and worldwide populations as references. Crude rates, worldwide-, Europe- and USA-population standardized rates will be referred to, from now on, as CR, WSR, ESR and USR, respectively. The statistical analyses were performed using the SAS software.
Ethical approval
This study was approved by the French legislation, and by all the French Societies involved in neuro-oncology: Société Française de Neuropathologie (SFNP), the Société Française de Neurochirurgie (SFNC) and the Club Neuro-Oncologie of the Société Française de Neurochirurgie (CNO-SFNC), and the Association des Neuro-Oncologues d’Expression Française (ANOCEF).
Results
Characteristics of the population
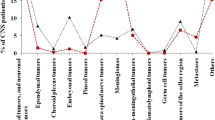
Between 2006 and 2011, 57,816 incident cases of PCNST were recorded. The case distribution according to the year of diagnosis is presented in Table 2 and shows no major fluctuation in time. Among the patients, 46.4% were male, corresponding to a ratio male/female of 0.86. The mean age was 52.6 years (51.9 for males and 53.3 for females). The median ages at diagnosis for all cases, male cases and female cases were 56 (range 0–99), 56 (range 0–96) and 56 (range 0–99) years, respectively. At diagnosis, 4068 patients (7.0%) were aged under 20, and 2948 patients (5.1%) were under 15. The tumor distribution according to the histology group is shown in Fig. 1. Tumors of the neuroepithelial tissue accounted for about 44% (including gliomas 39%) while tumors of the meninges accounted for about one-third and tumors of the cranial and paraspinal nerves for about 10% of cases. The characteristics of all the cases [number of cases, median ages by gender, number of cryopreserved samples, and type of surgery (biopsy or resection)] according to the histological subtype are presented in Table 1. Resection was performed in 79.1% of patients for whom surgical data was available, i.e. 73.2% of all patients (and 64.9% of tumors of the neuroepithelial tissue, 62.3% of glioblastomas, 93.1% of ependymal tumors, 90.4% of embryonal tumors, 98.0% of meningiomas…) (Table 1). 13 508 samples (23.4%) were cryopreserved, among which 7048 gliomas, 3481 meningiomas, and a number of rare subtypes of PCNST (Table 1).
Incidence data
The CR for all newly diagnosed PCNST with histological confirmation was 15.5/105 per person-years (Table 3). To allow international comparisons, age-standardized IRs were calculated: USR 14.1/105, ESR 14.5/105, and WSR 12.0/105. For patients aged <15, the CR for all PCNST was 4.34/105. Table 3 shows the standardized IR for all the histological subtypes of PCNST. Concerning rare tumors, examples of CR are the following: CRanaplastic gangliogliomas: 0.018/105, among rare subtypes of meningiomas: CRchordoid meningiomas: 0.021/105 and CRanaplastic meningiomas: 0.056/105, among medulloblastomas: CRall medulloblastomas: 0.179/105 (0.56/105 for patients aged <15 years), and CRdesmoplastic medulloblastomas: 0.025/105, CRall hemangiopericytomas: 0.041/105, CRchoroid plexus carcinoma: 0.006/105, CRcentral neurocytoma: 0.042/105.
Discussion
Our study reports, for the first time, data on all histological subtypes of PCNST according to the WHO classification, together with their IRs, at a national level. It provides the very first data as regards to IRs for many rare subtypes of PCNST. This work was made possible by the collaboration of neurosurgeons and neuropathologists, with the methodological support of epidemiologists, and emphasizes the relevance of creating multidisciplinary networks and databases involving clinicians, pathologists and epidemiologists.
Differences and similarities between histological population-based studies and registries for PCNST
The IRs for all PCNST in our study are: CR 15.5/105 (compared with 17.6/105 in the Gironde Registry [6]), USR 14.1/105 (compared with 22.0/105 in the CBTRUS [4]), ESR 14.5/105 (compared with 18.1/105 in the Austrian Brain Tumor Registry [5]), and WSR 12.0/105 (Table 4). IRs in our study are lower than reported by the PCNST registries in Western countries [4–6]. However, the difference can mainly be explained by the fact that these registries include cases without histological confirmation. Indeed, non-histological cases represent 28.7% of cases in the data from the CBTRUS, 19.1% in the Austrian Brain Tumor Registry and 20.7% in the Gironde Registry. It is to be noted that the difference in incidence between a histological collection and a registry (which includes non-histologically proven cases) may provide interesting data as regards to the proportion of patients with a histological validation, as only a very small proportion of cases receives a tumor treatment without histological confirmation.
The absence of histological diagnosis is more frequent in the following situations: non-malignant tumors and/or tumors with a slow growth such as meningiomas or pituitary tumors [24]; elderly and/or frail patients for whom no oncological treatment is proposed; tumors located in poorly accessible CNS regions (for example, some cases of brainstem tumors); and rare cases for whom the diagnosis can be made on cerebrospinal fluid markers (for example, some cases of CNS germ-cell tumors). On the contrary, our results are similar with the literature concerning major histology groups with uncertain or malignant evolution (for example, USR for neuroepithelial tissue tumors is 6.16/105 in our study compared with 6.62/105 in the CBTRUS [4], USR for lymphomas and hematopoietic neoplasms is 0.45/105 compared with 0.46/105 in the CBTRUS [4] and 0.50/105 in the Gironde registry [6]).
Regarding the sex ratio for all PCNST, 46.4% of cases are male in our study, compared to 42.1% in the data from the CBTRUS [4]. The difference can be explained by the higher IR for meningiomas in the CBTRUS, as these tumors preferentially affect women. Median age for all PCNST was 56 in our study compared to 59 in the data from the CBTRUS [4], probably due to the non-inclusion of non-histological cases in our study. Among pediatric patients (aged < 15), our IR (CR = ESR = USR = 4.3/105) was comparable to that of other studies: 3.9/105 in the French National Registry of Childhood Solid Tumors [16], 4.1/105 in the German registry ([14], http://www.kinderkrebsregister.de/fileadmin/kliniken/dkkr/pdf/jb/jb2013_2014/jb2014_s.pdf), and 5.26/105 in the CBTRUS [25].
Strengths
Our study provides interesting data as regards IRs of rare tumors (Table 3). We will discuss a few examples. Among mixed glial-neuronal tumors, anaplastic gangliogliomas are rare tumors of unknown incidence, with only one study from the “Surveillance, Epidemiology and End-Results” (SEER) database reporting data on 58 patients between 1973 and 2007 [26]. In that study, the USR for anaplastic gangliogliomas was 0.002/105, compared with a USR of 0.018/105 in our study. The sex-ratio was comparable, with a predominance of males. However, we found a significant difference in the median age (39.5 years old in our study vs. 25.5 in the SEER study). Meningiomas are among the most common PCNST [4, 22] and comprise histological subtypes that are considered as rare tumors. For example, chordoid meningiomas are a rare subtype of WHO grade II meningiomas, with an unknown incidence. Their incidence was not reported in studies from the CBTRUS [4], and only small clinical and/or pathological series have been published so far [27, 28]. We found a CR of 0.02/105, with a median age of 52 years old and a sex-ratio of 0.34. With a total of 79 patients with a chordoid meningioma registered by the FBTDB, the French series could bring interesting data regarding the prognostic factors and treatment strategies for these patients. We also described CR for atypical meningiomas (0.398/105), clear cell meningiomas (0.013/105), anaplastic meningioma (0.056/105), rhabdoid meningioma (0.002/105), and papillary meningioma (0.003/105). Among mesenchymal tumors, CNS hemangiopericytomas are rare, accounting for about 0.4% of all PCNST [2]. As the diagnosis of hemangiopericytoma requires a histological confirmation (and now a STAT6 immunohistochemistry), the IR found in our study is comparable to that of a study from the SEER database (USR 0.03/105 in the SEER study compared with 0.036/105 in our study), which further validates our results [29]. Likewise, the IR found for medulloblastomas in our study is comparable for children to that of the Canadian pediatric registry (CR 0.48/105 compared with WSR 0.57/105 in our study, data not shown), and for all patients to that of the SEER database in the USA (USR 0.15/105 compared with 0.20/105 in our study) [30, 31]. For all other rare tumors (excepted germ-cells tumors as these tumors can sometimes be diagnosed without a biopsy), for which the epidemiological and incidence data are rare, our study brings essential data on descriptive epidemiology, and provides a basis for clinical population-based studies.
Furthermore, the methodology of the FBTDB allows for an exhaustive recording of histological cases. Indeed, all the neurosurgery departments and pathologic labs involved in PCNST in metropolitan France participated in the data recording. Moreover, the collection of annual listing of cases from all the pathology departments ensured the exhaustiveness of the data collection. Such a collection is of high value, especially for rare tumors or tumors for which a clinical and/or radiological diagnosis is difficult. For these tumors, reaching a histological diagnosis is essential for clinical data analyses, oncological care management and outcomes at the population level. The number of cryopreserved tumors for each histological subtype of PCNST is described in Table 1. This information, even though it cannot be extrapolated to other countries where the practice may vary, is important as it gives an idea on the number of biological samples that could be used for research purposes. The percentage of resection versus biopsy is also provided for more than 42,000 tumors and for each histological subtype (Table 1). This data and the analysis of surgical practice at the population level are useful for the public health organizations.
In metropolitan France, the FBTDB not only allows a comprehensive description of the epidemiology of PCNST with histological validation, but it also provides a base for clinical and population studies. These works are useful in many areas, such as the validation of prognostic factors or therapeutic strategies [32–34], or the search for risk factors [35]. This is particularly true for rare tumors for which collaborations at the national level are necessary to be able to provide data on a significant number of patients.
Moreover, histological population-based studies have a great value in countries where the declaration of PCNST is not mandatory, in particular for rare subtypes of tumors. They are easier and cheaper than general registries, as the collection of non-histological cases is very time-consuming. As few patients receive active anti-tumor treatments without a histological confirmation, a good knowledge of the number of cases to be expected is of great importance for the public health system.
Limitations
One of the limits of our study is that, due to its methodology, the FBTDB does not collect cases without histological confirmation, which causes an obvious bias. For a proportion of histological subtypes, our IRs are lower than that of other registries. We have previously explained that this difference mainly concerned patients who do not have antitumor treatment. Therefore, histological population-based studies are essential to validate prognostic factors or to study oncological care management on tumors that need histological confirmation before treatment, but are not exhaustive enough to determine the exact incidence of all PCNST. This bias is more pronounced for non-malignant tumors.
Another weakness of the FBTDB is the absence of a systematic pathological review for all the PCNST. In France, a pathological review is performed for patients included in clinical trials or in a national biological database [36, 37], or when the diagnostic is considered as difficult by the pathologist. Of course, in these cases, the FBTDB includes the pathological review in the final diagnosis. Recently, the French pathologists and clinicians, in accordance with the French National Cancer Institute, have decided to perform a pathologic review for all rare PCNST, as part of a national project entitled “TUCERA”. But it will take time and money before it is done in a systematic manner for all PCNST in France.
The analysis of molecular markers is increasingly performed in France for the PCNST, and is now part of the diagnosis for some histological subtypes (i.e., diffuse astrocytomas, oligodendrogliomas) in the recently published new WHO classification. With the development of these markers and personalized oncology, we can hypothesize that both the pathological and molecular analysis of the tumor will soon become mandatory before any treatment for most PCNST. Therefore, the FBTDB will now imperatively need to collect these molecular markers as well as the new integrated diagnosis [3]. However, the current data based on the 2007 WHO classification will serve as a reference for future epidemiological studies using the new classification.
Perspectives
In the field of personalized oncology, population-based studies will need to collect not only the histological diagnosis data, but also molecular, clinical, imaging, quality of life, therapeutic and outcome data to evaluate the best medical strategies at the population level. This is especially crucial for rare tumors, as for these, clinical trials are very difficult to conduct. We believe that this challenging objective will be made possible in the near future thanks to the rapid expansion of health care information systems and the improvement of computer interface systems and health information technologies.
Conclusion
This study is the first to detail all histological subtypes of PCNST, including all rare tumors, and their IRs, at a national level. Above all, this work shows the importance of multidisciplinary networks and databases involving clinicians, pathologists and epidemiologists. Histological population-based studies have many perspectives in the field of clinical epidemiology and are complementary to brain tumor registries.
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) (2007) WHO classification of tumours of the central nervous system. IARC, Lyon
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C et al (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17(Suppl 4):iv1–iv62
Wöhrer A, Waldhör T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mösenbacher U et al (2009) The austrian brain tumour registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol 95(3):401–411
Baldi I, Gruber A, Alioum A, Berteaud E, Lebailly P, Huchet A et al (2011) Descriptive epidemiology of CNS tumors in France: results from the Gironde registry for the period 2000–2007. Neuro Oncol 13(12):1370–1378
Crocetti E, Trama A, Stiller C, Caldarella A, Soffietti R, Jaal J et al (2012) Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer 48(10):1532–1542
DeAngelis LM (2001) Brain tumors. N Engl J Med 344(2):114–123
Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Pineros M et al (2014) Cancer incidence in five continents, vol X. IARC, Lyon
Johannesen TB, Angell-Andersen E, Tretli S, Langmark F, Lote K (2004) Trends in incidence of brain and central nervous system tumors in Norway, 1970–1999. Neuroepidemiology 23(3):101–109
Klaeboe L, Lonn S, Scheie D, Auvinen A, Christensen HC, Feychting M et al (2005) Incidence of intracranial meningiomas in Denmark, Finland, Norway and Sweden, 1968–1997. Int J Cancer 117(6):996–1001
Deltour I, Johansen C, Auvinen A, Feychting M, Klaeboe L, Schüz J (2009) Time trends in brain tumor incidence rates in Denmark, Finland, Norway, and Sweden, 1974–2003. J Natl Cancer Inst 101(24):1721–1724
Arora RS, Alston RD, Eden TO, Estlin EJ, Moran A, Geraci M et al (2010) Are reported increases in incidence of primary CNS tumours real? An analysis of longitudinal trends in England, 1979–2003. Eur J Cancer 46(9):1607–1616
Kaatsch P, Rickert CH, Kühl J, Schüz J, Michaelis J (2001) Population-based epidemiologic data on brain tumors in German children. Cancer 92(12):3155–3164
McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M et al (2012) Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro Oncol 14(9):1194–1200
Desandes E, Guissou S, Chastagner P, Lacour B (2014) Incidence and survival of children with central nervous system primitive tumors in the french national registry of childhood solid tumors. Neuro Oncol 16(7):975–983
Ruiz-Tovar M, López-Abente G, Pollán M, Aragonés N, Ardanaz E, Moreo P et al (1999) Brain cancer incidence in the province of Zaragoza and Navarre (Spain): effect of age, period and birth cohort. J Neurol Sci 164(1):93–99
Caldarella A, Crocetti E, Paci E (2011) Is the incidence of brain tumors really increasing? A population-based analysis from a cancer registry. J Neurooncol 104(2):589–594
Bauchet L, Rigau V, Mathieu-Daudé H, Figarella-Branger D, Hugues D, Palusseau L et al (2007) French brain tumor data bank: methodology and first results on 10,000 cases. J Neurooncol 84(2):189–199
Rigau V, Zouaoui S, Mathieu- Daudé H, Darlix A, Maran A, Trétarre B et al (2011) French brain tumor database: 5-year histological results on 25756 cases. Brain Pathol 21(6):633–644
Zouaoui S, Rigau V, Mathieu-Daudé H, Darlix A, Bessaoud F, Fabbro-Peray P et al (2012) French brain tumor database: general results on 40,000 cases, main current applications and future prospects. Neurochirurgie 58(1):4–13
Zouaoui S, Darlix A, Rigau V, Mathieu- Daudé H, Bauchet F, Bessaoud F et al (2015) Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006–2010. Neurochirurgie. pii: S0028-3770(15):00073–9.
Kleihues P, Cavenee WK (eds) (2000) World health classification of tumors. Tumors of the nervous system. Pathology and genetics. IARC Scientific Publications, Lyon
Dolecek TA, Dressler EV, Thakkar JP, Liu M, Al-Qaisi A, Villano JL (2015) Epidemiology of meningiomas post-Public Law 107–206: The Benign Brain Tumor Cancer Registries Amendment Act. Cancer 121(14):2400–2410
Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL et al (2015) Alex’s lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 10):x1–x36
Selvanathan SK, Hammouche S, Salminen HJ, Jenkinson MD (2011) Outcome and prognostic features in anaplastic ganglioglioma: analysis of cases from the SEER database. J Neurooncol 105(3):539–545
Couce ME, Aker FV, Scheithauer BW (2000) Chordoid meningioma: a clinicopathologic study of 42 cases. Am J Surg Pathol 24(7):899–905
Di Ieva A, Laiq S, Nejad R, Schmitz EM, Fathalla H, Karamchandani J et al (2015) Chordoid meningiomas: incidence and clinicopathological features of a case series over 18 years. Neuropathology 35(2):137–147
Sonabend AM, Zacharia BE, Goldstein H, Bruce SS, Hershman D, Neugut AI et al (2014) The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: a Surveillance, Epidemiology, and End Results analysis. J Neurosurg 120(2):300–308
Smoll NR, Drummond KJ (2012) The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 19(11):1541–1544
Johnston DL, Keene D, Kostova M, Strother D, Lafay-Cousin L, Fryer C et al (2014) Incidence of medulloblastoma in Canadian children. J Neurooncol 120(3):575–579
Bauchet L, Mathieu-Daudé H, Fabbro-Peray P, Rigau V, Fabbro M, Chinot O et al (2010) Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol 12(7):725–735
Scott JG, Bauchet L, Fraum TJ, Nayak L, Cooper AR, Chao ST et al (2012) Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer 118(22):5595–5600
Zouaoui S, Darlix A, Fabbro- Peray P, Mathieu-Daudé H, Rigau V, Fabbro M et al (2014) Oncological patterns of care and outcomes for 265 elderly patients with newly diagnosed glioblastoma in France. Neurosurg Rev 37(3):415–423
Darlix A, Zouaoui S, Virion JM, Rigau V, Mathieu-Daudé H, Blonski M et al (2014) Significant heterogeneity in the geographical distribution of diffuse grade II/III gliomas in France. J Neurooncol 120(3):547–555
Menei P, Figarella-Branger D, Bauchet L, Loiseau H, Denyset M, Roman T et al (2014) French research infrastructures to develop and validate glioma biomarkers. Neurosurgery 75(2):E195–E196
Labreche K, Simeonova I, Kamoun A, Gleize V, Chubb D, Letouzé E et al (2015) TCF12 is mutated in anaplastic oligodendroglioma. Nat Commun 6:7207
Acknowledgements
The authors wish to acknowledge Dr. Hélène de Forges for reviewing the manuscript and all the doctors and professors who helped and completed the cards for the recording of the PCNST in France: Abbey Toby A., Adam C., Adle-Biassette H., Adreux, Albagnac V., Algros M.P., Amat C., Ansart F., Arbez-Gindre F., Arbion F., Arrivets P., Aubriot Lorton M.H., Auvigne I., Averous G., Pinel-briquel N., Badsi A., Baglin A.C., Baldet P., Barbey C., Bazille C., Bedgedjian I., Bedossa P., Benabidallah S., Benali A., Bergemer Fouquet A.M., Bergouignan M.A., Bernier M., Bertocchi C., Beuvon F., Billotet C., Blechet C., Bondoin L., Bonneau C., Bonyhay G., Bordier, Borrelly C., Bost Bezeaud F., Boudjadi S., Bouvier C., Brambilla E., Branquet D., Bressenot A., Broche C., Brouchet A., Brousse N., Brousset P., Bruniau A., Burel-Vandenbos F., Camparo P., Cahn V., Calvet P., Camo J., Camparo P., Carbonnelle Puscian A., Carloz E., Cassagnau E., Cathelineau D., Caulet S., Caveriviere P., Cazals-Hatem D., Chapon F., Charlotte F., Taranger-Charpin C., Chatelain D., Chenard M.P., Chretien F., Christov C., Clairotte A., Cohen C., Colombat M., Conan V., Conan-Charlet V., Cormier B., Costa K., Costes V., Costes-Charlet N., Coulon A., Couvelard A., Cremades, Croue A., Cruel T., Danjoux M., Darrasse D., Daumas-Duport C., Degano-Valmarx S., Delage Corre M., Delattre C., Delisle M.B., Delsol M., Denoux Y., Deschamps L., Desrousseaux M., Diebold M.D., Donsbeck A.V., Dosda A., Dreux N., Dubost G., Duga I., Dujardin F., Dumollard J.M., Durand L., Duval H., Eimer S., Etchandy M.P., Eyremandi R.P., Fabre B., Fallet C., Felce dachez M., Felix S., Fernandez C., Fetissof F., Feutry C., Fornes P., Figarella-Branger D., Figuccio M., Fornes P., Fouet, Fregeville M., Fromont G., Gaillard F., Gallois S., Gaspard C., Gay G., Gonguet A.M., Gontier M.F., Gonzalez, Goujon J.M., Gray F., Grignon Y., Gros P., Guedj N., Guettier-Bouttier C., Guillou P.J., Guymar S., Gyenes C., Hassoun, Haudebourg J., Heitzmann A., Henin D., Hennequin V., Heymann M.F., Istier L., Jaubert F., Jonas J., Jouan H., Jourdan F., Jouvet A., Justrabo, Kaci R., Kantelip B., Kemeny J.L., Kerdraon O., Kerdraon R., Kermanach P., Khaddage A., Kleinclaus I., Kopp N., Krzisch S., Kujas M., Labrousse F., Lacroix C., Lamant. L, Lannes B., Lantuejoul S., Laquerriere A., Laurent C., Le Gall F., Le Houcq M., Lechapt E., Leclair F., Leduc F., Leger F., Lerintiu F., Levillain P., Lhermitte B., Liprandi A., Lomazzi S., Longchampt E., Loussouarn D., Maitre F., Majek-Zakine E., Manent A.M., Maran A., Marcon N., Mareel A., Marie B., Martin L., Maues de Paula A., Maurage C.A., Mazerolles C., Mergey E., Meyronnet D., Michalak S., Michenet P., Michiels J.F., Milin S., Miquel C., Mohr M., Mohra, Mokhtari K., Monpon C., Morand Dusserre I., Moreau A., Moreau M., Moreno S., Mosnier J.F., Musso Rigal C., Neuville A., Oksman A., Oukabli M., Palasse J., Parent M., Patey M., Pellissier J.F., Peoc’h M., Philippe A., Pialat J., Pluot M., Polivka M., Pommepuy I., Ponnelle T., Pradere Labat M., Quintana M., Quintin-Roue I., Quintyn-Ranty M.L., Ranfaing E., Raoux D., Raynaud P., Reis Borges R., Renaudin K., Renjard L., Reyre J., Richard, Richard S., Rigau V., Ringenbach F., Roger P., Rouquette I., Rousseau A., Rousselet M.C., Rousselot C., Ruchoux, Sabourin J.C., Saïkali S., Saingra B., Saint-Andre J.P., Saint Blancard P., Saint Pierre G., Saint-Paul M.C., Salameire D., Salon C., Sarrouy J., Savin C., Sawan B., Schill H., Selves J., Seurat P.L., Sevestre H., Sorbara R., Soulard P., Soulard R., Souraud J.B., Straub P., Streichenberger N., Sturm N., Talagas M., Terrier J.P., Toquet C., Tortel M.C., Trouillas J., Tubiana A., Uro-Coste E., Valmary S., Van-Tran S., Vandenbos F., Varlet P., Vasiljevic A., Vaunois B., Verdier D., Veresezon L., Vic P., Viennet G., Vignaud J.M., Villa C., Vital A., Warter A., Weinbreck N., Yacoub M., Yaziji N., Yriarte-Laurent M.C., Yver M., Zafrani E., Zidane Marinnes M., Abi Lahoud G., Achim V., Adetchessi T., Aesch B., Agha M., Aghakhani N., Akkhabar, Al Hallak R., Albarello P., Almairach F., Al Nader E., Albert A., Aldea S., Alfieri A., Ali Benali M., Aliamus A., Allano V., Allaoui M., Alliez B., Alliez J.R., Amlashi S.F.A., Aouad N., Arthuis F., Ashraf A., Assaker R., Atta, Auque J., Autricque A., Ayache D., Barat J.L., Baron M.H., Baroncini M., Barrey C., Bataille B., Bauchet L., Baussart B., Bayram M., Bazin A., Beauchesne P., Beaudic Y., Beaurain J., Bedou G., Belliard H., Bellow F., Beltechi R., Ben hamouda H., Ben ismaïl M., Ben yahia M., Benabid A.L., Benezech J., Benhima H., Bennis S., Berger C., Bernard C., Bernard M.H., Berthelot J.L., Besson G., Bieron V., Billant J.B., Billon-Grand R., Bitar A., Bizette C., Blanc J.L., Blanquet A., Blond S., Blondet E., Blonski M., Boch A.L., Boetto S., Boissonnet H., Boone M., Bord E., Borha A., Bouali I., Bouazza S., Bougeard R., Bouillot P., Bourdain, Bourgeois P., Bousigue J.Y., Bousquet C., Bousquet O., Bousquet P., Boyer P., Brassier G., Bresson D., Bret P., Bruneau M., Brunon J., Buffenoir Billet K., Buffenoir K., Cabal P., Caillaud P., Caille J.M., Caire F., Campello C., Capelle L., Cardoso M., Carnin C., Carpentier A., Carron R., Cartalat-Carel S., Cesari J.B., Chabane A., Chabardes S., Chabolle F., Champeaux K., Chaynes P., Chays A., Chazal J., Chibbaro S., Chinot O., Chirossel J.P., Chobaut J.C., Choplain J.N, Choukri M., Cioloca C., Civit T., Clemenceau S., Colnat S., Comoy J., Cornelius J., Cornu P., Coste A., Coubes P., Coulbois S., Crampette L., Cristini A., Cuny E., Cuttaree H., Czorny A., Dagain A., Dam Hieu P., Dandine J.B., Darrouzet V., Dautheribes M., David P., De Germay B., De Greslan T., De Soultrait F., Debono B., Decq P., Delalande O., Delaretti, Delhaye M., Delion M., Delmas J.M., Delattre J.Y., Delpy P., Delsanti C., Derlon J.M., Derrey S., Deruty R., Desenclos C., Desgeorges M., Desplat A., Destandau J., Destrieux C., Devaux B., Dezamis E., Dhellemmes P., Di Rocco F., Di Tommaso L., Diabira S., Diaz A., Dimitriu C., Djindjian M., Do L., Doe K., Dorfmuller G., Dorwling-carter D., Dran G., Dubois G., Ducolombier A., Ducray F., Duffau H., Dufour H., Dufour T., Duhem R., Dulou R., Dumas B., Duntze J., Dupard T., Duplessis E., Durand A., Durandeau A., Dutertre G., Duthel R., El Fertit H., Emery E., Espagno C., Esposito P., Fabre T., Faillot T., Farah W., Farizon F., Faure A., Faure P., Fesselet J., Fichten A., Finiels P.J., Fischer D., Fischer Lokou D., Fono S., Fontaine D., Fotso M.J., Fournier D., Francois P., Frank B., Freger P., Freppel S., Froelich S., Fuentes J.-M., Fuentes S., Gadan R., Gaillard S., Garrel R., Gay M., George B., Giacomelli R., Gigaud M., Gil Robles S., Gimbert E., Ginguene C., Goasguen O., Godard J., Godfrin G., Gomez A., Gosset J.F., Goutagny S., Gras R., Grayeli B., Graziani N., Grellier P., Grisoli F., Grosskopf D., Guarnieri J., Guegan Y., Guenot M., Guerrier B., Gueye E.M., Guinguene C., Guy G., Guyotat J., Haddad E., Haegelen C., Hallacq M., Hallacq P., Hamcha, Hamdi S., Hamel O., Hamlat A., Hansen F., Hatem O., Hattou M., Hayek G., Henaux P.L., Henry, Herman P., Heyman D., Hladky J.P., Hoffmann D., Honnorat J., Huot J.C., Iakovlev G., Ibrahim R., Irthum B., Ischac R., Jacquet G., James S., Jan M., Jarraya B., Jouanneau E., Joud A., Joulin, Kaczmarek D., Kaddoum H., Kalamarides M., Karachi C., Katranji H., Kaya J.M., Kehrli P., Keravel Y., Khalfallah M., Khalil T., Khouri K., Khouri S., Klap P., Klein O., Koubaïssi W., Koudsie A., Kuzeawu A., Laccoureye L., Lacerda P., Lagarrigue J., Langlois O., Lapierre F., Lapras C., Le Fay, Le Franc M., Le Guerinel C., Le Nen D., Le Rhun E., Legars D., Lejeune J.P., Lemaire J.J., Lena G., Lepeintre J.F., Leriche B., Lescanne D., Lescure J.P., Leston J.M., Leveque M., Liguoro D., Linne M., Lioret E., Lisii D., Listrat A., Litre F., Litrico S., Loiseau H., Lonjon M., Lonjon N., Lopes M., Lorgis V., Lot G., Louis E., Louveau A., Lubrano V., Lungu G., Maarrawi J., Maghreu, Magro E., Mahla K., Maillard A., Maingot M., Maitrot D., Makiese O., Malca S., Mandat S., Mandonnet E., Manisor M., Mansour M., Manzo N., Marchal J.C., Marchal T., Marie J.P., Marnet D., Martin S., Mascott C., Mazon A., Memia Zolo D., Mendes Martins V., Menegalli D., Menei P., Mercier P., Merlet Chicoine I., Merlot I., Mertens P., Metellus P., Mineo F., Mineo J.F., Mireau E., Monteil P., Montessuy R., Mora A, Morandi X., Morar S., Moraru C., Moreau J.J., Morel C., Mortada J., Mostofi K., Mottolese C., Moubarak K., Mourier L., Muckensturm B., Mulnet D., Nahas F., Namaki H., Nataf F., Navarro S., Nguyen J.P., Njee Bugha T., Njee T., Noël G., Nogues L., Noudel R., Nouet A., Nseir R., Nuti C., Orabi M., Orenstein D., Page P., Palfi S., Pallud J., Palombi O., Pampin S., Paquis P., Paradot Mouton G., Parker F., Pasqualini E., Passagia J.G., Patru M.C, Pech Gourg G., Pejeredou, Pelissou-Guyotat I., Pellet W.J., Peltier J., Pencalet P., Penchet G., Peragut J.C., Peyre M., Pernot P., Perrin G., Person H., Peruzzi P., Petit D., Pierre-Kahn A., Pinaudeau M., Pinelli C., Plas J.Y., Polo G., Poncet J.L., Porhiel V., Postelnicu A., Pouit B., Prades J.M, Privat J.M., Proust F., Puget S., Rabehenoina C., Rabhi M., Ragragui O., Ramirez C., Raoul S., Rasendrarijao D., Rech F., Redondo A., Regis J., Remond J., Renard J.L., Reynier Y., Reyns N., Ricard D., Ricci Franchi A.C., Richet A., Riem T., Riffaud L., Rigoard P., Robert G., Robert R., Robier A., Roche P.H., Rodriguez M.-A., Rodriguez V., Roualdes G., Roualdes V., Rougier A., Roujeau T., Rousseaux P., Roux F., Roux F.X., Sabatier J.F., Sabatier P., Sabbah M., Sacko O., Sainte-Rose C., Sakka L., Salmon B., Sallansonnet M., San Galli F., Sankaredja J.M., Sautreaux J.L., Sauvaget, Scarone P., Scavarda D., Scherpereel B., Schmidt E., Segnarbieux F., Seguin, Seigneuret E., Seizeur R., Sichez j.P., Sid Hamed S., Silhouette B., Simbert, Simon A., Simon E., Sinardet D., Sindou M., Sleiman M., Sol J.C., Sorin A., Soumare O., Srour A., Srour R., Stecken J., Sterkers O., Stilhart B., Suleiman N., Szathmari A., Tadie M., Taha S., Taillandier L., Taillia H., Tarek A., Ternier J., Thiebaut J.B., Thines L., Thomassin J.M., Tobenas Dujardin A.C., Tonnelle Duhem V., Tourneux H., Toussaint P., Touta A., Touzet G., Tran Ba Huy P., Travers N., Tremoulet M., Turak B., Turner F., Valery C., Vallee B., Van Effenterre R., Van Raay Y., Vassal F., Velut S., Verez J., Vidal J., Vignes J.R., Vincentelli Ange F., Villette L., Vinchon M., Voirin J., Von Langsdorf D., Vongsouthi. C, Wager M., Yordanova Y., Zaïri F., Zanaret M., Zemmoura I., Zerah M.
Funding
This work was conducted with the financial support, as Grants, of the French National Cancer Institute (INCa), the Ligue Nationale Contre le Cancer, the Société Française de Neuropathologie (SFNP), the Société Française de Neurochirurgie (SFNC) and the Club of Neuro-Oncologie of the Société Française de Neurochirurgie (CNO-SFNC), the Association des Neuro-Oncologues d’Expression Française (ANOCEF), the Roche Laboratory, the Association pour la Recherche sur les Tumeurs Cérébrales Nord et Sud (ARTC), for the collection, management, analysis and interpretation of the data, and of the SIRIC Montpellier Cancer (Grant “INCa-DGOS-Inserm 6045”) for the design and conduct of the study and for the collection, management, analysis and interpretation of the data.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: In the first line of Table 1 (line “Tumors of neuroepithelial tissue”), column “Male, n”, the number was corrected to read 14,621.
Amélie Darlix and Sonia Zouaoui have equally contributed to this work.
With the participation of the Société Française de Neuropathologie (SFNP), the Société Française de Neurochirurgie (SFNC) and the Club Neuro-Oncologie of the Société Française de Neurochirurgie (CNO-SFNC), and the Association des Neuro-Oncologues d’Expression Française (ANOCEF).
An erratum to this article is available at http://dx.doi.org/10.1007/s11060-016-2340-5.
Rights and permissions
About this article
Cite this article
Darlix, A., Zouaoui, S., Rigau, V. et al. Epidemiology for primary brain tumors: a nationwide population-based study. J Neurooncol 131, 525–546 (2017). https://doi.org/10.1007/s11060-016-2318-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2318-3