Abstract
With emerging drug delivery technologies becoming accessible, more options are expected to become available to patients with glioblastoma (GBM) in the near future. It is important for clinicians to be familiar with the underlying mechanisms and limitations of intratumoral drug delivery, and direction of recent research efforts. Tumor-adjacent brain is an extremely complex living matrix that creates challenges with normal tissue intertwining with tumor cells. For convection-enhanced delivery (CED), the role of tissue anisotropy for better predicting the biodistribution of the infusate has recently been studied. Computational predictive methods are now available to better plan CED therapy. Catheter design and placement—in addition to the agent being used—are critical components of any protocol. This paper overviews intratumoral therapies for GBM, highlighting key anatomic and physiologic perspectives, selected agents (especially immunotoxins), and some new developments such as the description of the glymphatic system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The idea of delivering drugs or larger therapeutic molecules directly into the brain or tumor bed—thereby circumventing the blood–brain barrier (BBB)—continues to be extremely attractive for neurosurgeons. While current therapy for newly-diagnosed glioblastoma (GBM) includes the maximum safe cytoreductive surgery followed by concurrent temozolomide and fractionated external beam radiotherapy, the median overall survival is only 14.6 months with a “progression-free” interval of only 6.9 months [1]. While there are many options for recurrent GBM [2], there currently is no established standard of care [3]. The highly invasive character of GBM cells confounds our ability to effectively eliminate them with surgical resection; subsequent radiation and chemotherapy entail considerable localized tissue damage and systemic toxicity. Successful delivery of therapeutic agents to residual GBM cells remains a very significant challenge, especially beyond areas of contrast enhancement, in relatively normal brain. Since Ehrlich described the BBB in 1885, it has been widely appreciated that drugs and other therapeutic macromolecules have extremely limited penetration into CNS when delivered systemically [4, 5].
Neurosurgeons and their colleagues have therefore explored multiple modes of intratumoral therapy for GBM, including instillation of chemotherapy, induction of an immunologic response, gene therapy, interstitial wafer chemotherapy, convection-enhanced delivery (CED) and (recently) nanoparticle administration, to name but a few (Fig. 1) [8, 13, 14]. Fortunately, as the bioengineering and neurosurgical communities advance the field of drug delivery, intratumoral therapy for GBM continues to evolve and is still promising. The purpose of this focused review is to provide the practicing neuro-oncologist and neurosurgeon with insights into intratumoral drug delivery for GBM, including anatomic and physiologic considerations, and recent therapeutic agents, highlighting immunotoxic therapy.
GBM is treated with cytoreductive surgery when possible followed by chemotherapy and radiation therapy as the standard of care. When there is recurrent GBM, neurosurgeons have multiple modes of intratumoral therapy including: instillation of chemotherapy, nanoparticles, gene therapy, interstitial wafer chemotherapy, and convection-enhanced delivery
Anatomic and physiologic considerations
Successful therapy for GBM will likely be based on continued improvements in our understanding not only of the therapeutic agent, but also the nature of brain parenchyma adjacent to (and beyond) the resection cavity. Tumor-adjacent brain is not just a sponge or gel; rather, it is an extremely complex living matrix which includes neurons, glia, immune cells, cellular processes, blood vessels, fiber tracts, extracellular matrix, defined extracellular spaces, and the interstitial space. In patients, these components are not normal brain; they have been variably affected by radiation and chemotherapy, and the infiltrating malignant cells themselves. Molecules delivered into brain parenchyma are affected by all of these factors, which may vary from patient to patient. Drug binding, clearance, destruction, and/or uptake by non-neoplastic cells all have potential adverse effects on therapeutic efficacy and can increase toxicity.
Anatomically, brain parenchyma and tumor—adjacent brain are predominantly cellular, and the interstitial fluid “bathing” the cells plays a critical role in their homeostasis, and drug delivery. Therapeutic agents vary in their solubility and movement (such as diffusion) within interstitial fluid. The interstitial space of the brain—being both extracellular and extravascular—is separated from ventricular CSF by ependyma, and from the subarachnoid CSF by the glia limitans (a layer of interdigitating astrocytic processes with an overlying basement membrane). The perivascular (or Virchow-Robin) space is an extension of the subarachnoid space. Use of an Ommaya reservoir effectively delivers drug to the intraventricular CSF, but not necessarily to the brain interstitial fluid [6]. It has been calculated that the concentration of a drug being directly infused into brain parenchyma decreases logarithmically with each millimeter of distance from the catheter used for CED [6]. It is because of such considerations in scale, that while in small animal models treatment efficacy is high, such results in do not translate into success when dealing with the large brains and ventricular anatomy of humans. In the mouse brain, the entire parenchyma is within 5 mm of the CSF surface. In the human brain, this distance reaches 50 mm, effectively precluding therapeutic drug distribution into brain parenchyma from the CSF space alone [6].
The lack of a lymphatic system for the brain has long puzzled some neuroscientists, who have wondered exactly how the brain clears extracellular proteins and toxic substances. Recently, it has been shown that CSF enters the parenchyma along subarachnoid “paravascular” spaces, mixes with interstitial fluid, and then is cleared along paravenous drainage pathways [6]. This circulation has been dubbed the “glymphatic system”, is dependent on aquaporin-4 water channels, and is reportedly more active during sleep. While interstitial fluid movement undoubtedly affects drug movement, other variables also need to be studied in more detail clinically. The resistance of brain tissue is a major factor which reduces delivery to brain beyond the catheter tip [6]. Such resistance can also unintentionally direct flow along the catheter tract, although this problem has been minimized by modifications in catheter design [7]. If a substance is being infused into a tumor cavity, gliosis at the cavity wall creates an additional barrier to the brain interface. In addition, intratumoral pressure gradients siphon drug towards areas of less resistance such as the cisterns and interstitial spaces. These barriers for drug delivery, create challenges in effective drug distribution.
Gene therapy, interstitial wafers, and chemotherapy
The concept of directly delivering genetic material to modify tumor cell phenotype has been appealing to neuro-oncologists for decades. The transfer of genetic material into a patient’s cell for therapeutic processes is the general definition for “gene therapy [7].” A broader definition includes transfer of genetic material to boost the immune system, enzyme prodrug therapy, oncoloytic therapy, transfer of tumor suppressor or anti-angiogenesis genes, or transfer of antisense oligonucleotides [7]. There are challenges with gene therapy which are inherent in the mode of drug delivery, and transduction efficiency. For instance, to date, intracerebral injection allows localized delivery to a few tumor cells at the site of the injection, but does not penetrate to other tumor cells deep in (otherwise normal) parenchyma.
The genetic material is typically transfected through viral vectors that ideally transduce tumor cells while avoiding normal cells. The major limitation with the viral transfection is the host immune response which limits the viral spread to a few tumor cells. This limitation of viral transfection therapy must be addressed through new drug delivery methods that bypass the host’s immune system.
Interstitial BCNU wafers provide an FDA-approved localized drug delivery method that neurosurgeons can employ when treating recurrent GBM. 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) delivered into the tumor bed by biodegradable polymer wafers is thought to treat a 2 cm margin of brain surrounding the tumor cavity, where 90 % of GBMs recur [8]. Animal studies suggest that penetration of BCNU occurs mostly in the first 4 days with therapeutic concentrations of the drug, at 1 cm beyond the margins [9, 10]. Reported and potential complications of BCNU wafers include infection, impaired wound healing, CSF leak, tumor cyst formation, seizures, and cerebral edema [8]. The limited success of BCNU wafers could be related to the efficacy of BCNU itself on malignant tumor cells, as well as the concentration and depth of penetration into the parenchyma [8].
In addition to wafer implantation, there have been clinical trials of the direct infusion of other chemotherapeutic agents. It is important to appreciate that inadvertent injection of chemotherapy into an Ommaya reservoir (for instance) has been reported to be lethal [11]. Paclitaxel is an antineoplastic agent that promotes the assembly of microtubules into a metastable state which cells cannot disassemble [12]. On infusion of paclitaxel for recurrent grade III or IV gliomas, Lidar et al. demonstrated an overall median survival of 7.5 months. However, there were a significant number of treatment-related adverse events including transient neurological deterioration from cerebral edema (20 %), bacterial infections (15 %), and chemical meningitis (30 %) [13]. The lack of specificity and the chemical meningitis associated with paclitaxel in the CSF space made it an unattractive agent for GBM treatment. With all of these agents, apart from the challenges of drug delivery, there are additional challenges including the heterogeneous nature of GBM, and the ability of GBM cells to develop resistance, and otherwise elude therapy.
Convection-enhanced delivery
CED is a technique that propels therapeutic molecules via continuous infusion directly into the tumor bed or tumor–adjacent brain, thus circumventing the BBB. This is often accomplished through direct stereotactic intracerebral catheter placement, with a pump used as the continuous source of fluid carrying the therapeutic agent. The pump provides a constant microfluidic flow rate that is administered to promote the efficacy of fluid transfer to the brain parenchyma. In previous trials, the flow rate administered in patients is at 0.5 mL/h for 96 h [14]. The key limitation of higher flow rates of fluid is backflow. Backflow or reflux is described as the fluid discharge around the catheter shaft back out of the brain instead of into the parenchyma. Backflow-free catheter designs are being developed that has increased the flow rates to higher values that include: channel-inducing catheters and dual-action backflow-free catheters [15]. Pumps that provide a longer continuous lower flow rate are being utilized to prevent backflow such as the Alaris® pumps and Medtronic Synchromed-II pumps [16, 17]. These pump systems are implantable and provide continuous flow at flow rates at 5μL/min.
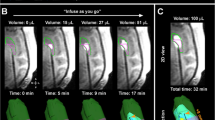
To date, immunotoxins have been most extensively tested in clinical trials utilizing CED (Table 1). The initial CED trials had demonstrated, through small phase I and II trials, a good safety profile and some limited efficacy in small cohorts. TF-CRM 107, a diphtheria toxin fused to Tranferrin-C, had some initial promise through a phase I trial demonstrating greater than 50 % decrease in tumor volume on MRI in 9 of 15 patients [18]. These results were not reproduced in the phase II trial with a 39 % response rate in 44 patients, therefore the phase III trial was aborted [19]. Around the same time, the PRECISE trial was initiated that compared the efficacy of citredekin besudotox with Gliadel chemotherapy wafers through a randomized trial. Eventhough the survival benefit was not significant (36.4 vs. 35.3 weeks), 68 % of the catheters were misplaced and the progression free survival was significant (17.7 vs. 11.4 weeks; p < 0.0008) [20]. The PRECISE trial highlighted the need to have improved computational modeling and image guidance in catheter placement, and there is a belief that the trial failed to demonstrate efficacy due to this misplacement of catheters.
The fluid carries, or advects, agents through the interstitial space by means of a small, constant pressure gradient [21, 22]. If CED is applied after resection of a brain tumor, the microinfusion catheters placed under stereotactic guidance target the peritumoral region with bulk flow, supplementing intrinsic diffusivity of either small or large therapeutic compounds to greatly enhance their distribution within the brain [21]. Since CED depends on fluid flow to carry the agent, it is possible to achieve a relatively constant concentration of drug spanning a predictable distance from the infusion site before the drop-off [23]. This provides superior localization to residual tumor cells, given accurate catheter placement [23, 24]. Another theoretical advantage relates to the BBB itself, which would be expected to conversely limit the egress of the therapeutic agent from the brain, enhancing effectiveness and limiting systemic exposure [23].
Given that the efficacy of any drug delivered by CED will be dependent on the ability to achieve sufficient concentrations within the targeted region, much attention has been placed on the factors that dictate optimal catheter placement [24, 25]. If catheters are inaccurately placed, leakage of infusate into the intraventricular spaces and subarachnoid space may result in poor drug delivery and distribution [25]. Currently, catheters do generate backflow in the tissue-free region along the outer surface of the catheter [25–28]. In addition, any large (i.e. millimeter sized) bubbles may redirect flow away from the catheter tip in unpredictable ways. Thus, optimization of devices and of various protocols constitute an extremely important part of improving the efficacy of CED [29–32].
Future CED efforts will benefit from the expanded use of reliable computer modeling, and real-time monitoring of catheter placement and the infusion process [33, 34]. Failures in early clinical trials involving CED may have been due (at least in part) to an incomplete understanding of tissue physiology (e.g. inhomogeneities in fluid pathways of the brain). It is very encouraging that the role of tissue anisotropy for better predicting the drug biodistribution by CED has recently been studied, both experimentally and with computer models [35–38]. Advances in experimental techniques are currently aimed at achieving a better understating of the biomechanical interactions between convective drug transport, and the complexities of the brain parenchyma [39, 40]. Vigorous use of interventional MR, with software enabling rapid imaging of catheter placement, will greatly aid future studies of CED.
Intratumoral delivery of nanoparticles
Biocompatible iron-oxide nanoparticles have recently been directly delivered to the tumor bed in GBM patients, then stimulated by an alternating magnetic field to induce heat. The heat produced by the magnetic nanoparticles, in addition to 5 fractions of stereotactic radiotherapy of 5 × 2 Gy per week, was thought to induce a synergistic cytotoxicity [41]. A single arm clinical trial in 2 centers in Germany investigated this therapy in 66 patients (59 recurrent GBM patients). The median overall survival was 13.4 months, while the median overall survival from diagnosis of primary tumor was 23.2 months. The tumor volume at entry correlated significantly with survival. There were no systemic side effects of therapy, such as iron toxicity. 4 patients exhibited worsening of hemiparesis following nanoparticle instillation but did have a degree of weakness before the procedure [41]. The major limitations of this study in recurrent GBM patients were two-fold: the indefinite exclusion of subsequent MR imaging due to artifact from the iron oxide, and the need for removal of all metal from within 40 cm of treatment area, including all dental work.
Conclusions
Despite progress on multiple fronts, including the identification of favorable prognostic markers such as IDH mutations, the outlook for most patients with GBM remains dismal [2]. This review provides background for neurosurgeons and neuro-oncologists seeking information on intratumoral therapy (such as CED) for their GBM patients (Table 2). BCNU wafers remain an FDA-approved therapeutic option. Gene therapy, and intratumoral chemotherapy have been tried, while CED of immunotoxins has been studied more extensively. CED remains a promising technique for administering anti-cancer agents in the context of experimental trials for GBM patients. Notably, advances have been made in understanding the basic neurophysiology of the brain parenchyma, e.g. clarifying the nature of the circulation of interstitial fluid. Advances in bioengineering have included using tissue anisotropy for improved prediction of drug biodistribution, understanding the complexities of convective drug transport, and improving techniques for catheter placement.
A critical task that needs to be achieved clinically in order to allow for successful treatment of GBM patients with CED is to be able to distribute the therapeutic agent over large areas of the brain in a predictable, sustainable, and controlled fashion. Recently, preliminary studies of magnetic drug targeting [42, 43] and ultrasound -assisted methods [44] have been reported. Such techniques may soon be able to generate the additional guidance needed to steer agents placed within the tumor bed to desired target locations deep within the brain parenchyma, beyond the reach of even the most deftly-guided intravascular microcatheters, for extended time periods. The possibly of intratumoral therapy for GBM patients, while challenging, continues to offer an intriguing possibility for neurosurgeons.
References
Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Stupp R et al (2012) NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 48(14):2192–2202
Juratli TA, Schackert G, Krex D (2013) Current status of local therapy in malignant gliomas: a clinical review of three selected approaches. Pharmacol Ther 139(3):341–358
Kanu OO et al (2009) Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets 13(6):701–718
Engelhard HH, Groothuis DG (1999) The blood–brain barrier: structure, function, and response to neoplasia. In: Berger MS, Wilson CB (eds) The gliomas. W.B. Saunders Company, Philadelphia, pp 115–121
Iliff JJ et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4(147):47ra111
Bansal K, Engelhard HH (2000) Gene therapy for brain tumors. Curr Oncol Rep 2(5):463–472
Engelhard HH (2000) The role of interstitial BCNU chemotherapy in the treatment of malignant glioma. Surg Neurol 53(5):458–464
Fung LK et al (1996) Chemotherapeutic drugs released from polymers: distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm Res 13(5):671–682
Strasser JF et al (1995) Distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea and tracers in the rabbit brain after interstitial delivery by biodegradable polymer implants. J Pharmacol Exp Ther 275(3):1647–1655
Meggs WJ, Hoffman RS (1998) Fatality resulting from intraventricular vincristine administration. J Toxicol Clin Toxicol 36(3):243–246
Jordan MA et al (1993) Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci U S A 90(20):9552–9556
Lidar Z et al (2004) Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 100(3):472–479
Sampson JH, B.M., Raghavan R, Mehta AI, Friedman AH, Reardon DA, Petry NA, Barboriak DP, Wong TZ, Zalutsky MR, Lally-Goss D, Bigner DD (2010) Co-localization of gadolinium-DTPA with high molecular weight molecules after intracerebral convection-enhanced delivery in man. Neurosurgery, In Press
Krauze MT et al (2005) Effects of the perivascular space on convection-enhanced delivery of liposomes in primate putamen. Exp Neurol 196(1):104–111
Sonabend AM et al (2011) Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro Oncol 13(8):886–893
Sillay K et al (2013) Convection enhanced delivery to the Brain: preparing for gene therapy and protein delivery to the Brain for functional and restorative Neurosurgery by understanding low-flow neurocatheter infusions using the Alaris((R)) system infusion pump. Ann Neurosci 20(2):52–58
Laske DW, Youle RJ, Oldfield EH (1997) Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med 3(12):1362–1368
Weaver M, Laske DW (2003) Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol 65(1):3–13
Kunwar S et al (2007) Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 25(7):837–844
Bobo RH et al (1994) Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 91(6):2076–2080
Raghavan R et al (2006) Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus 20(4):E12
Ding D et al (2010) Convection-enhanced delivery of free gadolinium with the recombinant immunotoxin MR1-1. J Neurooncol 98(1):1–7
Sampson JH et al (2010) Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113(2):301–309
Sampson J. H. et al. (2007) Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. Neurosurgery, 60(2 Suppl 1): p ONS89-98; discussion ONS98-9
Tanner P. G. et al. (2007) Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas: technical note. Neurosurgery, 61(4): p E880-2; discussion E882
Raghavan R et al (2010) Fluid infusions from catheters into elastic tissue: I. Azimuthally symmetric backflow in homogeneous media. Phys Med Biol 55(1):281–304
Ivanchenko O. et al. (2010) Design of backflow-free catheters based on micro-fluid dynamics, in BMES 2010 annual fall meeting, Austin
Raghavan R. (2010) Intraparenchymal delivery and its discontents. Drug delivery to the central nervous system, p 85–135
Li D et al. (2010) Optimal catheter placement for chemotherapy, in proceedings of 20th european symposium on computer aided process engineering (ESCAPE), p 223–228
Somayaj MB et al (2008) Systematic design of drug delivery therapies. Comp Chem Eng 32:89–98
Zhang L et al (2007) Discovery of transport and reaction properties in distributed systems. AIChE J 53(2):381–396
Sampson JH et al (2007) Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro-oncology 9(3):343–353
Raghavan R, and M. Brady (2011) Predictive models of pressure-driven infusions into brain parenchyma. Phys Med Biol, In Press
Linninger AA et al (2008) Computational methods for predicting drug transport in anisotropic and heterogeneous brain tissue. J Biomech 41(10):2176–2187
Linninger AA et al (2008) Prediction of convection-enhanced drug delivery to the human brain. J Theor Biol 250(1):125–138
Linninger AA et al (2008) Rigorous mathematical modeling techniques for optimal delivery of macromolecules to the brain. IEEE Trans Biomed Eng 55(9):2303–2313
Ivanchenko O, Sindhwani N, Linninger A (2012) Exact solution of the convection-diffusion problem in cylindrical geometry. AIChE J 58(4):1299–1302
Sindhwani N et al (2011) Methods for determining agent concentration profiles in agarose gel during convection-enhanced delivery. IEEE Trans Biomed Eng 58(3):626–632
Ivanchenko O, Sindhwani N, Linninger A (2010) Experimental techniques for studying poroelasticity in brain phantom gels under high flow microinfusion. J Biomech Eng 132(5):051008
Maier-Hauff K et al (2011) Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 103(2):317–324
Lueshen E et al (2014) Intrathecal magnetic drug targeting using gold-coated magnetite nanoparticles in a human spine model. Nanomedicine (Lond) 9(8):1155–1169
Lueshen E et al (2014) Implant-Assisted intrathecal magnetic drug targeting to aid in the therapeutic nanoparticle localization for potential treatment of central nervous system disorders. J Biomed Nanotechnol 11(2):253–261
Wang P, Olbricht WL (2011) Fluid and solid mechanics in a poroelastic network induced by ultrasound. J Biomech 44(1):28–33
Kunwar S et al (2006) Safety of intraparenchymal convection-enhanced delivery of cintredekin besudotox in early-phase studies. Neurosurg Focus 20(4):E15
Vogelbaum MA et al (2007) Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery 61(5):1031–1037; discussion 1037–1038
Rand RW et al (2000) Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res 6(6):2157–2165
Weber F et al (2003) Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol 64(1–2):125–137
Schlingensiepen R et al (2005) Intracerebral and intrathecal infusion of the TGF-beta 2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: toxicology and safety. Oligonucleotides 15(2):94–104
Patel SJ et al (2005) Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery 56(6):1243–1252; discussion 1252–1253
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehta, A.I., Linninger, A., Lesniak, M.S. et al. Current status of intratumoral therapy for glioblastoma. J Neurooncol 125, 1–7 (2015). https://doi.org/10.1007/s11060-015-1875-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1875-1