Abstract
Gliosarcoma is classified by the World Health Organization as a variant of glioblastoma. These tumors exhibit biphasic histologic and immunophenotypic features, reflecting both glial and mesenchymal differentiation. Gliosarcomas can be further classified into primary (de novo) tumors, and secondary gliosarcomas, which are diagnosed at recurrence after a diagnosis of glioblastoma. Using a retrospective review, patients seen at MD Anderson Cancer Center between 2004 and 2014 with a pathology-confirmed diagnosis of gliosarcoma were identified. 34 patients with a diagnosis of gliosarcoma seen at the time of initial diagnosis or at recurrence were identified (24 primary gliosarcomas (PGS), 10 secondary gliosarcomas (SGS)). Molecular analysis performed on fourteen patients revealed a high incidence of TP53 mutations and, rarely, EGFR and IDH mutations. Median overall survival (OS) for all patients was 17.5 months from the diagnosis of gliosarcoma, with a progression free survival (PFS) of 6.4 months. Comparing PGS with SGS, the median OS was 24.7 and 8.95 months, respectively (from the time of sarcomatous transformation in the case of SGS). The median OS in SGS patients from the initial diagnosis of GB was 25 months, with a PFS of 10.7 months. Molecular analysis revealed a higher than expected rate of TP53 mutations in GS patients and, typical of primary glioblastoma, IDH mutations were uncommon. Though our data shows improved outcomes for both PGS and SGS when compared to the literature, this is most likely a reflection of selection bias of patients treated on clinical trials at a quaternary center.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GB) is the most common primary malignant brain tumor in adults, accounting for 45 % of all reported cases [1]. GB is classified using WHO criteria as a grade IV glioma, which can be genetically partitioned into primary GB, which arises de novo, and secondary GB, which arises by malignant transformation from grade II or III diffuse gliomas. Molecularly, secondary GBs are defined by the presence of isocitrate dehydrogenase (IDH) mutations, which occur only rarely in primary GB (>80 vs. <5 %, respectively) [2–4]. The latest WHO classification recognizes few distinct histological variants of grade IV diffuse gliomas, consisting of classic GB, gliosarcoma, small cell GB, GB with oligodendroglioma component, and giant cell GB. Each variant is characterized by varying prognostic outcomes [5, 6].
Gliosarcoma (GS) accounts for less than 2 % of all diffuse gliomas. It is seen most commonly in patients between ages 40 and 60, and has a male predominance (M:F 1.9:1) [7]. Historically, prognosis is poor, with a median survival of 4–11.5 months [8–10]. These tumors most commonly arise in the temporal lobes, followed by parietal and frontal lobes. GSs are histologically characterized by two distinct cellular components: glial, which expresses glial fibrillary acidic protein (GFAP) and is reticulin poor, and sarcomatous, which lacks GFAP expression and is reticulin rich [5]. In the literature, GS exhibits a genetic profile similar to that of primary GB, except for absent or minimal EGFR amplification/overexpression; conversely, this is one of the most frequent molecular genetic alterations in primary GB.
Despite the differences in molecular profile, histologic appearance, and greater propensity to develop metastatic disease compared to GB, GS is typically treated with the same regimens as those used for GB. We have reviewed a series of patients with GS to better understand the clinical and molecular features of GS so that new treatment strategies could be considered.
Materials and methods
We conducted a retrospective data and tissue analysis of all adult GS patients in the MD Anderson Cancer Center (MDACC) institutional database from 7/2004 through 07/2014 under a protocol with waiver of consent approved by the Institutional Review Board. All patients were age 18 or greater, had undergone a biopsy or surgical resection with a pathological diagnosis of GS. Patients were further classified as primary GS (PGS) if the tumor arose de novo or secondary GS (SGS) in patients with histologically confirmed GS who had previously been diagnosed with GB that had been resected and irradiated [11–13]. Patients that were perceived as potentially likely to benefit from molecular characterization by their treating physicians underwent genomic profiling after informed consent on an Institutional Review Board-approved prospective protocol (NCT01772771). We collected demographic, treatment, and survival data from the database. Death was confirmed by review of medical records, death certificate, and/or the social security index database. Survival analyses were performed using Kaplan–Meier curves, the Cox proportional hazard method and log-rank test. When the date of death was not known, the record was censored (for OS) in the analysis as of the date of last follow-up. Statistical significance was considered at a p value ≤0.05.
Histologic and immunophenotypic characterization/histochemical preparations
Microscopic sections were cut at 4–6 microns for hematoxylin and eosin-stained sections and immunohistochemical (IHC) studies. IHC studies were performed on an automatic Leica immunostainer (Leica Biosystems, Buffalo Grove, IL), and included Ki-67 antigen (Dako, clone MIB-1, 1:100 dilution), GFAP (BD Biosciences, 4A11, clone 1B4, 2E1, 1:7000), S-100 (BioGenex, clone 15E2E2, 1:900), IDH1 R132H mutant protein (Dianova, clone H09, 1:40), p53 (mouse monoclonal, Dako, Cat#M7001, dilution 1:100, clone DO-7), EGFR (mouse monoclonal, Source: Calbiochem, cat#GR01, dilution 1:150, clone 528) and PTEN (mouse monoclonal, Source: Dako, cat#M3627, dilution 1:100, clone 6H2.1). All controls were appropriate.
Molecular characterization
Mutation analyses were performed using 11-gene (n = 3), and 50-gene (n = 2) mutation analyses to identify frequently reported hotspot mutations in a CLIA Certified Molecular Diagnostics Laboratory at MD Anderson Cancer Center using either a PCR-based primer extension assay or next generation sequencing (NGS) based analysis Ampliseq Cancer Panel (Life Technologies, Carlsbad, CA). The 11-gene sequence analysis included AKT1, BRAF, GNAQ, GNAS, IDH1, IDH2, KRAS, MET, NRAS, PIK3CA, and RET. The 50-gene NGS-based sequence analyses included APC, BRAF, CDKN2A, CTNNB1, EGFR, IDH1, IDH2, KIT, KRAS, NRAS, PDGFRA, PIK3CA, PTEN, PTPN11, SMARCB1, and TP53. Details on the 11 gene and the 50 gene panel used are available in Supplementary Table I. Nine of the cases were interrogated by targeted gene whole exome DNA sequencing at the MD Anderson Institute for Personalized Cancer Therapy. For details on the 202 gene panel methods see Supplementary Data I.
A minimum of 250× coverage was required at a given base for the interpretation of a wild-type or variant call. Although, the NGS platform is capable of achieving a much higher analytical sensitivity, for clinical purposes, we determined the effective lower limit of detection of this assay (analytical sensitivity) to be in the range of 5 % (one mutant allele in the background of 19 wild type alleles) to 10 % (one mutant allele in the background of nine wild type alleles) by taking into consideration the depth of coverage at a given base and the ability to confirm low level mutations using independent conventional platforms. We required that the tumor nuclei represent at least 20 % of the nuclei in the tested sample to avoid false negative results.
Results
Demographics and clinical presentation
We identified 34 patients with a diagnosis of GS seen at MDACC at the time of initial diagnosis or at recurrence between 7/2004 and 7/2014. Most of the patients were men (71 %), with a median age of onset for both genders being 55.5 years (range, 35–74). Twenty-one patients (62 %) were Caucasian, eight (23 %) were Hispanic, three (9 %) were African American; the rest included a Native American and other races (6 %). Of the 34 patients, 24 were classified as PGS, with the remaining 10 patients having SGS. GS was associated with previous brain radiation therapy (RT) in nine patients (26 %) (all of these were SGS patients). The most common location of GS was the temporal lobe (53 %), followed by the frontal lobe (41 %), parietal lobe (21 %), occipital lobe (9 %) and brainstem (3 %). Nine patients (26 %) presented with bilobar involvement. Fifteen patients (44 %) presented with multiple symptoms. Clinical presentation included headaches (59 %), hemiparesis (21 %), seizures (18 %), hemihypoesthesia (15 %), and, less frequently, symptoms associated with elevated intracranial pressure (i.e. nausea/vomiting), visual field deficits and language deficits (12 % each). The Karnofsky performance status (KPS) at GS diagnosis ranged between 60 and 100, with 76 % of patients having a KPS between 80 and 100. Five (15 %) patients had a history of cancer other than CNS, consisting of basal cell carcinoma, melanoma, prostatic adenocarcinoma, and thyroid cancer of unknown type. One patient with neurofibromatosis type 1 (Case 14) developed two tumor phenotypes other than GS: B lymphoblastic leukemia/lymphoma, and schwannoma. Demographics and clinical presentation are listed in Table 1.
Tumor characteristics
Ten patients (29 %) had a history of sarcomatous transformation from GB (SGS). In 40 % of these patients this dedifferentiation occurred at the time of first recurrence, with a median time to sarcomatous transformation of 13.5 months. The progression free survival (PFS) in SGS patients (with a prior history of GB) was 3.1 months from the time of sarcomatous transformation. Three patients (9 %) had a history of a lower grade diffuse glioma, with malignant transformation of a WHO grade II astrocytoma (Case 2), anaplastic glioma and a brainstem astrocytoma (Case 14). The presence of IDH1 mutation was checked on only 1 of these 3 patients (Case 2) and a mutation in codon 132 (R132H) was detected.
Seven patients (21 %) developed evidence of tumor dissemination during the course of their disease, with 5 (15 %) displaying leptomeningeal dissemination (LMD) (3 PGS, 2 SGS patients). The diagnosis of LMD was made based on characteristic imaging findings (2 patients) and cytology (3 patients). In the remaining 2 patients (6 %; 1 PGS and 1 SGS patient), extra-axial spread occurred to the infratentorial region outside the temporal bone, cervical lymph nodes, and temporal bone. Extra-axial metastasis occurred at the time of sarcomatous transformation in one patient with SGS, and at 29 months from diagnoses in a PGS patient.
Pathologic and molecular studies
All tumors were diagnosed as grade IV gliosarcoma by 2007 WHO criteria [5]. No other heterologous elements (e.g. bone, cartilage, smooth or striated muscle, lipomatous elements) were present in our samples. On 14 patients tissue was available to perform mutation analysis. (Table 2) The main reason tissue was not available for analysis was that most patients had their surgery at outside institutions (n = 15). Of the tissues analysed, mutations were identified in TP53 (n = 8), NF1 (n = 3), RB1 (n = 2), PTEN (n = 2), IDH1 (n = 1), PIK3CA (n = 1), NF2 (n = 1) and EGFR (n = 1). Supplementary Table II presents details on other mutated genes found in these 13 patients.
Treatment
Treatment approaches were variable across our patient population. Extent of resection, as defined radiologically, varied between gross total resection, which was achieved in 22 patients (53 %), and a subtotal resection in the remainder of patients (47 %). None of the patients underwent biopsy. Six of the 10 patients (60 %) with SGS had a subtotal resection at the time of sarcomatous transformation, in contrast to PGS, in which a gross total resection was achieved in 14 out of 23 patients at presentation (61 %). In one case no data was available on post-surgical treatment. Twenty four patients (70 %) received concurrent chemoradiation as initial treatment after diagnosis of GS, with 1 of these patients being a SGS who received a second course of chemoradiation, having received a first course at diagnosis of GB more than 4 years prior. Of the 10 patients with SGS, 8 had received concurrent chemoradiation at the time of GB diagnosis. The remaining 2 patients were treated with sequential radiation followed by chemotherapy at GB diagnosis since they were diagnosed prior to the publication of the EORTC/NCI-C data published demonstrating the superiority of chemoradiation [14]. 6 of the remaining 9 SGS patients received chemotherapy alone, 2 received chemotherapy and radiation during their clinical course, and 1 patient was placed on hospice care 3 months after re-resection. (Table 3) Through the course of disease, 26 patients received multiple chemotherapeutic agents, 3 received oncolytic viral therapy and chemotherapy, 2 received RT followed by chemotherapy, and 1 patient was treated with targeted vaccine therapy and chemotherapy. Only 1 patient did not receive any treatment after surgery.
Bevacizumab-based regimen was used as salvage therapy in 16 patients (47 %), comprising 11 patients with PGS and 5 with SGS. Eight patients received bevacizumab plus another agent at initiation of therapy, with the rest receiving single agent bevacizumab. The chemotherapeutic agents given in combination with bevacizumab were carboplatin (3 patients), irinotecan (2 patients) and CCNU, temozolomide, etoposide, erlotinib, and trebananib (one patient each). Two patients had switching of the chemotherapeutic agent used in combination with bevacizumab at the time of clinical or radiographic progression.
Patients outcomes
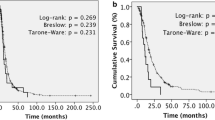
Median overall survival (OS) for all patients was 17.5 months from the diagnosis of GS, with a PFS of 6.4 months. When separating PGS from SGS, the median OS was 24.7 and 8.95 months, respectively, with PFS of 7 and 3.1 months, respectively. The median OS for patients with a specific prior diagnosis of GB was 25 months from the diagnosis of GB, with a PFS of 10.7 months from the diagnosis of glioblastoma. Patients who had a GTR at the time of first diagnosis lived longer than those with a STR—24.7 vs 10.1 months, though given the small numbers in each group, this did not reach statistical significance (p = 0.2).
Overall, patients treated with bevacizumab had a PFS of 4.2 months from initiation of treatment and an OS of 8.4 months from the time of starting bevacizumab. In all 5 of the 10 SGS patients treated with bevacizumab, this antiangiogenic therapy was started at the time of GS diagnosis, with a PFS of 3.8 months and OS of 7.3 months from initiation of treatment. In the case of PGS, median PFS was 4.2 months and median OS was 8.7 months after initiation of bevacizumab (Fig. 1).
Discussion
GS was initially described by Stroebe in 1898 and was thought to be similar to GB in terms of age of onset, location, and clinical prognosis [15]. In 1958, Feigin et al. defined GS as a subtype of GB with features of sarcoma and proposed its origin from proliferating vessels within GB [16, 17].
The glial component is usually of astrocytic origin, less commonly exhibiting an oligodendroglial or ependymal phenotype [18–20]. The current 2007 WHO classification of brain tumors lists GS as a variant of GB that exhibits a biphasic histological pattern consisting of glial and sarcomatous components that stain positively for GFAP and pericellular reticulin deposition respectively. The sarcomatous component may demonstrate a wide range of phenotypes (e.g., fibroblastic, smooth muscle, adipose tissue, cartilage, bone, etc.) [21–27].
GS can be further classified into primary de novo (PGS) and secondary GS (SGS), which are diagnosed at recurrence after a prior diagnosis of GB. In our series, 24 out of 34 patients were PGS. One of the clinical features that distinguishes PGS from GB is its location of origin [8]. PGS almost never arises infrantentorially. There is a predilection to involve the temporal lobes (as seen in our case series), although some reports indicate a higher incidence in the frontal lobes [11, 28].
The pathogenesis of GS is not known. The two main hypotheses that have been advanced to explain the biphasic nature of GS are the “polyclonal” and the “monoclonal” hypotheses. In the polyclonal hypothesis, the glial and mesenchymal components are thought to differentiate from different stem cells, with the mesenchymal element developing from fibroblasts, vascular adventitia, vascular smooth muscle cells, or monohistiocytic cells [29]. In the monoclonal hypothesis, the mesenchymal component is postulated to develop from glial precursors during tumor progression. Most studies have supported the monoclonal theory based largely on the observation that molecular characterization has shown that both glial and mesenchymal components share common genetic aberrations in most instances [27, 29–31]. GS karyotyping has shown gains on chromosomes 7, X, 9q, 12q, and 20q, and losses on chromosomes 10, 9p, 13q, and 17. Similar genetic alterations are seen in both the glial and sarcomatous components in most published literature on the subject [25, 29, 30, 32]. With respect to specific molecular alterations, in one case series comprising 26 patients, 7.7 % showed an IDH1 mutation, whilst other reports have shown overexpression of PDGFA-driven pathways [33, 34].
In the present series, eight of the 11 cases that underwent genomic sequencing for TP53 mutation showed a somatic mutation (73 %) (Table II). Three cases had evidence of p53 overexpression by IHC; no immunohistochemical or sequencing data on TP53 was available in 1 case. Interestingly, one case was TP53 wild-type by sequencing whilst staining showed strong nuclear staining in the sarcomatous component (approx. 30 % of the tissue sampled) and rare positivity in the glial component (Case 10). This has been reported and might be indicative of a downstream alteration of the TP53 pathway or a mutation away from the ‘hotspots’ analyzed [35]. In those cases that were positive by IHC, both components of the tumor variably stained positive for p53, supporting the monoclonal hypothesis. One identical TP53 mutation occurred in 2 patients (Case 7 and 9; c.535C > T). These mutations were found in patients with PGS and SGS, suggesting that they may occur early in gliomagenesis. In a similar molecular review of 19 GS cases by Reis et al., only 5 of the 19 patients had evidence of TP53 mutations (26 %), although another 6 had evidence of p53 overexpression. The higher incidence of TP53 mutations detected in our series could be related to an increased number of ‘hotspots’ screened, and comes closer to the frequency of TP53 mutations described in secondary glioblastoma (60–65 %) [31, 36]. However, in view of the relatively small number of patients, the possibility that the increased number of TP53 mutations are a chance event cannot be completely excluded.
As expected, an EGFR mutation was present in only one of the tumors in this study (1 out of 10 tumors sequenced), whilst all 3 tumors analyzed by IHC did not show evidence of EGFR overexpression. Conversely, PTEN mutations were present in 2 of the 10 tumors sequenced. This reflects the fact that the mutational profile of GS lies somewhere in between that seen in primary glioblastoma (where EGFR mutations are more frequent) and secondary glioblastoma (where PTEN mutations are less frequent) [36]. Of the three GS that arose from lower grade tumors, 1 of them had sequencing showing the presence of an R132H IDH1 mutation (Case 4). In the other 2 cases, one of them (Case 14) was IDH wild type whilst the other case did not have molecular sequencing performed [36].
As compared to classic GB, which rarely spreads extracranially and only occasionally disseminates into cerebrospinal fluid, this is a common feature of GS. Extracranial metastasis is thought to occur in about 11 % of cases with lung and liver, the most commonly involved sites of GS metastasis reported in the literature [7]. In most of the cases in which the metastatic tumor was evaluated, histopathological analysis revealed that the metastatic focus was solely composed of the sarcomatous component, generating the belief that this component that has a propensity for hematogenous dissemination. This may account for the reason why GS has a higher rate of tumor dissemination than GB [7, 37–42]. Prior to our case series, there were only 5 other cases describing LMD secondary to GS, which could be a reflection that there is an increased awareness of the risk of dissemination from GS [43]. On the other hand, extracranial spread (0.06 %) was lower than what is reported in the literature (11 %) [7].
All of the patients with PGS were initially treated with concurrent chemoradiation with temozolomide. In the largest retrospective study of chemoradiation in GS patients, 22 patients were treated uniformly with concurrent chemoradiation with temozolomide and achieved an overall median survival of 18.5 months. Of these 22 patients, extent of resection was known in 19, with 10 of these undergoing a GTR and the remaining 9 having a STR. This did not have a statistically significant impact on survival, similar to the results in our series. However, the mean age for this previously reported cohort was 37.5 years, with all patients having a KPS of more than 70, indicating potential reasons for the better outcomes seen [44].
Historically, the outcome in PGS is poor with a reported median survival of 4 months if untreated. With treatment, the median survival has been reported to range between 6.25 and 11.5 months [10, 11, 28, 44–47]. However, overall (PGS and SGS together), the median OS in our series was 17.5 months, but was significantly longer if only patients with PGS were analyzed with a median OS of 24.7 months and PFS of 7 months. This might also be related to the fact that more PGS had a GTR than SGS (61 vs 40 %). The median OS for patients with SGS was the same as PGS if measured from the time of diagnosis of GB (25 months). The better survival for both PGS and SGS might reflect the selection of patients seen at quaternary referral centers, and the subsequent enrollment in clinical trials, since most of the patients in this study were part of a clinical trial. In fact internal data for primary GB patients enrolled in clinical trials at MD Anderson show a median OS of 25 months which is similar to the survival for PGS in our study (Unpublished data). One other possibility for the better outcomes seen in our patients compared to what is described in the literature is general improvements in post-progression care plus the approval of bevacizumab in the recurrent setting [48].
One of the features of our study is the results of bevacizumab treatment for recurrent GS. Han and colleagues described 4 PGS patients treated with bevacizumab at recurrence but give no subset analysis on their outcomes [8]. In our series, the median OS of 8.4 months from initiation of bevacizumab treatment is similar to that recently reported in patients with recurrent GB treated with single-agent bevacizumab [49]. In 10 out of the 16 patients started on bevacizumab, a second chemotherapeutic agent was added at the time of recurrence, but because of the small numbers of patients who were treated with different combinations it is not possible to make any conclusions on any additive effects these agents might have had on the survival outcomes. However, based on the latest analysis of the AVAGLIO trial, bevacizumab did not offer any survival benefit when given in the upfront setting in the mesenchymal group of GB in contrast to the IDH1 wild type proneural group, putting in doubt whether GS patients would derive any benefit [50, 51].
The pathogenesis of sarcomatous expression in GB is not known. Given the small patient population and the widespread use of RT for GB, it is not possible to infer that RT caused the transformation.
The strengths of this study is that it constitutes one of the largest cohort of patients with a diagnosis of gliosarcoma. However, our paper also has a number of limitations; these include the retrospective nature of this study, the limited number of patients in this cohort with tissue available to perform mutational analysis on and also the fact that due to the rarity of this tumor, 34 patients is still a relatively small number of patients. Though difficult due to the rarity of this tumor, multi institution molecular integrated prospective trials specific for both PGS and SGS might be the only way to reveal unique molecular alterations and therapeutic targets that will help foster the best treatment strategies and outcomes for this tumor type.
References
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 4):iv1–iv63. doi:10.1093/neuonc/nou223
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. doi:10.1056/NEJMoa0808710
Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A (2008) Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol 116:597–602. doi:10.1007/s00401-008-0455-2
Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (2009) IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 174:1149–1153. doi:10.2353/ajpath.2009.080958
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Karsy M, Gelbman M, Shah P, Balumbu O, Moy F, Arslan E (2012) Established and emerging variants of glioblastoma multiforme: review of morphological and molecular features. Folia Neuropathol 50:301–321
Beaumont TL, Kupsky WJ, Barger GR, Sloan AE (2007) Gliosarcoma with multiple extracranial metastases: case report and review of the literature. J Neurooncol 83:39–46. doi:10.1007/s11060-006-9295-x
Han SJ, Yang I, Ahn BJ, Otero JJ, Tihan T, McDermott MW, Berger MS, Prados MD, Parsa AT (2010) Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer 116:1358–1366. doi:10.1002/cncr.24857
Han SJ, Yang I, Tihan T, Prados MD, Parsa AT (2010) Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol 96:313–320. doi:10.1007/s11060-009-9973-6
Galanis E, Buckner JC, Dinapoli RP, Scheithauer BW, Jenkins RB, Wang CH, O’Fallon JR, Farr G Jr (1998) Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg 89:425–430. doi:10.3171/jns.1998.89.3.0425
Perry JR, Ang LC, Bilbao JM, Muller PJ (1995) Clinicopathologic features of primary and postirradiation cerebral gliosarcoma. Cancer 75:2910–2918
Han SJ, Yang I, Otero JJ, Ahn BJ, Tihan T, McDermott MW, Berger MS, Chang SM, Parsa AT (2010) Secondary gliosarcoma after diagnosis of glioblastoma: clinical experience with 30 consecutive patients. J Neurosurg 112:990–996. doi:10.3171/2009.9.jns09931
Han SJ, Yang I, Tihan T, Chang SM, Parsa AT (2010) Secondary gliosarcoma: a review of clinical features and pathological diagnosis. J Neurosurg 112:26–32. doi:10.3171/2009.3.jns081081
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Stroebe H Über Entstehung und Bau der Hirngliome. Zieglers Beiträge. 1895. Bd XVIII 3
Feigin I, Allen LB, Lipkin L, Gross SW (1958) The endothelial hyperplasia of the cerebral blood vessels with brain tumors, and its sarcomatous transformation. Cancer 11:264–277
Paulus W, Bayas A, Ott G, Roggendorf W (1994) Interphase cytogenetics of glioblastoma and gliosarcoma. Acta Neuropathol 88:420–425
Agale SV, Bhatia VO, D’Costa GF, Velho V, Domkundwar S, Mandal S Gliosarcoma Arising in Anaplastic Ependymoma with Heterologous Sarcomatous Component: A Rare Phenomenon. Spine Neurosurg
Rodriguez FJ, Scheithauer BW, Perry A, Oliveira AM, Jenkins RB, Oviedo A, Mork SJ, Palmer CA, Burger PC (2008) Ependymal tumors with sarcomatous change (“ependymosarcoma”): a clinicopathologic and molecular cytogenetic study. Am J Surg Pathol 32:699–709. doi:10.1097/PAS.0b013e318158234e
Kepes JJ, Bastian FO, Weber ED (1996) Gliosarcoma developing from an irradiated ependymoma. Acta Neuropathol 92:515–519
Banerjee AK, Sharma BS, Kak VK, Ghatak NR (1989) Gliosarcoma with cartilage formation. Cancer 63:518–523
Barresi V, Cerasoli S, Morigi F, Cremonini AM, Volpini M, Tuccari G (2006) Gliosarcoma with features of osteoblastic osteosarcoma: a review. Arch Pathol Lab Med 130:1208–1211
Barut F, Kandemir NO, Ozdamar SO, Gul S, Bektas S, Gun BD, Bahadir B (2009) Gliosarcoma with chondroblastic osteosarcomatous differentation: report of two case with clinicopathologic and immunohistochemical features. Turk Neurosurg 19:417–422
Hayashi K, Ohara N, Jeon HJ, Akagi S, Takahashi K, Akagi T, Namba S (1993) Gliosarcoma with features of chondroblastic osteosarcoma. Cancer 72:850–855
Barnard RO, Bradford R, Scott T, Thomas DG (1986) Gliomyosarcoma. Report of a case of rhabdomyosarcoma arising in a malignant glioma. Acta Neuropathol 69:23–27
Haddad SF, Moore SA, Schelper RL, Goeken JA (1992) Smooth muscle can comprise the sarcomatous component of gliosarcomas. J Neuropathol Exp Neurol 51:493–498
Biernat W, Aguzzi A, Sure U, Grant JW, Kleihues P, Hegi ME (1995) Identical mutations of the p53 tumor suppressor gene in the gliomatous and the sarcomatous components of gliosarcomas suggest a common origin from glial cells. J Neuropathol Exp Neurol 54:651–656
Meis JM, Martz KL, Nelson JS (1991) Mixed glioblastoma multiforme and sarcoma. A clinicopathologic study of 26 radiation therapy oncology group cases. Cancer 67:2342–2349
Actor B, Cobbers JM, Buschges R, Wolter M, Knobbe CB, Lichter P, Reifenberger G, Weber RG (2002) Comprehensive analysis of genomic alterations in gliosarcoma and its two tissue components. Genes Chromosomes Cancer 34:416–427. doi:10.1002/gcc.10087
Boerman RH, Anderl K, Herath J, Borell T, Johnson N, Schaeffer-Klein J, Kirchhof A, Raap AK, Scheithauer BW, Jenkins RB (1996) The glial and mesenchymal elements of gliosarcomas share similar genetic alterations. J Neuropathol Exp Neurol 55:973–981
Reis RM, Konu-Lebleblicioglu D, Lopes JM, Kleihues P, Ohgaki H (2000) Genetic profile of gliosarcomas. Am J Pathol 156:425–432. doi:10.1016/s0002-9440(10)64746-3
Mueller W, Lass U, Herms J, Kuchelmeister K, Bergmann M, von Deimling A (2001) Clonal analysis in glioblastoma with epithelial differentiation. Brain Pathol 11:39–43
Lee D, Kang SY, Suh YL, Jeong JY, Lee JI, Nam DH (2012) Clinicopathologic and genomic features of gliosarcomas. J Neurooncol 107:643–650. doi:10.1007/s11060-011-0790-3
Reis RM, Martins A, Ribeiro SA, Basto D, Longatto-Filho A, Schmitt FC, Lopes JM (2005) Molecular characterization of PDGFR-alpha/PDGF-A and c-KIT/SCF in gliosarcomas. Cell Oncol 27:319–326
Simmons ML, Lamborn KR, Takahashi M, Chen P, Israel MA, Berger MS, Godfrey T, Nigro J, Prados M, Chang S, Barker FG 2nd, Aldape K (2001) Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res 61:1122–1128
Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res 19:764–772. doi:10.1158/1078-0432.ccr-12-3002
Gjerdrum LM, Bojsen-Moller M (1999) October 1998—61 year old male with brain tumor and oral, lung, and palpebral masses. Brain Pathol 9:421–422
Ojeda VJ, Sterrett GF (1984) Cerebral gliosarcoma, pulmonary adenoid-cystic carcinoma, and pulmonary metastatic gliosarcoma: report of an untreated case. Pathology 16:217–221
Weaver D, Vandenberg S, Park TS, Jane JA (1984) Selective peripancreatic sarcoma metastases from primary gliosarcoma. Case report. J Neurosurg 61:599–601. doi:10.3171/jns.1984.61.3.0599
Robert M, Wastie M (2008) Glioblastoma multiforme: a rare manifestation of extensive liver and bone metastases. Biomed Imaging Interv J 4:e3. doi:10.2349/biij.4.1.e3
Beauchesne P (2011) Extra-neural metastases of malignant gliomas: myth or reality? Cancers (Basel) 3:461–477. doi:10.3390/cancers3010461
Mandel JJ, Yust-Katz S, Cachia D, Wu J, Liu D, de Groot JF, Yung AW, Gilbert MR (2014) Leptomeningeal dissemination in glioblastoma; an inspection of risk factors, treatment, and outcomes at a single institution. J Neurooncol 120:597–605. doi:10.1007/s11060-014-1592-1
Mansouri B, Barboriak DP, Kilani RK (2013) Gliosarcoma metastatic to the leptomeninges and dura. J Neuroimaging 23:245–247. doi:10.1111/j.1552-6569.2011.00641.x
Singh G, Mallick S, Sharma V, Joshi N, Purkait S, Jha P, Sharma MC, Suri V, Julka PK, Mahapatra AK, Singh M, Kale SS, Sarkar C (2012) A study of clinico-pathological parameters and O(6)-methylguanine DNA methyltransferase (MGMT) promoter methylation status in the prognostication of gliosarcoma. Neuropathology 32:534–542. doi:10.1111/j.1440-1789.2012.01297.x
Morantz RA, Feigin I, Ransohoff J 3rd (1976) Clinical and pathological study of 24 cases of gliosarcoma. J Neurosurg 45:398–408. doi:10.3171/jns.1976.45.4.0398
Parekh HC, O’Donovan DG, Sharma RR, Keogh AJ (1995) Primary cerebral gliosarcoma: report of 17 cases. Br J Neurosurg 9:171–178
Lutterbach J, Guttenberger R, Pagenstecher A (2001) Gliosarcoma: a clinical study. Radiother Oncol 61:57–64
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16:2443–2449. doi:10.1158/1078-0432.ccr-09-3106
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953. doi:10.1016/s1470-2045(14)70314-6
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722. doi:10.1056/NEJMoa1308345
Phillips H, Sandmann T, Li C, Cloughesy T, Chinot OL, Wick W, Nishikawa R, Mason W, Henriksson R, Saran F, Lai A, Moore N, Hegde P, Abrey L, Bourgon R, Garcia J, Bais C (2014) BI-22correlation of molecular subtypes with overall survival (OS) in avaglio, a randomized, placebo-controlled study of bevacizumab (BEV) plus radiotherapy (RT) and temozolomide (TMZ) for newly diagnosed glioblastoma (GBM). Neuro Oncol 16:v28. doi:10.1093/neuonc/nou239.22
Acknowledgments
We thank Dr Funda Meric-Bernstam MD and Dr Kenna R Shaw PhD for their invaluable support in preparation of this manuscript. This work was supported in part by the Sheikh Khalifa Al Nahyan Ben Zayed Institute for Personalized Cancer Therapy, and the MD Anderson Cancer Center Support grant (P30 CA016672). A Olar was supported by the National Institutes of Health/National Cancer Institute (Training Grant No. 5T32CA163185). T.S. Armstrong serves as consultant for Immunocellular therapeutics;is on the advisory board for Roche; receives research support from Merck and Genentech. M.R. Gilbert reports research support from Genentech, Merck, Glaxo Smith Kline; receives honoraria from Merck, Genentech, AbbVie; and serves on the advisory board for Genetech, AbbVie, Heron Therapeutics. JF de Groot serves as a consultant for Celldex and Deciphera Pharmaceuticals; serves on the advisory board for Genentech, Novartis, Celldex, is on the Data and Safety Monitoring Board for VBL Therapeutics and receives research support from Sanofi-Aventis, AstraZeneca, EMD-Serono, Eli Lilly, Novartis, Deciphera Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
D. Cachia, C. Kamiya-Matsuoka, A. Olar, M. D. Cykowski, report no disclosures.
Additional information
David Cachia and Carlos Kamiya-Matsuoka have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cachia, D., Kamiya-Matsuoka, C., Mandel, J.J. et al. Primary and secondary gliosarcomas: clinical, molecular and survival characteristics. J Neurooncol 125, 401–410 (2015). https://doi.org/10.1007/s11060-015-1930-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1930-y