Abstract
Carbohydrate and nitrogen storage in seedlings can improve field growth and survival. However, carbohydrate and nitrogen reserves may compete. For instance, carbohydrate reserves can be accumulated due to sink limitation on growth triggered by nutrient deficiency. Few studies have addressed the effects of nutrient supply on carbohydrate and nutrient storage and subsequent field performance of Quercus and Pinus species with different shoot growth strategies at the early growing stage. We exposed seedlings of Pinus tabuliformis Carr and Quercus mongolica Fisch to three exponential fertilizer treatments (10, 50, 100 mg N) in the nursery for 18 weeks to assess the combined response of growth and storage of carbohydrate and nitrogen in seedlings during their first year of growth in the nursery, as well as their subsequent field performance over the next 2 years. At the end of nursery stage, high nutrient supply increased nitrogen storage in both species, increased carbohydrate concentration in Q. mongolica but decreased that in P. tabuliformis seedlings. P. tabuliformis showed higher winter mortality than Q. mongolica but field mortality of P. tabuliformis decreased with an increase in fertilizer dosage. Our findings suggest that P. tabuliformis seedlings exposed to 100 mg N per plant can mitigate the field winter mortality although it could be still under deficiency status. Given lower winter mortality in Q. mongolica seedlings, being exposed to at least 100 mg N per plant in the nursery can produce nutrient-loaded seedlings, and therefore can be considered for restoration programs in areas affected by low winter temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Application of fertilizers during the nursery culture can effectively increase seedling size/biomass and nutrient storage in forest plantations. Initial seedling size or biomass is associated with the ability to outcompete weeds (Cuesta et al. 2010) and produce new roots (Grossnickle 2005). Consequently, larger seedlings show higher post-planting survival (Andivia et al. 2021; Villar-Salvador et al. 2015) and growth due to a large surface area for photosynthesis (Villar-Salvador et al. 2012).
Nitrogen and carbohydrate reserves are key requirements for plant growth and survival. Deciduous trees rely on the remobilization of N and carbohydrate stored in the previous growing season to support budburst and new growth (Hoch et al. 2003; Millard and Grelet 2010; Wang et al. 2019).Evergreen species rely on remobilization of N storage (> 74%) for new fine-root growth in early spring (Uscola et al. 2015). The amount of N remobilized depends on the amount of N stored (Millard and Grelet 2010) if no environmental factor inhibits the growth of sink organs. Although the driver of remobilization of C reserves is not fully understood (Brüggemann et al. 2011), some observations emphasized that carbohydrate reserves impact field growth and survival. For instance, fast growth leads to low carbohydrate reserves due to the growth competes with carbohydrate storage (Wiley et al. 2013), leading to a higher winter mortality (Luo et al. 2021); or high carbohydrate storage competes with growth, leading to lower field growth (Landhausser et al. 2012). Thus, the balance between growth and carbohydrate reserves could play an important role in field performance. In addition, N and carbohydrate reserves frequently affect plant cold and drought tolerance (O'Brien et al. 2014; Villar-Salvador et al. 2015). Carbohydrate reserves also act as important carbon sources for respiration during periods of resource shortage (Mooney 1972; Myers and Kitajima 2007; Weber et al. 2019). Consequently, N and carbohydrate reserves strongly affect the growth rate and survival after planting in the field (Landhausser et al. 2012; Luo et al. 2021; Oliet et al. 2009; Villar-Salvador et al. 2013; Villar-Salvador et al. 2004), as well as the seedling’s capacity to recover foliage after disturbances (Millard et al. 2001; Palacio et al. 2020). However, the relationship among growth, N and carbohydrate storage are rarely investigated together, especially the effect on field performance.
Carbohydrates are well known as an indicator of sink limitation on growth under stressful conditions (Hoch 2015; Körner 2003). Nutrient deficiency can induce sink limitation on growth (Birk and Matson 1986), under which carbohydrate reserves can be accumulated due to low demand of photosynthates for growth, accompanied by decreased N reserves and growth. Nutrient loading (involving both nutrient sufficiency and luxury uptake described in (Salifu and Jacobs 2006) can lead to less plasticity in growth responding to nutrient supply, that is, seedlings with nutrient loading respond less in biomass to nutrient supply. This switch between two nutritional states stipulated by Timmer (1997), whose model defines nutrient deficiency as when plants grow, i.e., when the nutritional status of the plant increases with increased fertilization; and nutrient loading as when the internal nutrient concentration increases without the plant’s total biomass changing (Salifu and Jacobs 2006; Timmer 1997). Nutrient loading involves fertilization in excess of the demand required for growth in nursery culture, leading to “luxury uptake” of nutrients. Thus, we expect nutrient loading increases storage of both N and carbohydrates due to the reduced need of both to sustain. The relationship among growth, N and carbohydrate reserves seems to be complex. Few studies have addressed how a switch from nutrient deficiency to nutrient loading may trigger an integrated response involving seedling biomass and storage of carbohydrate and N. How these three dimensions interact responding to nutrient supply and further effect on field performance needs to be elucidated.
Understanding this integrated response may be particularly important for the success of restoration programs in areas affected by high winter mortality, which frequently occurred in Northern China (Luo et al. 2021, 2022; Wang et al. 2015). Quercus and Pinus species are widely used in restoration programs across the world, from Mediterranean to certain temperate regions, since they are dominant in many temperate and Mediterranean regions of the Northern Hemisphere and, consequently, play a basic role in ecosystem functioning (Rogers 2009; Torres et al. 2021). The difference between the two species in seed size and shoot growth strategy indicates different initial nutrient supply to seedlings and different nutrient demands along the growing season. How these two species respond to different nutrient supply at early nursery stage helps to better understand the integrated response among growth, N and carbohydrate reserves. With small seeds, soil nutrient supply plays important role in seedling growth for Pinus species with determinate shoot growth strategy (Pallardy 2008), which grow mostly in early nursery stage. Conversely, although the resources from big acorns are almost sufficient during early growing stage (at least 4 weeks after emergency) for Quercus species (Milberg and Lamont 1997; Shi et al. 2018b; Villar-Salvador et al. 2010; Yi and Ju 2020) with semi-determinate shoot growth strategy (Hanson et al. 1986), which shows recurrent shoot growth during growing seasons, soil nutrient supply may also matter during later growing season (Oliet et al. 2009; Villar-Salvador et al. 2013; Villar-Salvador et al. 2004).
These considerations suggest that species with different shoot growth strategies and soil nutrient requirements at the early nursery stage may respond differently to nutrient supply, which can lead to later differences in field performance. Therefore, our study aimed to assess how changes in nutrient supply affect growth (in terms of seedling biomass) and storage (carbohydrate and N reserves). These questions were addressed using seedlings of two temperate species, Pinus tabuliformis Carr and Quercus mongolica Fisch. All seedlings were fertilized with doses of nitrogen-phosphorus-potassium (NPK) fertilizer based on an exponential regime (10, 50, and 100 mg) during the first year of growth in the nursery, and field performance was measured during the 2 years after outplanting. We tested the following hypotheses (1) lower dose nutrient supply can lead to an increase in carbohydrate concentration due to sink limitation on growth and higher dose nutrient supply can cause a decrease in carbohydrate concentration due to eased sink limitation on growth by nutrient supply. (2) Species with different shoot growth strategies and soil nutrient requirements at the early nursery stage may respond differently in carbohydrates to nutrient supply. Carbohydrate concentration in Quercus species responds to both nutrient deprivation and nutrient excess, due to sink limitation and low plasticity, respectively, that stimulate in both cases reserves building. (3) An increase in seedling size, N and carbohydrate storage would increase field growth and survival of both species.
Materials and methods
Seedling production
On February 23, 2017, seeds of Q. mongolica were collected from the National Seed Orchard for Quercus mongolica (Qigou Forest Farm, 41° 00′ N and 118° 27′ E, 526 m a.s.l.) and planted in hard plastic container cells (D60, Stuewe & Sons, Inc., Oregon, USA). The cells with a diameter of 6.4 cm, depth of 36 cm, and volume of 983 ml were arranged in 48 trays with 20 cells, resulting in a density of 127 cells m−2. The growing medium was a 3:1 mixture of peat (pH 6.0, screening 0–6 mm; Pindstrup Seeding, Ryomgaard, Denmark) and perlite (diameter 5 mm; Xinyang Jinhualan Mining, Henan, China). A total of 960 seedlings were cultivated in 48 trays, and these trays were placed on raised benches inside a ventilated polyethylene greenhouse under natural photoperiod conditions at the Chinese Academy of Forestry Sciences in Beijing (40° 40′ N, 116° 14′ E).
On March 4, 2017, seeds of P. tabuliformis were collected from the National Seed Orchard for Chinese Pine (Qigou Forest Farm, 41° 00′ N and 118° 27′ E, 526 m.a.s.l.) and planted in hard plastic container cells (SC10 Super Ray Leach “Cone-tainer” ™ Single Cell System, Stuewe & Sons Inc., Oregon, USA). The cells with a depth of 21 cm and volume of 164 ml were arranged within trays (Ray Leach “Cone-tainers”™ RL98c, Stuewe & Sons Inc., Oregon, USA) at 49 cells per tray, resulting in a density of 528 cells m−2. The growing medium was the same 3:1 mixture of peat and perlite as for Q. mongolica. A total of 588 seedlings were cultivated in 12 trays, and these trays were placed on raised benches inside a ventilated polyethylene greenhouse under natural photoperiod conditions at the Chinese Academy of Forestry Sciences in Beijing (40° 40′ N, 116° 14′ E). Details on the seed traits of both species are listed in Table 1.
Fertilizer treatments
The fertilizer treatments began after seedling emergence on March 30, 2017. Seedlings were fertilized once a week based on an exponential fertilization regime (Timmer 1997), which was described in detail by Wang (2015). A solution was prepared by dissolving a 20N-20P-20K fertilizer that contained micronutrients (Peters Professional, ICL, Summerville, SC, USA) in tap water, and each seedling was supplied with 20 ml doses. By the end of the nursery cultivation period (July 27, 2017), each plant had received a total of 18 fertilizer applications, with doses corresponding to 10 mg (low), 50 mg (moderate), and 100 mg (high) nitrogen per seedling. For each species, all seeds were sown in the trays, and each tray was randomly arranged in each block for Pinus tabuliformis, and 4 trays were randomly arranged in each block for Quercus mongolica. The specific fertilization schedule is depicted in Fig. 5.
Seedlings were watered to container capacity approximately twice a week, with one watering event coinciding with fertilization. The average greenhouse temperature was 25 °C during the day and 20 °C at night; temperature measurements were made at 30-min intervals using a JL-18 thermometer (Huayan Instrument and Equipment, Shanghai, China). The seedlings were moved outdoors to harden between late October and late November 2017, during which the average day temperature was 9 °C and average night temperature was 6 °C. Between December 1, 2017, and late February 2018, the seedlings were stored outdoors in a manually dug hole to avoid freezing damage and mortality over the winter.
Field experiment
The outplanting of seedlings was carried out at the Beijing Forestry University Northern Experimental Base at Pingquan in Hebei province (41° 13′ N, 118° 40′ E, 765 m.a.s.l.). The field site was in an area that experiences a temperate continental monsoon climate, characterized by seasonal drought that is presented as a dry winter, dry spring, and wet summer. The long-term mean annual temperature at this site is 7.3 °C and the total annual rainfall is 540 mm, 70% of which occurs during summer (June–September).
Precipitation and temperature data were recorded using an on-site weather station between January 2018 and December 2019. The annual precipitation varied from 398 in 2018 to 452 mm in 2019, while the corresponding mean annual temperature varied from 8.1 in 2018 to 8.4 °C in 2019. In the winter of 2018 (December-February), the air temperature dropped below − 20 °C during 15 days. The plot for outplanting had a slope < 2% and a soil depth of 45–60 cm. The soil texture in the surface layer (0–20 cm) comprised sand (73%), silt (11%), and clay (16%), while the soil pH was 6.2 and the organic carbon concentration was 0.7%. The soil in this plot was considered moderately fertile, with a total N concentration of 629 mg kg−1, an available P concentration of 139 mg kg−1, and an available K concentration of 113 mg kg−1 (Shi et al. 2018a).
P. tabuliformis seedlings were outplanted on April 15, 2018, and Q. mongolica seedlings on May 15, 2018. Seedlings were planted in manually dug holes (0.4 × 0.4 × 0.4 m) that were spaced 1 m apart. We used a randomized complete block design with four blocks, with all of treatments in each block randomly distributed. In the case of Q. mongolica, 60 seedlings were planted in each block, resulting in 240 seedlings per treatment. In the case of P. tabuliformis, 35 seedlings were planted in each block, resulting in 140 seedlings per treatment. Manual weeding was performed as required.
Plant sampling and measurements
To determine nutrient status in seeds, we randomly selected 25 acorns of Q. mongolica and 200 seeds of P. tabuliformis and assigned them to five random groups per species. On March 13, 2018, 1 month before outplanting, we randomly sampled five seedlings in each of the four trays per treatment from each species (n = 20 seedlings per fertilization treatment and species). After separating the seedlings into needles, stems, and roots, the growing medium was washed gently from the roots. Plant parts and seeds were then oven-dried at 70 °C for at least 48 h. We measured the mass of the stems, needles, dry roots, and seeds, and the root mass fraction was calculated as the root mass/total plant mass × 100.
Dried samples were analyzed to determine concentrations of nutrients and total non-structural carbohydrates (TNCs), which comprised soluble sugars (glucose, fructose and sucrose) and starch. Composite samples of each organ were made by pooling together the five samples of seedlings from the same tray for P. tabuliformis, and individual seedling organs were collected to determine nutrient and TNC concentrations for Q. mongolica. After grinding, plant material and seeds were passed through a 0.25-mm screen sieve and wet-digested using the H2SO4–H2O2 method. Then we used the standard Kjeldahl digestion method with water distillation (UDK-159, VelpScientifica, Italy) to determine total N concentration and content (Bremner 1996).
To determine TNC concentration and content, samples were ground to a powder in a ball mill. To extract soluble sugars, approximately 100 mg of finely ground plant material was boiled in distilled water (10 ml) for 30 min. The rest of the plant materials were used to determine the content of starch, which was extracted with perchloric acid for 10–15 min, followed by centrifugation and decantation. TNC concentration was determined using the anthrone method (Hansen and Moller 1975). Total glucose content was calculated photometrically at 630 nm in a UV–visible spectrophotometer (Agilent 8453, Shanghai, China). Absorbance values were converted to moles of glucose based on a standard curve derived from pure glucose (catalog no. S2974, Xilong, Guangdong, China), and these values were then converted to total glucose equivalents (Hansen and Moller 1975). TNC content was defined as the sum of the contents of soluble sugars and starch. TNC content for specific organs was calculated by multiplying TNC concentration by the mass of the organ.
We used vector diagnosis of nitrogen to detect whether seedlings reached sufficient status or still in deficient status. The methods of vector analysis were described in detail in the reference (Haase 1995).
We also recorded field mortality, stem height, and root collar diameter (RCD) 2 days after planting (T0, spring 2018, only height and RCD recorded) and at the end of each growing season in late October 2018 (T1) and 2019 (T2), as well as at the end of the first winter in early spring 2019 (T1-AW). Stem height was measured using a ruler, and RCD was measured using a Vernier caliper. These data were used to calculate stem volume (SV) based on the following equation:
Seedling growth was calculated as the net increase in stem volume between T0 and T1 and between T1 and T2.
Statistical analysis
A mixed linear model with block as a random factor was used for each species to analyze the effect of fertilizer treatments on seedling morphology, nutrient content, and TNC concentration. A similar analysis was performed to assess seedling outplanting growth, but here we used field block as a random factor. Finally, a generalized mixed model for a binomial distribution and a logit link function was used to analyze the effects of the treatments on outplanting survival; once again, block was used as a random factor. The post hoc Tukey multiple comparison test was performed to detect the statistical differences. We used bartlett.test() and shapiro.test() in R to test normality and homogeneity of the residuals. Log or square root transformation of data was used to meet normality. The data were analyzed using the packages “lmerTest” and “multcomp” in R 3.6.3.
Results
Effect of fertilization on growth and nutrient status
Fertilization dose significantly increased plant mass in P. tabuliformis but not in Q. mongolica (Table 1). While low-dose fertilization increased root mass fraction in P. tabuliformis, none of the three treatments affected root mass fraction in Q. mongolica.
Fertilization increased N concentration in both species, at the level of the whole plant and in each plant fraction (Fig. 1, Tables 2, 4). Similarly. N content in P. tabuliformis increased with increasing fertilizer dosage, while in Q. mongolica only the highest dosage increased N content over the remaining fertilization treatments.
Effect of fertilization on plant nitrogen concentration (x-axis) and content (y-axis) in Pinus tabuliformis and Quercus mongolica seedlings. Values are mean ± SE. Different lowercase and uppercase letters indicate significant differences (p < 0.05) among treatments based on ANOVA and the Tukey post-hoc test. HF, high-dose fertilization; LF, low-dose fertilization; MF, moderate-dose fertilization; N, nitrogen
Fertilization had a significant effect on the concentration of plant soluble sugars and TNCs in both species, but no effect on starch concentrations. In P. tabuliformis seedlings, the concentration of plant soluble sugars and TNCs decreased with an increase in fertilizer dosage (Fig. 2, Table 4). For stem, the concentration of stem soluble sugars, starch and TNC significantly increased with an increase in fertilizer dosage, while the opposite trend was observed in needle soluble sugars and root TNCs. Conversely, fertilization significantly increased the concentration of soluble sugars and TNCs in Q. mongolica seedlings in both at the level of the whole plant and organ. While, stem starch decreased with an increase in fertilizer dosage. In P. tabuliformis, plant TNC content increased with an increase in fertilizer dosage, but the difference in TNC content of P. tabuliformis seedlings was not significant after application of moderate or high fertilizer doses (Table 2, Fig. 2). No significant effect on plant TNC content was observed in Q. mongolica.
Effects of fertilization on plant total non-structural carbohydrate (TNC) concentration (x-axis) and content (y-axis) in Pinus tabuliformis and Quercus mongolica seedlings. Values are mean ± SE. Different lowercase and uppercase letters indicate significant differences (p < 0.05) among treatments based on ANOVA and the Tukey post-hoc test. HF, high-dose fertilization; LF, low-dose fertilization; MF, moderate-dose fertilization; TNC, non-structural carbohydrate
Carbohydrate and nitrogen reserves ratio (N/C reserves) at the level of whole plant increased with an increase in fertilizer doses in both species, but difference was not significant after application low and moderate fertilizer doses in Q. mongolica (Table 4). The results of nitrogen diagnosis showed that 100 mg N supply can produce seedlings with luxury consumption for Q. mongolica but P. tabuliformis seedlings were still under deficiency status under 100 mg N supply (Fig. 2A).
Effect of fertilization on field performance
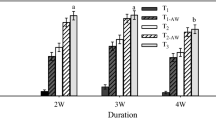
Seedling field mortality during each growing season was < 10%. However, during the first winter, we observed higher mortality in P. tabuliformis seedlings (27–36%) than in Q. mongolica seedlings (8–10%; Fig. 3). At the end of the second growing season (T2), P. tabuliformis seedlings showed higher variation in accumulated mortality across treatments (39–58%) than Q. mongolica seedlings (19–22%). Furthermore, at T2, there was a significant decrease in P. tabuliformis seedling mortality with an increase in fertilizer dosage (χ2 = 9.9, p = 0.007; Fig. 3). In contrast, fertilization in the nursery had no effect on mortality in Q. mongolica seedlings (T1: χ2 = 0.24, p = 0.89; T1-AW: χ2 = 0.68, p = 0.711; T2: χ2 = 0.53, p = 0.767; Fig. 3).
Mortality of Pinus tabuliformis (left) and Quercus mongolica (right) seedlings at the end of the first (T1) and second growing season (T2), as well as after the first winter (T1-AW), as a function of the fertilizer treatment applied in the nursery. Values are mean ± SE. Different lowercase and uppercase letters indicate significant differences (p < 0.05) among treatments based on ANOVA and the Tukey post-hoc test. HF, high-dose fertilization; LF, low-dose fertilization; MF, moderate-dose fertilization
An increase in fertilizer dosage led to an increase in stem volume in both species during the two growing seasons (Fig. 4). However, this effect was not significant in P. tabuliformis seedlings during the second growing season.
Stem volume increase in Pinus tabuliformis (left) and Quercus mongolica (right) seedlings in the field during the first and second growing seasons. Values are mean ± SE. Different lowercase and uppercase letters indicate significant differences (p < 0.05) among treatments based on ANOVA and the Tukey post-hoc test. HF, high-dose fertilization; LF, low-dose fertilization; MF, moderate-dose fertilization
There is a significant positive relationship between initial starch content and the field growth in first year (SV1-0) in both species, while positive relationship between SV1-0 and initial N content was only observed in P. tabuliformis (Table 5).
Discussion
Differences in carbohydrate allocation and seedling growth in response to nutrient supply
Our findings show that plant N concentration increased with an increase in N content in both species (Fig. 1), indicating that the seedlings were in the process of storing N for future use, as described in Fig. 3 of Sanz Pérez et al. (2007). We also found that plant biomass in P. tabuliformis seedlings increased with an increase in fertilizer dosage, while no such effect was observed in Q. mongolica seedlings (Table 1). We found that seedling growth in P. tabuliformis was N-limited, especially under the low-dose treatment. A supply of 100 mg N can produce nutrient-sufficient P. tabuliformis seedlings due to maximum growth obtained but not yet reach luxury consumption (Wang et al. 2015). Thus, P. tabuliformis seedlings can be considered nutrient-deficient (Salifu and Jacobs 2006) under 10 and 50 mg N treatments. This was also supported by the results of high N/C reserves under high fertilizer doses (Table A2) and the results of nutrient diagnosis (Fig. 2A). An increase in fertilization led simultaneously to seedling growth and nutrient storage in our P. tabuliformis seedlings when they receive a supply of 10–100 mg N.
In P. tabuliformis seedlings, plant carbohydrate concentration decreased with an increase in carbohydrate content, while the reverse was true for the Q. mongolica seedlings (Fig. 2). This indicates that the seedling growth was favored over carbohydrate storage in P. tabuliformis with an increase in fertilizer dose, especially within N-limited conditions (< 100 mg N). Our results correspond to those obtained for pine (Fife and Nambiar 1997), birch (McDonald et al. 1986) and other Quercus species (Sanz Pérez et al. 2007; Vizoso et al. 2008). Low nutrient supply can increase carbohydrate concentration due to sink limitation on growth (Körner 2003). With an increase in nutrient supply, sink limitation on growth caused by nutrients was partly alleviated and more photosynthates are allocated to growth instead of being allocated to carbohydrate storage. Thus, we observed opposite trends of carbohydrate and N concentration in P. tabuliformis in current study. Given determinate shoot growth strategy in most of Pinus species (Pallardy 2008), more growth demand during the early growing season may exacerbate the N limitation under low doses of fertilizer. Thus, this would lead to higher TNC reserves under low doses of fertilizer at the end of shoot growing season. Species with determinate shoot growth strategy would invest more photosynthates into TNC reserves once shoot ceased growth. For the current study, since trees were harvested at the end of hardening stage, we infer that the difference in TNC reserves at the end of shoot growing season keeps pace with that at the end of hardening stage though more photosynthates invested into TNC reserves.
In contrast, seedling growth in Q. mongolica was not limited by N supply even under the low-dose fertilizer treatment, that is, seedling size was not affected by the fertilizer treatments. Similar results were frequently reported in Quercus species (Kabeya and Sakai 2005; Sanz Pérez et al. 2007; Uscola et al. 2014; Yi and Wang 2016). Low response in biomass can be caused by two reasons. On the one hand, acorn reserves can cover seedling demands during the first year of growth (Kennedy et al. 2004; Milberg and Lamont 1997; Shi et al. 2018b).On the other hand, low plasticity to resources availability of Quercus species may be partly responsible for the constant seedling biomass under the three fertilizer treatments (Sanz Pérez et al. 2007).
In addition, we observed that both plant N concentration and content increased with an increase in dosage of fertilizer. Considering together with low plasticity in biomass, this indicates N storage of Q. mongolica seedlings was favored when they receive a supply of 10–100 mg N. In fact, acorn nutrient reserves can be remobilized completely to support seedling growth within 4 weeks after emergence (Shi et al. 2018b). Thus, given a semi-determinate shoot growth strategy in Quercus species, nutrient supply can meet nutrient demand during the later growing stage and further accelerate nutrient storage. Similar results were frequently reported in in evergreen Quercus species (Cuesta et al. 2010; Oliet et al. 2009; Villar-Salvador et al. 2004) and deciduous Quercus species (Salifu and Jacobs 2006; Shi et al. 2019). This implies that an external nutrient supply can accelerate the production of nutrient-loaded oak seedlings (Salifu and Jacobs 2006; Timmer 1997) due to low plasticity in growth. In this study, we infer that 50 mg N supply would produce nutrient-sufficient Q. mongolica seedlings and 100 mg N may accelerate seedlings to reach luxury consumption stages (Salifu and Jacobs 2006).
Q. mongolica seedlings favored carbohydrate storage over growth in response to an increase in nutrient supply. Our results are similar to those with rice (Guo et al. 2022) that leaf soluble sugars significantly increased under high doses of fertilizer and Salix viminalis that TNC amounts increased in perennial organs under high N supply when all leaves senesced. (Bollmark 1999). However, some opposite effects were observed in Quercus species (Kabeya and Sakai 2005; Sanz Pérez et al. 2007). The discrepancy may be caused by the difference in seedling phenological stages. In our study, we harvested seedlings at the end of dormant stages, which is comparable to the stage in Salix viminalis (Bollmark 1999). Thus, this implies that photosynthates increased by high N reserves (Evans and Clarke 2018; Niinemets 1999; Poorter et al. 2009) can be allocated more to carbohydrate storage instead of being used for growth (Hoch 2015), as evidenced by the lack of significant change in structural biomass. Furthermore, it seems that sink limitation on growth in Q. mongolica was caused by other factors other than nutrient (Birk and Matson 1986), such as low plasticity.
Taken together, we infer fertilization supply increases carbohydrate reserves when seedlings are at the stage of luxury consumption (Fig. 1 in (Salifu and Jacobs 2006)) since the growth will not be limited by nutrients and more photosynthates caused by high N reserves are allocated to carbohydrate storage instead of being used by growth.
High carbohydrate and N storage improve field performance
Studies have shown that nutrient and carbohydrate storage can improve the rate of subsequent growth (Landhausser et al. 2012; Uscola et al. 2015; Villar-Salvador et al. 2004; Wang et al. 2019), as well as the ability to resprout (Kabeya and Sakai 2005). When exposed to high doses of fertilizer in our study, both species showed increased field growth, perhaps because the high nutrient and carbohydrate storage facilitated remobilization after transplanting, increasing leaf area and subsequent C acquisition in a positive feedback loop (Pokharel and Chang 2016; Vizoso et al. 2008). This idea is also supported by that the positive relationship between plant N, starch and soluble sugars content and stem volume increase during the first growing season (SV1-0) respectively in P. tabuliformis, and positive relationship between plant starch content and SV1-0 in Q. mongolica (Table 5). It appears that starch content, known as the major carbohydrate storage, contributes more to the field growth. It seems that a minimum amount of fertilizer was needed to achieve this better growth, since low or moderate doses of fertilizer were ineffective in our experiments.
The overall winter mortality was lower in Q. mongolica seedlings than in P. tabuliformis seedlings. Mortality during the first growing season was comparable for the two species while most mortality difference occurred in winter, indicating that seedlings likely had been exposed to temperatures that exceeded their freezing tolerance. P. tabuliformis seedlings showed almost twice higher winter mortality in all three doses of fertilizer than that in Q. mongolica. This indicates Q. mongolica seedlings showed better winter stress resistance in the field than P. tabuliformis seedlings under the same amount of fertilizer treatment. This is supported by the difference in freezing tolerance for the two species that great damage would be caused at temperature − 28.9 °C in Q. mongolica (PFAFdatabase) in USDA hardiness 5–8 zones) and at temperature − 23.3 °C in P. tabuliformis (Bannister and Neuner 2001). In our study site, minimum temperature ranged between − 20 and − 26.3 °C for 15 days in the winter of 2018 (Table 6), which is beyond the minimum freezing temperature for P. tabuliformis. Moreover, precipitation during the winter was low (Table 6), which might have induced some water stress in the seedlings, thereby aggravating frost damage (Fernández-Pérez et al. 2018). Hence, the winter stress resistance in Q. mongolica may be attributed to much higher plant TNC concentration than that in P. tabuliformis.
However, within P. tabuliformis, the mortality of seedlings was lower when exposed to high doses of fertilizer, for which plant N and stem TNC reserves was higher. This indicates that higher N and TNC reserves led to less field mortality probably due to increased cold tolerance(Greer et al. 2000; Heredia-Guerrero et al. 2014; Toca et al. 2017). Since soluble sugars play important role in osmoregulation maintaining hydraulic integrity (Chen et al. 2020) and nitrogen is related to N-dependent cryoproteins (Griffith and Yaish 2004), high stem soluble sugar concentration (similar trend in starch and TNC) and N concentration can also partly explain the lower winter mortality under high fertilization treatment. Similar results were observed in another study conducted in the same site on P. tabuliformis where high winter mortality was attributed to low N and TNC reserves (Luo et al. 2021). In addition, exposed to high doses of fertilizer, plant N and TNC content was higher as well as stem volume increase during the first growing season was higher. This indicates that optimized growth and root mass led by higher N, TNC storage and initial seedling size under high doses of fertilizer can help to outcompete weeds and to exploit field resources (Luo et al. 2021). No difference in winter mortality was observed in Q. mongolica, which may be attributed to less morphological plasticity responding to fertilizer application and a higher intrinsic frost resistance.
Conclusions and implications for forest plantations
Although there was low morphological plasticity in the response of Q. mongolica to soil fertility, the dosage of fertilizer administered can accelerate the production of nutrient-loaded seedlings, which can in turn increase seedling storage of nutrients and carbohydrates as well as improve field growth. An increase in carbohydrate storage can also increase nutrient storage in nutrient-loaded seedlings such as Q. mongolica. Given that P. tabuliformis seedlings experience high winter mortality perhaps caused by exceeding freezing tolerance, we recommend producing P. tabuliformis seedlings applying a higher fertilizer rate than 100 mg N to promote nutrient and carbohydrate storage and help to mitigate the field mortality. We also recommend producing nutrient-loaded Q. mongolica seedlings by growing them in a nursery with fertilization of at least 100 mg N per plant. Such seedlings may be effective for restoration programs targeting areas affected by winter low temperature.
References
Andivia E, Villar-Salvador P, Oliet JA, Puértolas J, Dumroese RK, Ivetić V, Molina-Venegas R, Arellano EC, Li G, Ovalle JF (2021) Climate and species stress resistance modulate the higher survival of large seedlings in forest restorations worldwide. Ecol Appl 31:e02394. https://doi.org/10.1002/eap.2394
Bannister P, Neuner G (2001) Conifer Cold Hardiness. Springer, Netherlands
Birk EM, Matson PA (1986) Site fertility affects seasonal carbon reserves in loblolly pine. Tree Physiol 2:17–27. https://doi.org/10.1093/treephys/2.1-2-3.17
Bollmark L, Sennerby-Forsse L, Ericsson T (1999) Seasonal dynamics and effects of nitrogen supply rate on nitrogen and carbohydrate reserves in cutting-derived Salix viminalis plants. Can J For Res 29(1):85–94. https://doi.org/10.1139/x98-183
Bremner JM (1996) Nitrogen-total. Methods Soil Anal 5:1085–1121
Brüggemann N, Gessler A, Kayler Z, Keel SG, Badeck F, Barthel M, Boeckx P, Buchmann N, Brugnoli E, Esperschütz J, Gavrichkova O, Ghashghaie J, Gomez-Casanovas N, Keitel C, Knohl A, Kuptz D, Palacio S, Salmon Y, Uchida Y, Bahn M (2011) Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences 8:3457–3489. https://doi.org/10.5194/bg-8-3457-2011
Chen X, Zhao P, Ouyang L, Zhu L, Ni G, Schäfer KVR (2020) Whole-plant water hydraulic integrity to predict drought-induced Eucalyptus urophylla mortality under drought stress. For Ecol Manag 468:118179. https://doi.org/10.1016/j.foreco.2020.118179
Cuesta B, Villar-Salvador P, Puertolas J, Jacobs DF, Benayas JMR (2010) Why do large, nitrogen rich seedlings better resist stressful transplanting conditions? A physiological analysis in two functionally contrasting Mediterranean forest species. For Ecol Manag 260:71–78. https://doi.org/10.1016/j.foreco.2010.04.002
Evans JR, Clarke VC (2018) The nitrogen cost of photosynthesis. J Exp Bot 70:7–15. https://doi.org/10.1093/jxb/ery366
Fernández-Pérez L, Villar-Salvador P, Martínez-Vilalta J, Toca A, Zavala MA (2018) Distribution of pines in the Iberian Peninsula agrees with species differences in foliage frost tolerance, not with vulnerability to freezing-induced xylem embolism. Tree Physiol 38:507–516. https://doi.org/10.1093/treephys/tpx171
Fife DN, Nambiar EKS (1997) Changes in the canopy and growth of Pinus radiata in response to nitrogen supply. For Ecol Manag 93:137–152. https://doi.org/10.1016/S0378-1127(96)03917-5
Greer DH, Robinson LA, Hall AJ, Klages K, Donnison H (2000) Frost hardening of Pinus radiata seedlings: effects of temperature on relative growth rate, carbon balance and carbohydrate concentration. Tree Physiol 20:107–114. https://doi.org/10.1093/treephys/20.2.107
Griffith M, Yaish MWF (2004) Antifreeze proteins in overwintering plants: a tale of two activities. Trends Plant Sci 9(8):399-405. https://doi.org/10.1016/j.tplants.2004.06.007
Grossnickle SC (2005) Importance of root growth in overcoming planting stress. New For 30:273–294. https://doi.org/10.1007/s11056-004-8303-2
Guo C, Yuan X, Yan F, Xiang K, Wu Y, Zhang Q, Wang Z, He L, Fan P, Yang Z, Chen Z, Sun Y, Ma J (2022) Nitrogen application rate affects the accumulation of carbohydrates in functional leaves and grains to improve grain filling and reduce the occurrence of chalkiness. Front Plant Sci 13:921130. https://doi.org/10.3389/fpls.2022.921130
Haase R (1995) Vector analysis and its use.
Hansen J, Moller I (1975) Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal Biochem 68:87–94. https://doi.org/10.1016/0003-2697(75)90682-x
Hanson PJ, Dickson RE, Isebrands JG, Crow TR, Dixon RK (1986) A morphological index of Quercus seedling ontogeny for use in studies of physiology and growth. Tree Physiol 2:273–281. https://doi.org/10.1093/treephys/2.1-2-3.273
Heredia-Guerrero N, Oliet JA, Villar-Salvador P, Benito LF, Penuelas JL (2014) Fertilization regime interacts with fall temperature in the nursery to determine the frost and drought tolerance of the Mediterranean oak Quercus ilex subsp ballota. For Ecol Manag 331:50–59. https://doi.org/10.1016/j.foreco.2014.07.022
Hoch G (2015) Carbon reserves as indicators for carbon limitation in trees. Progr Bot (Genet Physiol Systemat Ecol) 76:321–346. https://doi.org/10.1007/978-3-319-08807-5_13
Hoch G, Richter A, Korner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26:1067–1081. https://doi.org/10.1046/j.0016-8025.2003.01032.x
Kabeya D, Sakai S (2005) The relative importance of carbohydrate and nitrogen for the resprouting ability of Quercus crispula seedlings. Ann Bot 96:479–488. https://doi.org/10.1093/aob/mci200
Kennedy PG, Hausmann NJ, Wenk EH, Dawson TE (2004) The importance of seed reserves for seedling performance: an integrated approach using morphological, physiological, and stable isotope techniques. Oecologia 141:547–554. https://doi.org/10.1007/s00442-004-1686-0
Körner C (2003) Carbon limitation in trees. J Ecol 91:4–17. https://doi.org/10.1046/j.1365-2745.2003.00742.x
Landhausser SM, Pinno BD, Lieffers VJ, Chow PS (2012) Partitioning of carbon allocation to reserves or growth determines future performance of aspen seedlings. For Ecol Manag 275:43–51
Luo N, Villar-Salvador P, Li G, Wang J (2021) The dark side of nursery photoperiod reduction on summer plantation performance of a temperate conifer: high winter mortality mediated by reduced seedling carbohydrate and nitrogen storage. For Ecol Manag 491:119171
Luo N, Grossnickle SC, Li G (2022) The effect of nursery drought preconditioning on summer plantation performance of Pinus tabuliformis: high winter mortality mediated by trade off between seedling carbohydrate and field growth. New For 53:301–317. https://doi.org/10.1007/s11056-021-09857-y
McDonald AJS, Ericsson A, Lohammar T (1986) Dependence of starch storage on nutrient availability and photon flux density in small birch Betula pendula Roth). Plant Cell Environ 9:433–438. https://doi.org/10.1111/j.1365-3040.1986.tb01757.x
Milberg P, Lamont BB (1997) Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol 137:665–672. https://doi.org/10.1046/j.1469-8137.1997.00870.x
Millard P, Grelet GA (2010) Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol 30:1083–1095. https://doi.org/10.1093/treephys/tpq042
Millard P, Hester A, Wendler R, Baillie G (2001) Interspecific defoliation responses of trees depend on sites of winter nitrogen storage. Funct Ecol 15:535–543. https://doi.org/10.1046/j.0269-8463.2001.00541.x
Mooney HA (1972) The carbon balance of plants. Annu Rev Ecol Syst 3:315–346. https://doi.org/10.1146/annurev.es.03.110172.001531
Myers JA, Kitajima K (2007) Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J Ecol 95:383–395. https://doi.org/10.1111/j.1365-2745.2006.01207.x
Niinemets Ü (1999) Research review. Components of leaf dry mass per area – thickness and density – alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144:35–47. https://doi.org/10.1046/j.1469-8137.1999.00466.x
O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A (2014) Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat Clim Change 4:710–714. https://doi.org/10.1038/nclimate2281
Oliet JA, Tejada M, Salifu KF, Collazos A, Jacobs DF (2009) Performance and nutrient dynamics of holm oak (Quercus ilex L.) seedlings in relation to nursery nutrient loading and post-transplant fertility. Eur J Forest Res 128:253–263. https://doi.org/10.1007/s10342-009-0261-y
Palacio S, Paterson E, Hester AJ, Nogués S, Lino G, Anadon-Rosell A, Maestro M, Millard P (2020) No preferential C-allocation to storage over growth in clipped birch and oak saplings. Tree Physiol. https://doi.org/10.1093/treephys/tpaa011
Pallardy SG (2008) Physiology of woody plants.
PFAFdatabase https://pfaf.org/user/Plant.aspx?LatinName=Quercus+mongolica.
Pokharel P, Chang SX (2016) Exponential fertilization promotes seedling growth by increasing nitrogen retranslocation in trembling aspen planted for oil sands reclamation. For Ecol Manag 372:35–43. https://doi.org/10.1016/j.foreco.2016.03.034
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. https://doi.org/10.1111/j.1469-8137.2009.02830.x
Rogers PSJSRSR (2009) The ecology and silviculture of oaks.
Salifu KF, Jacobs DF (2006) Characterizing fertility targets and multi-element interactions in nursery culture of Quercus rubra seedlings. Ann Forest Sci 63(3):231–237. https://doi.org/10.1051/forest:2006001
Sanz Pérez V, Castro Díez P, Valladares F (2007) Growth versus storage: responses of Mediterranean oak seedlings to changes in nutrient and water availabilities. Ann For Sci 64:201–210. https://doi.org/10.1051/forest:2006104
Shi W, Grossnickle SC, Li G, Su S, Liu Y (2018a) Fertilization and irrigation regimes influence on seedling attributes and field performance of Pinus tabuliformis Carr. For Inst For Great Britain 92:97–107. https://doi.org/10.1093/forestry/cpy035
Shi WH, Villar-Salvador P, Jacobs DF, Li GL, Jiang XX (2018b) Simulated predation of Quercus variabilis acorns impairs nutrient remobilization and seedling performance irrespective of soil fertility. Plant Soil 423:295–306. https://doi.org/10.1007/s11104-017-3518-0
Shi W, Villar-Salvador P, Li G, Jiang X (2019) Acorn size is more important than nursery fertilization for outplanting performance of Quercus variabilis container seedlings. Ann for Sci 76:22. https://doi.org/10.1007/s13595-018-0785-8
Timmer VR (1997) Exponential nutrient loading: a new fertilization technique to improve seedling performance on competitive sites. New for 13:279–299. https://doi.org/10.1023/A:1006502830067
Toca A, Oliet JA, Villar-Salvador P, Maroto J, Jacobs DF (2017) Species ecology determines the role of nitrogen nutrition in the frost tolerance of pine seedlings. Tree Physiol 38:96–108. https://doi.org/10.1093/treephys/tpx165
Torres I, Moreno JM, Morales-Molino C, Arianoutsou M (2021) Ecosystem services provided by pine forests. In: Ne’eman G, Osem Y (eds) Pines and their mixed forest ecosystems in the Mediterranean Basin. Springer International Publishing, Cham
Uscola M, Oliet JA, Villar-Salvador P, Díaz-Pinés E, Jacobs DF (2014) Nitrogen form and concentration interact to affect the performance of two ecologically distinct Mediterranean forest trees. Eur J Forest Res 133:235–246. https://doi.org/10.1007/s10342-013-0749-3
Uscola M, Villar-Salvador P, Gross P, Maillard P (2015) Fast growth involves high dependence on stored resources in seedlings of Mediterranean evergreen trees. Ann Bot 115:1001–1013. https://doi.org/10.1093/aob/mcv019
Villar-Salvador P, Planelles R, Enriquez E, Rubira JP (2004) Nursery cultivation regimes, plant functional attributes, and field performance relationships in the Mediterranean oak Quercus ilex L. For Ecol Manag 196:257–266. https://doi.org/10.1016/j.foreco.2004.02.061
Villar-Salvador P, Heredia N, Millard P (2010) Remobilization of acorn nitrogen for seedling growth in holm oak (Quercus ilex), cultivated with contrasting nutrient availability. Tree Physiol 30:257–263. https://doi.org/10.1093/treephys/tpp115
Villar-Salvador P, Puertolas J, Cuesta B, Penuelas JL, Uscola M, Heredia-Guerrero N, Benayas JMR (2012) Increase in size and nitrogen concentration enhances seedling survival in Mediterranean plantations. Insights from an ecophysiological conceptual model of plant survival. New For 43:755–770. https://doi.org/10.1007/s11056-012-9328-6
Villar-Salvador P, Peñuelas JL, Nicolás-Peragón JL, Benito LF, Domínguez-Lerena S (2013) Is nitrogen fertilization in the nursery a suitable tool for enhancing the performance of Mediterranean oak plantations? New For 44:733–751. https://doi.org/10.1007/s11056-013-9374-8
Villar-Salvador P, Uscola M, Jacobs DF (2015) The role of stored carbohydrates and nitrogen in the growth and stress tolerance of planted forest trees. New For 46:813–839. https://doi.org/10.1007/s11056-015-9499-z
Vizoso S, Gerant D, Guehl JM, Joffre R, Chalot M, Gross P, Maillard P (2008) Do elevation of CO2 concentration and nitrogen fertilization alter storage and remobilization of carbon and nitrogen in pedunculate oak saplings? Tree Physiol 28:1729–1739. https://doi.org/10.1093/treephys/28.11.1729
Wang J, Villar-Salvador P, Li G, Liu Y (2019) Moderate water stress does not inhibit nitrogen remobilization, allowing fast growth in high nitrogen content Quercus variabilis seedlings under dry conditions. Tree Physiol 39:650–660. https://doi.org/10.1093/treephys/tpy130
Wang J, Li G, Pinto J, Liu J, Shi W, Liu Y (2015) Both nursery and field performance determine suitable nitrogen supply of nursery-grown, exponentially fertilized Chinese pine. Silva Fennica 49 https://doi.org/10.14214/sf.1295.
Weber R, Gessler A, Hoch G (2019) High carbon storage in carbon-limited trees. New Phytol 222:171–182. https://doi.org/10.1111/nph.15599
Wiley E, Huepenbecker S, Casper BB, Helliker BR (2013) The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol 33:1216–1228
Yi X, Ju M (2020) Soil nitrogen assimilation of 1-year oak seedlings: Implication for forest fertilization and management. For Ecol Manag 456:117703. https://doi.org/10.1016/j.foreco.2019.117703
Yi X, Wang Z (2016) The importance of cotyledons for early-stage oak seedlings under different nutrient levels: a multi-species study. J Plant Growth Regul 35:183–189. https://doi.org/10.1007/s00344-015-9516-7
Acknowledgements
This study was supported by the National Natural Science Foundation of China (32171764).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Figs.
5,
Vector nomograms of relative changes in dry weight, nutrient content, and nutrient concentration occurring at the level of whole plant of seedling cultured under three fertilization regimes (10 mg, 50 mg and 100 mg N supply). Seedling status treated with 10 mg N is normalized to 100. Arrows reflect progressions in increase of fertilizer doses from 10 to 50 mg and 100 mg N supply, respectively. Note differences in axis scale between Pinus tabuliformis and Quercus mongolica
6 and Tables
3,
4,
5,
6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, N., Wei, N. & Li, G. Growth versus storage: response of Pinus tabuliformis and Quercus mongolica seedlings to variation in nutrient supply and its associated effect on field performance. New Forests 55, 81–99 (2024). https://doi.org/10.1007/s11056-023-09966-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-023-09966-w