Abstract
Both age of patients and tumor location are associated with tumor origin, genetic characteristics, and prognosis. The objective of this study was to investigate the relationship between tumor location and age at diagnosis in a large cohort of patients with a primary diagnosis of glioma. We consecutively enrolled a cohort of 200 adults with glioblastoma and another cohort of 200 adults with diffuse low-grade gliomas. The magnetic resonance images of all tumors were manually segmented and then registered to a standard brain space. By using voxel-by-voxel regression analysis, specific brains regions associated with advanced age at tumor diagnosis were localized. In the low-grade gliomas cohort, the brain regions associated with advanced age at tumor diagnosis were mainly located in the right middle frontal region, while a region in the left temporal lobe, particularly at the subgranular zone, was associated with lower age at tumor diagnosis. In the glioblastoma cohort, the brain regions associated with advanced age at tumor diagnosis were mainly located in the temporal lobe, particularly at the posterior region of the subventricular zones. A region in the left inferior frontal region was associated with lower age at tumor diagnosis. Significant differences in the age of patients were found between tumors located in the identified regions and those located elsewhere in both cohorts. The current study demonstrated the correlation between tumor location and age at diagnosis, which implies differences in the origin of gliomas in young and older patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common primary brain tumors and account for about half of all central nervous system neoplasms. They are characterized by biological heterogeneity and can be classified into a variety of histological subtypes. Glioblastoma multiformes (GBMs; WHO grade IV) have poor prognosis with a median survival period of 12–14 months even under multimodal treatments [1]. Diffuse low-grade gliomas (LGGs; WHO grade II), including oligodendrogliomas, astrocytomas, and oligoastrocytomas [2], have better prognoses than high-grade gliomas do. The median overall survival period for patients with LGGs was 118 months [3], with most patients dying from the disease.
Age is an important clinical factor in gliomas that correlates with incidence, malignancy, and survival outcome [4, 5]. Increased age is associated with higher incidence of tumor and higher degree of malignancy in all types of gliomas [5, 6]. A randomized clinical trial on GBM demonstrated that the median lifespan of patients aged ≥60 was significantly shorter than that of younger patients [7]. Additionally, an age of <40 years was found to be a good predictor of survival outcome [8, 9] and was associated with a lower tumor proliferative index in patients with LGG [10]. Further, age plays an important role in tumorigenesis and is associated with tumor-related genetic abnormalities, such as isocitrate dehydrogenase 1 (IDH1) mutation and glioma CpG island methylator phenotype [11]. Malignant gliomas likely originate from neural progenitor cells or their glial progenitor progeny. A previous animal experiment suggested that the neural progenitor cells in aged mice overexpressed Ras, abrogated p53, and Rb. Therefore, increased malignancy was seen for gliomas in the aged mice than their younger counterparts [12]. On the other hand, neural progenitor cells are associated with decreased regeneration in aged mice [13]. As a result, age-related decline in stem-like cells may induce increased cellular deficits.
In addition, the anatomic location of the tumor was also revealed as a prognostic factor [7, 14–16] associated with tumor-specific genetic changes. For instance, IDH1 mutations are more likely to occur in gliomas located at the frontal lobe [17–20] and predict a better prognosis. Similarly, 1p/19q co-deletion [19] was found to predominantly occur in frontal lobe gliomas. In addition, O6-methylguanine DNA methyltransferase promoter methylation was found to be prone to hemispheric lateralization [21]. This anatomic specificity of genomic mutation was also found in meningiomas [22]. Moreover, tumor location also plays a role in tumorigenesis, since tumor precursor cells are specifically distributed in the brain. Different types of oligodendrogliomas were shown to arise from various precursor cells that were region-specific in origin during brain development [23].
Both age and tumor location are important clinical factors and have been associated with tumorigenesis, tumor-specific genetic changes, and prognosis of patients with glioma. However, the potential intrinsic association between age and tumor location has not been investigated. Voxel-based regression analysis has been increasingly used for determining the correlation between the location of brain lesions and clinical manifestations [24–27]. This approach was performed in the current study to map the relationship between tumor location and age at diagnosis in a large cohort of patients with primary diagnosis of gliomas.
Methods
Patients
A consecutive enrollment of 200 patients with LGG and 200 patients with GBM, surgically treated between December 2007 and November 2012, was conducted for the current study. Clinical information was collected from the Chinese Glioma Genome database (http://www.cgga.org.cn). All patients participating in this study met the following criteria: (1) no prior craniotomy or biopsy, (2) pathological confirmation of diffuse LGG (WHO grade II) or primary GBM, (3) no history of radiotherapy or chemotherapy, (4) documentation of the age of patient at diagnosis. Clinical and radiologic characteristics of all patients are summarized in Table 1. The current study was approved by the ethics committee of Beijing Tiantan Hospital, and written informed consent was obtained from all patients.
Imaging acquisition and tumor registration
Magnetic resonance (MR) imaging scans for the majority of patients were acquired using a Magnetom Trio 3.0 Tesla (Siemens AG, Erlangen, Germany) scanner. The T2-weighted image parameters included TR = 5800 ms, TE = 110 ms, field of view = 240 × 188 mm2, flip angle = 150°, voxel size = 0.6 × 0.6 × 5 mm3. Other MR images were collected on a Magnetom Verio 3.0 Tesla (Siemens AG, Erlangen, Germany) (n = 31) or a HD 1.5 Tesla (GE Medical System, Milwaukee, USA) (n = 26) scanner. If a patient underwent more than one MR scan, the first scan by which the tumor was observed was used in this study. Based on the findings that brain areas with T2 signal abnormality without contrast enhancement on the post-contrast T1-weighted image could still contain high-density of tumor cells [28, 29], T2-weighted images were used as a reference sequence in this study. All tumor masks (outline of the tumor boundary) were manually delineated on all slices of the T2-weighted sequence where the tumor was present using MRIcro (http://www.mccauslandcenter.sc.edu/mricro/) by two board-certified neurosurgeons blinded to the clinical information. All the tumor masks were subsequently re-evaluated by an experienced neuroradiologist. If the discrepancy was 5 % or more, the neuroradiologist determined the lesion border to be used in the study. Each masked tumor region was registered to a Montreal Neurological Institute (MNI) brain template using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). The volume and centroid of a tumor were calculated using Matlab (R2012a, MathWorks, Natick, MA, USA). The centroid of a registered tumor region was defined as the location of the mean coordinates of all voxels contained in the masked region in three orthogonal directions. All computed tumor centroids were rendered on a template brain to show the distribution of the tumors and age of the corresponding patients.
Voxel-based lesion-symptom mapping (VLSM) analysis
We first examined the overlap between the normalized tumor masked regions for the LGG group and the GBM group, respectively. Consequently, we performed VLSM analysis [24, 30] for each cohort of patients in order to localize brain regions associated with the age of tumor diagnosis. The general linear model (Y = βX + ε) was applied to each voxel independently. In this case, Y represents involvement of the lesion (1 = tumor presence and 0 = tumor absence) in each patient; X represents the matrix of clinical characteristics (X1 = age of patient, X2 = sex of patient, and X3 = tumor volume); β represents the model parameter to be estimated and shows the correlation of the voxel to clinical characteristic (age in this case), while ε represents the residual. Consequently, the VLSM approach provided a t-statistic value that indicated the association of the specific voxel to the age of patient at tumor diagnosis. By applying the general linear model, voxels that significantly correlated with patient age were identified by regressing out the influence of sex and tumor volume. This result was further corrected using a permutation test (n = 500) [31, 32]. The t value of the voxel that was higher than the t values in more than 95 % of permutations was preserved in the results, and considered statistically significant in this study [31].
Evaluation of VLSM-identified regions
The VLSM-identified brain regions were validated by examining the average age of patients with tumors that occurred within the different clusters. Tumor involvement was identified based on whether a tumor centroid was located in the VLSM-identified cluster. A two sample independent t test was used to compare the mean age of patients between the subgroups of tumors involving the brain regions associated with advanced age and those associated with lower age.
Results
Demographic and clinical information
During our study period, 400 primary gliomas were diagnosed, including 200 LGGs and 200 GBMs. The median age at diagnosis was 38 years (range 15–68 years) in the LGG group and 53.5 years (range 16–77 years) in the GBM group. The overall information on the LGG and GBM cohorts are summarized in Table 1.
Lesion volume
There were no significant differences in T2-hyperintense lesion volumes between older (≥45 years, 84.5 ± 72.9 cm3) and young (<45 years, 79.4 ± 61.3 cm3) patients with LGGs (p = 0.621). Similarly, no significant difference in tumor volume was found between older (≥45 years, 130.9 ± 71.3 cm3) and young (<45 years, 137.3 ± 82.3 cm3) patients with GBMs (p = 0.578). These results suggested that the age at diagnosis was not associated with tumor size in patients with LGGs or GBMs.
Radiographic characteristics
A statistical power map for the VLSM analysis [31] was generated and showed that most brain areas were highly reliable for statistical analysis (Fig. S1). Centroids of tumor lesions were rendered onto a standard MNI brain template based on the age of patients (Fig. S2; Table S1).
VLSM analysis findings
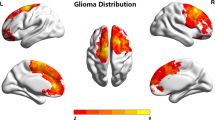
Our VLSM analysis identified a specific association between the anatomic location of tumor and the age of patient at diagnosis. The brain regions that significantly correlated to patient age were mapped on a standard brain space (Fig. 1). In the LGG cohort, the brain region (Cluster 1) associated with advanced age at tumor diagnosis was mainly located in the right middle frontal region, while the brain region (Cluster 2) associated with low age at tumor diagnosis was preferentially located in the left temporal lobe, particularly at the subgranular zone (Fig. 1A, B). The voxels with the highest t value in Cluster 1 (t max = 3.33, x = 103, y = 162, z = 68) and Cluster 2 (t max = 3.26, x = 42, y = 101, z = 52) had the strongest correlation effects according to our method.
VLSM identifying age-associated brain regions in patients with gliomas. A voxel-wise regression model is performed correlating the age of the patient at diagnosis and the location of tumor in LGG and GBM cohort, respectively. In LGG cohort, the right middle frontal region is significantly associated with advanced age of patients at tumor diagnosis, while the left temporal is associated with lower age at tumor diagnosis. In the GBM cohort, the bilateral temporal lobes, particularly the posterior region of the SVZ, are significantly associated with advanced age at tumor diagnosis, and the left inferior frontal region is associated with lower age. Only significant voxels are demonstrated based on a permutation correction (n = 500). LGG low-grade gliomas, GBM glioblastoma multiformes, VLSM voxel-based lesion-symptom mapping, SVZ subventricular zone
In the GBM cohort, the brain region (Cluster 3) associated with advanced age at tumor diagnosis was mainly located in the bilateral temporal lobe, particularly at the posterior region of the subventricular zone (SVZ). Meanwhile, the brain region associated with low age at tumor diagnosis was preferentially located in the left inferior frontal region (Fig. 1C, D). The voxel with the highest t value in Cluster 3 (t max = 4.07, x = 56, y = 75, z = 72) and Cluster 4 (t max = 4.24, x = 88, y = 170, z = 53) had the strongest correlation effects.
Age differences in VLSM-identified regions
Significant differences in the age of patient were found between tumors involving VLSM-regions associated with advanced age and those associated with lower age in both cohorts (Fig. 2). The mean age of patients with LGGs involving Cluster 1 (46.7 ± 15.5 years) were significantly higher than that in Cluster 2 (29.5 ± 7.0 years) (p < 0.0001, t test). Similarly, a significantly higher age was observed in patients with GBMs involving Cluster 3 (59.1 ± 9.2 years) than those involving Cluster 4 (35.5 ± 13.6 years) (p < 0.0001, t test). These results validated that the age-associated brain regions identified by VLSM in patients with gliomas.
Comparison of patient age at diagnosis and tumors involving the VLSM-identified regions. In the LGG cohort, patients with tumors involving VLSM-identified Cluster 1 associated with advanced age are significantly older than patients with tumors involving VLSM-identified Cluster 2 associated with lower age (46.7 ± 15.5 vs. 29.5 ± 7.0 years, p < 0.0001, t test). Similarly, a significantly higher age is observed in patients with GBMs involving Cluster 3 associated with advanced age than those with GBMs involving Cluster 4 associated with lower age (59.1 ± 9.2 vs. 35.5 ± 13.6 years, p < 0.0001, t test). LGG low-grade gliomas, GBM glioblastoma multiformes, VLSM voxel-based lesion-symptom mapping
Discussion
This is the first voxel-level investigation correlating patient age and tumor location in 200 patients with GBMs and 200 patients with LGGs. Preferential brain regions associated with tumor occurrence at different ages of patients were identified by voxel-based analysis. This quantitative three-dimensional statistics provides new evidence to the anatomic correlates for age groups in gliomas, which were different between LGG cohort and GBM cohort.
Age and location are both considered to be associated with tumor origin, genetic characteristics, and prognosis. The associations between the age at diagnosis and location of tumors were suggested by several previous studies. For example, a study delineated two subgroups of the posterior fossa ependymomas that had distinct clinical and molecular features. This study demonstrated that the ependymomas located in the lateral parts of the fourth ventricle were more likely to occur in children and had frequent relapse and poor prognosis, while tumors located near the midline were more prevalent in adults and had favorable prognosis [33]. Based on the occurrence of H3F3A (histone H3, family 3A) mutations (K27M, G34R/V), a recent study on GBMs identified two specific subtypes with specificity of age and location. GBMs with H3F3A-K27M mutation were predominantly located in the midline structure (thalamus, pons, and spinal cord) and were commonly seen in children, while H3F3A-G34R/V mutant tumors most likely arose from the cerebral hemispheres and mostly occurred in adults [34]. These findings suggested that different brain tumors might arise from distinct precursors with anatomical and age group specificity, thus providing a reasonable explanation for the association between patient age at diagnosis and tumor location in the current study.
Previous studies have suggested a relationship between gliomagenesis and neural stem cells [35, 36], which were preferentially located in two specific brain regions: the SVZ along the wall of the lateral ventricles, and the subgranular zone in the dentate gyrus of the hippocampus [37]. A voxel-based analysis with a large number of patients (n = 358) demonstrated that GBMs frequently occurred in the posterior SVZ [21], which was confirmed in a group of Chinese patients with GBMs [38]. In addition, GBMs that originated from SVZ were identified with a distinct phenotype, were more likely to present as multifocal on MR contrast enhancement at diagnosis, recurred away from the initial lesion [39], and had decreased progression-free survival and overall survival [40]. This study also demonstrated that GBMs were more likely to be located in the SVZ in older patients compared to younger patients, which supported the idea of age-associated location of gliomas.
Age-related differences in histopathological and molecular profiles of glioblastomas have been identified. For instance, IDH1 mutation was found to rarely occur in primary adult GBMs but present in nearly half of young (<40 years) patients with GBM [41]. In this study, we found that GBMs in young patients are preferentially located in the frontal lobe, in accordance with a previous study showing that GBM with IDH1 mutation was prone to occur in younger patients in the frontal lobe [17]. These findings suggested that potential genetic associations might exist between age at diagnosis and tumor location.
Previous studies have found that LGGs were prone to involve the eloquent areas [42], particularly the secondary functional areas [16]. Our voxel-wise statistical analysis showed that LGGs were preferentially located in the anterior frontal lobe in the elderly group and in the inferior temporal lobe in the young group. These differences in the results from different studies may be due to several reasons: (1) Inconsistent proportions of LGG subtypes may be due to patients from different races [43, 44]; (2) Certain histological subtypes may have originated from specific groups of cells with distinct preferential location [35]; (3) A brain-lobe based study insufficiently used the tumor location information by ignoring the tumor volume; (4) Tumors involving multiple brain regions may be over or less calculated. This study used voxel-based statistics in order to avoid this bias; (5) Furthermore, both previous and the current studies reviewed patients treated at a single-institute, which may lead to inclusion discrepancies.
Notably, we found that LGGs in the elderly group were more likely to invade the insular lobe than those in the young group. Tumors involved in the insular region were found to be associated with decreased extent of resection and worse prognosis [45, 46]. On the other hand, insular LGG tends to possess unfavorable molecular characteristics including wild-type IDH1 and the absence of 1p19q deletion [47, 48]. Therefore, poor prognosis of insular LGG is associated with patient age, tumor location, and genetic signature. Our results suggest that certain specific tumor subtypes have certain age and location characteristics.
Since previous studies suggested that areas showing T2 signal abnormality but no enhancement on a post-contrast T1-weighted image could still contain high-density tumor cells [28, 29], we used T2-weighted images, and not post-contrast T1-weighted images, as a reference sequence for tumor segmentation in this study. The computed tumor centroid was the geographic center of a tumor, which should not be directly considered as the biologic origin of a tumor. In addition, the VLSM analysis for identifying—clusters used age as a continuous variable. The cut-off of 45 years was only used to delineate the proportion of young and older patients with tumors involving the VLSM-identified brain regions.
Our analysis has several limitations. In order to acquire maximally accurate results, only tumors with centroids (a 1 mm3 size voxel) located in the VLSM cluster were included in the two cohorts (young group and aged group). The strictness of the inclusion criteria permitted only a limited number of patients to be included. Future studies with a larger number of clinical cases are needed to confirm the effect identified by the current study. Since our group-based neuroimaging studies used individual cases, registration of anatomically distorted brain structures following tumor involvement into a standard brain space is still a challenge using algorithms. Despite the development of the automated tumor differentiation, manual segmentation is still considered to be the reference method [21]. However, the pathological border of a tumor (if present) could hardly be accurately identified on MR images [49, 50]. In order to minimize inaccuracy, every tumor mask was identified by two independent neurosurgeons and further re-evaluated by a senior neuroradiologist. Future studies could further the current investigation by evaluating the prognostic value of tumor location in patients from different age groups.
Conclusion
Our results suggested that tumor location possessed characteristics associated with age at diagnosis in LGG and GBM. This finding implies the biological characteristics of gliomas. Future studies will focus on exploring prognosis value in combination of age and tumor location.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Bauman G, Fisher B, Watling C, Cairncross JG, Macdonald D (2009) Adult supratentorial low-grade glioma: long-term experience at a single institution. Int J Radiat Oncol Biol Phys 75(5):1401–1407
Ohgaki H, Kleihues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64(6):479–489
Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG (2010) Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol 12(6):520–527
Barker FG 2nd, Chang SM, Larson DA, Sneed PK, Wara WM, Wilson CB, Prados MD (2001) Age and radiation response in glioblastoma multiforme. Neurosurgery 49(6):1288–1297 (discussion 1297–1288)
Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, Isaacson S, Rotman M, Asbell SO, Nelson JS et al (1993) Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 26(2):239–244
Karim AB, Afra D, Cornu P, Bleehan N, Schraub S, De Witte O, Darcel F, Stenning S, Pierart M, Van Glabbeke M (2002) Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys 52(2):316–324
Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, Mascarenhas F, Horiot JC, Parvinen LM, van Reijn M, Jager JJ, Fabrini MG, van Alphen AM, Hamers HP, Gaspar L, Noordman E, Pierart M, van Glabbeke M (1996) A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys 36(3):549–556
Fisher BJ, Naumova E, Leighton CC, Naumov GN, Kerklviet N, Fortin D, Macdonald DR, Cairncross JG, Bauman GS, Stitt L (2002) Ki-67: a prognostic factor for low-grade glioma? Int J Radiat Oncol Biol Phys 52(4):996–1001
Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A, Jeuken JW, Wesseling P, Reifenberger G, von Deimling A (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1010 diffuse gliomas. Acta Neuropathol 118(4):469–474
Mikheev AM, Stoll EA, Mikheeva SA, Maxwell JP, Jankowski PP, Ray S, Uo T, Morrison RS, Horner PJ, Rostomily RC (2009) A syngeneic glioma model to assess the impact of neural progenitor target cell age on tumor malignancy. Aging Cell 8(4):499–501
Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16(6):2027–2033
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS (2002) Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol 4(4):278–299
Jeremic B, Grujicic D, Antunovic V, Djuric L, Stojanovic M, Shibamoto Y (1994) Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol 21(2):177–185
Duffau H, Capelle L (2004) Preferential brain locations of low-grade gliomas. Cancer 100(12):2622–2626
Lai A, Kharbanda S, Pope WB, Tran A, Solis OE, Peale F, Forrest WF, Pujara K, Carrillo JA, Pandita A, Ellingson BM, Bowers CW, Soriano RH, Schmidt NO, Mohan S, Yong WH, Seshagiri S, Modrusan Z, Jiang Z, Aldape KD, Mischel PS, Liau LM, Escovedo CJ, Chen W, Nghiemphu PL, James CD, Prados MD, Westphal M, Lamszus K, Cloughesy T, Phillips HS (2011) Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol 29(34):4482–4490
Stockhammer F, Misch M, Helms HJ, Lengler U, Prall F, von Deimling A, Hartmann C (2012) IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure 21(3):194–197
Ren X, Cui X, Lin S, Wang J, Jiang Z, Sui D, Li J, Wang Z (2012) Co-deletion of chromosome 1p/19q and IDH1/2 mutation in glioma subsets of brain tumors in Chinese patients. PLoS ONE 7(3):e32764
Wang Y, Zhang T, Li S, Fan X, Ma J, Wang L, Jiang T (2015) Anatomical localization of isocitrate dehydrogenase 1 mutation: a voxel-based radiographic study of 146 low-grade gliomas. Eur J Neurol 22(2):348–354
Ellingson BM, Cloughesy TF, Pope WB, Zaw TM, Phillips H, Lalezari S, Nghiemphu PL, Ibrahim H, Naeini KM, Harris RJ, Lai A (2012) Anatomic localization of O6-methylguanine DNA methyltransferase (MGMT) promoter methylated and unmethylated tumors: a radiographic study in 358 de novo human glioblastomas. Neuroimage 59(2):908–916
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avsar T, Li J, Murray PB, Henegariu O, Yilmaz S, Gunel JM, Carrion-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioglu M, Kaymakcalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilguvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kilic T, Lifton RP, Noonan JP, Yasuno K, Gunel M (2013) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339(6123):1077–1080
Zlatescu MC, TehraniYazdi A, Sasaki H, Megyesi JF, Betensky RA, Louis DN, Cairncross JG (2001) Tumor location and growth pattern correlate with genetic signature in oligodendroglial neoplasms. Cancer Res 61(18):6713–6715
Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003) Voxel-based lesion-symptom mapping. Nat Neurosci 6(5):448–450
Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P (2010) Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain 133(Pt 3):880–894
Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D (2012) Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci USA 109(36):14681–14686
Rousseaux M, Honore J, Vuilleumier P, Saj A (2013) Neuroanatomy of space, body, and posture perception in patients with right hemisphere stroke. Neurology 81(15):1291–1297
Barajas RF Jr, Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, Parsa AT, Aghi MK, McDermott MW, Berger MS, Cha S, Chang SM, Nelson SJ (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol 14(7):942–954
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66(6):865–874
Wang Y, Qian T, You G, Peng X, Chen C, You Y, Yao K, Wu C, Ma J, Sha Z, Wang S, Jiang T (2015) Localizing seizure-susceptible brain regions associated with low-grade gliomas using voxel-based lesion-symptom mapping. Neuro Oncol 17(2):282–288
Kimberg DY, Coslett HB, Schwartz MF (2007) Power in voxel-based lesion-symptom mapping. J Cogn Neurosci 19(7):1067–1080
Medina J, Kimberg DY, Chatterjee A, Coslett HB (2010) Inappropriate usage of the Brunner–Munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia 48(1):341–343
Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R, Benner A, Hielscher T, Milde T, Remke M, Jones DT, Northcott PA, Garzia L, Bertrand KC, Wittmann A, Yao Y, Roberts SS, Massimi L, Van Meter T, Weiss WA, Gupta N, Grajkowska W, Lach B, Cho YJ, von Deimling A, Kulozik AE, Witt O, Bader GD, Hawkins CE, Tabori U, Guha A, Rutka JT, Lichter P, Korshunov A, Taylor MD, Pfister SM (2011) Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20(2):143–157
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tonjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu XY, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Fruhwald MC, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Durken M, Kulozik AE, Madden J, Donson A, Foreman NK, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm CM, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth AM, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski PP, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor MD, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister SM (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22(4):425–437
Sanai N, Alvarez-Buylla A, Berger MS (2005) Neural stem cells and the origin of gliomas. N Engl J Med 353(8):811–822
Vescovi AL, Galli R, Reynolds BA (2006) Brain tumour stem cells. Nat Rev Cancer 6(6):425–436
Hagg T (2009) From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist 15(1):20–27
Wang Y, Fan X, Zhang C, Zhang T, Peng X, Li S, Wang L, Ma J, Jiang T (2014) Anatomical specificity of O6-methylguanine DNA methyltransferase protein expression in glioblastomas. J Neurooncol 120(2):331–337
Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS (2007) Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol 9(4):424–429
Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S (2013) Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol 15(1):91–96
Jha P, Suri V, Singh G, Jha P, Purkait S, Pathak P, Sharma V, Sharma MC, Suri A, Gupta D, Mahapatra AK, Sarkar C (2011) Characterization of molecular genetic alterations in GBMs highlights a distinctive molecular profile in young adults. Diagn Mol Pathol 20(4):225–232
Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42(5):1044–1055 (discussion 1055–1046)
Yang P, Wang Y, Peng X, You G, Zhang W, Yan W, Bao Z, Wang Y, Qiu X, Jiang T (2013) Management and survival rates in patients with glioma in China (2004–2010): a retrospective study from a single-institution. J Neurooncol 113(2):259–266
Forst DA, Nahed BV, Loeffler JS, Batchelor TT (2014) Low-grade gliomas. Oncologist 19(4):403–413
Chang EF, Clark A, Jensen RL, Bernstein M, Guha A, Carrabba G, Mukhopadhyay D, Kim W, Liau LM, Chang SM, Smith JS, Berger MS, McDermott MW (2009) Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg 111(2):203–210
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26(8):1338–1345
Goze C, Rigau V, Gibert L, Maudelonde T, Duffau H (2009) Lack of complete 1p19q deletion in a consecutive series of 12 WHO grade II gliomas involving the insula: a marker of worse prognosis? J Neurooncol 91(1):1–5
Metellus P, Coulibaly B, Colin C, de Paula AM, Vasiljevic A, Taieb D, Barlier A, Boisselier B, Mokhtari K, Wang XW, Loundou A, Chapon F, Pineau S, Ouafik L, Chinot O, Figarella-Branger D (2010) Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol 120(6):719–729
Harpold HL, Alvord EC Jr, Swanson KR (2007) The evolution of mathematical modeling of glioma proliferation and invasion. J Neuropathol Exp Neurol 66(1):1–9
Swanson KR, Bridge C, Murray JD, Alvord EC Jr (2003) Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 216(1):1–10
Acknowledgments
We would like to thank Yuling Yang for tissue sample collection and clinical data retrieval. This work was supported by funding from the National 973 Program (No. 2015CB755500), the National 973 Program (No. 2011CB707804), and the Research Special Fund for Public Welfare industry of health (No. 201402008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yinyan Wang and Shuai Liu have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, S., Fan, X. et al. Age-associated brain regions in gliomas: a volumetric analysis. J Neurooncol 123, 299–306 (2015). https://doi.org/10.1007/s11060-015-1798-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1798-x