Abstract
Populus spp. are among the fast-growing tree species most prone to water deficit. The use of drought-resistant clones allows mitigating the negative effects of water deficit on the establishment and the productivity of unstable irrigated plantations in dryland areas. We evaluated the responses of four Populus × canadensis (‘Conti 12’, ‘Guardi’, ‘I-214’ and ‘I45/51’) and four Populus deltoides (‘Stoneville 67’, ‘Catfish 2’, ‘Dvina’ and ‘Australiano 129/60’) clones to a short-term water stress. Three water treatments were imposed on 6-month-old plants: well-watered (WW), 5 days of irrigation withdrawal followed by rewatering (−5 + RW), and 9 days of irrigation withdrawal followed by rewatering (−9 + RW). Populus clones presented different strategies to face water deficit, resulting in variable effects on plant growth. The small-leaved clone ‘Australiano 129/60’ showed strong stomatal control and maintained a high increase in leaf area during the water-shortage period. These strategies were associated with high relative growth under both water-shortage treatments. ‘Australiano 129/60’ showed to be a drought-resistant clone with the highest growth under both water-shortage treatments, appearing to be a promising clone for production in environments prone to short-term water stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cultivation of the genus Populus (poplars) is widespread throughout the world. Poplars are among the fastest-growing forestry species, and among the most susceptible to water deficit as their high productivity depends on large water requirements (Tschaplinski et al. 1994; Monclus et al. 2005). However, the variety of responses to water deficit among and within Populus species has encouraged the production of interspecific hybrids, and the selection of clones with varying range of growth rates under suboptimal water availability (Chen et al. 1997; Monclus et al. 2005; Bogeat-Triboulot et al. 2007; Giovannelli et al. 2007). The ability to survive under water deficit is called drought resistance, and according to Levitt (1980), it comprises three strategies: drought escape (vegetative dormancy, short life span), drought avoidance (stomatal closure, reduction of leaf area, extension of the root system, etc.) and drought tolerance (osmotic and elastic adjustment, resistance to cavitation, etc.). Hereafter, we will refer to poplar species and hybrids that are more or less “drought-resistant”, in relation to their capacity to reduce more or less the height growth with a limiting water supply.
Plants cope with water deficit by reducing transpiration mainly through stomatal closure. This maintains cell turgor, leaf growth, metabolism, and prevents xylem cavitation (Chen et al. 1997; Sparks and Black 1999; Raven 2002). However, closing stomata also reduces growth by limiting CO2 assimilation. Therefore, plants must balance water loss and CO2 assimilation in such a way that they do not die from desiccation or carbon starvation (McDowell et al. 2008). The effect of stomatal control on growth under water deficit will depend on the plant’s stomatal responses (magnitude of stomatal closure, stomata sensitivity, etc.) and on the duration and intensity of the water deficit (McDowell 2011).
Diversity in drought resistance in poplar species and hybrids is mediated by a variety of water relations responses. Some studies show that clones that are more resistant to drought in the field (i.e., those whose growth decreases less under drought conditions), gradually close their stomata in response to a decrease in soil water availability, concomitant with a decrease in leaf water potential. In the same vain, more sensitive genotypes (i.e., those whose growth decreases more under water shortage conditions) close stomata more quickly, maintaining higher leaf water potential, but reducing CO2 uptake and growth (Braatne et al. 1992; Silim et al. 2009). However, other studies relate higher resistance to water deficit to the ability to close stomata quickly in response to soil water shortage, thus avoiding defoliation and its negative impact on growth (Chen et al. 1997). Diverse results have also been reported on the impact of water stress on leaf growth and defoliation in poplars. Some genotypes maintain their leaf growth in drought, thus maintaining whole plant growth, while others defoliate, thus reducing whole plant growth to a greater extent (Giovannelli et al. 2007). Poplar productivity is positively associated to total plant leaf area, and the magnitude of decline in total plant leaf area is a good predictor of the magnitude of biomass decline (Roden et al. 1990; Marron et al. 2005; Monclus et al. 2005). Monclus et al. (2005) found that most of the productive clones (over 29 genotypes evaluated) displayed a low level of drought resistance (i.e., a large reduction in biomass), while the least productive genotypes presented a large range of drought resistance.
Poplar plantations in Mendoza are mostly distributed in small and medium-sized holdings, and are characterised by being an alternative activity to other agricultural production considered primary, such as vineyards and fruit production. The main uses of the wood produced in forest stands are the fruit crate or container industry, sawmilling and milling (Calderón 2010). Populus forest production in drylands is carried out under irrigation. A study model for this type of irrigated forest plantation is the province of Mendoza in western Argentina, with an arid climate and an average annual rainfall of 250 mm. Here, poplar production is based on traditional furrow irrigation with water from rivers whose flow comes from the melting of snow in the Andes. River water reaches the agricultural lands through a system of irrigation canals (Fig. S1a). This irrigation system has two main drawbacks. On the one hand, flaws in land levelling generate heterogeneous water distribution among and within forest stands (Fig. S1b). On the other hand, water availability in the farms is not constant and depends on the irrigation schedule established according to the flow of the rivers that feed the irrigation network. In dry years, water availability for irrigation is lower, and longer periods between irrigation events are common. In recent years, there has been a decrease in the water supply due to climate change. Moreover, the Intergovernmental Panel on Climate Change predicts that the current trend of snowfall reduction will continue in the coming years (IPCC 2019). With 6200 ha cultivated, the most widely grown clones in the region are P. × canadensis ‘Conti 12’ and ‘Guardi’, which were selected on the basis of their performance with high water availability, and their resistance to fungi Septoria musiva (Calderón 2010), but not for their performance under water stress. However, it is expected that genotypes from arid climates could present greater resistance and better performance in conditions such as those of Mendoza. For example, P. deltoides genotypes, which generally occur in semi-arid climates (Rood et al. 2000), are found commercially in the country and could be an interesting proposal for cultivation in the area. In the current climate change scenario, it is possible that there might be genotypes better suited to resist transient drought events than those currently planted in the region. This is especially important during the stand establishment, as the plants do not have a fully developed root system.
The aim of this study is to evaluate the growth of the widely used clones in the region and other commercial clones reported as being drought-resistant in the bibliography (Table 1) under short-term water stress, as an initial monitoring to determine which clones would be the most suitable for dryland forestry under an unstable irrigation system. We also want to understand some of the mechanisms that determine their performance under short-term water stress, such as their capacity to sustain leaf area growth and to control transpiration through stomata closure. In addition, we explored the relationship between growth under water stress, the growth capacity proper of each clone, and their resistance to water deficit (their ability to limit growth reduction under water stress). We hypothesize that the clones studied will show differences in their growth rate under short water stress events, and that pronounced stomatal closure and the ability to sustain a high leaf area are the main factors associated with a high growth rate under short-term water stress. To test this, we studied four clones of Populus × canadensis hybrids and four clones of Populus deltoides in a pot experiment under controlled irrigation. Our experiment will allow us to know and compare the behaviour of the clones in the face of a short-term water stress event. The results of this trial will allow us to select the most suitable genotypes for future experiments under field conditions.
Materials and methods
Growth conditions and experimental design
We studied four commercial clones of Populus × canadensis (‘Conti 12’, ‘Guardi’, ‘I-214’ and ‘I45/51’) and four clones of Populus deltoides (‘Stoneville 67’, ‘Catfish 2’, ‘Dvina’ and ‘Australiano 129/60’) (Table 1). We used ‘Conti 12’ and ‘Guardi’ due to their wide use in forest stands in the region. On the other hand, we chose commercial clones available in Argentina based on bibliographic evidence suggesting resistance to water deficit. We rooted 0.30 m-long-dormant cuttings in 2 l-pots using a peat-based commercial substrate (Dynamix 3, Agriservise; Buenos Aires, Argentina). After sprouting, plants were thinned to one shoot and transferred to 20 l-pots with a 3:2 v/v mixture of sand and a peat-based commercial substrate (Dynamix 5). We supplemented plants with 2.5 g of Hydrocomplex (12-11-18 NPK and micronutrients; Yara; Buenos Aires, Argentina) per pot weekly. Plants were watered with an automatized drip irrigation system and kept to the water holding capacity of the substrate until the beginning of the water-shortage experiment.
After the cuttings grew for six months, we imposed three differential water treatments. Treatments were named as: Well-watered (WW), −5 + RW (5 days of irrigation withdrawal + 23 days of re-watering), −9 + RW (9 days of irrigation withdrawal + 19 days of re-watering). In the WW treatment, the plants were watered to substrate saturation, to ensure that the pots were kept close to water holding capacity of the substrate throughout the experimental period. The −5 + RW and −9 + RW treatments consisted in applying transient water stress by suspending irrigation for 5 and 9 days, respectively. Thus, until day 5, the −5 + RW and −9 + RW plants were maintained under similar soil water content (SWC) conditions (Figs. 1, S4a). After day 5, the pots of treatment −5 + RW were watered again to achieve a SWC similar to that of treatment WW; from this point on, there began what we call the recovery period of treatment −5 + RW (Fig. 1). The suspension of irrigation of −9 + RW plants was maintained until day 9, when these plants reached the minimum values of SWC, differentiating from the other two treatments (Figs. 1, S4b). At the end of day 9, irrigation was resumed to SWC values similar to that of treatment WW; from this point on, there began what we call the recovery period of treatment −9 + RW. During the recovery period, WW, −5 + RW and −9 + RW plants were maintained under well-watered conditions until the end of the experiment (Fig. 1). We chose these periods of irrigation withdrawal to simulate frequent situations of surface runoff irrigation interruption or spacing of irrigation schedules (personal communication of local farmers to E. Rovida). The experiment was conducted in a greenhouse at IADIZA, Mendoza, Argentina (32° 53′ 49″ S, 68° 52′ 23″ W; 770 m a.s.l.; Fig. S2) during summer of 2017. The period of irrigation withholding plus irrigation resumption lasted a total of 28 days.
Progression of substrate water content (SWC) of different water-shortage treatments, and daily vapor pressure deficit (VPD) during the experimental period. Arrows indicate the moment when irrigation was resumed in −5 + RW and −9 + RW treatments. Well-watered plants (WW) were kept at substrate water holding capacity (≈ 20%). Dots represent the mean ± SE (n = 10). Data were analysed using a MLM followed by a DGC post-test. Asterisks indicate significant differences between the means among treatments
The experimental units (individual plants in a pot) were arranged systematically in the greenhouse according to the restrictions imposed by the irrigation system, so that the treatments and species were distributed homogeneously and without spatial biases (Fig. S2). We used ten independent replicates (n = 10) for each cell of the factorial experiment (clone × treatment) with a total number of 240 plants (N = 240).
We measured volumetric soil water content (SWC, % v/v) of all pots (n = 10) with soil humidity sensors (ECH2O 10HS; Decagon Devices, Pullman, USA) on days 0, 5, 9, 15, and 28. To determine the soil water availability under the different treatments, we assessed pre-dawn leaf water potential (ΨL PD), an indirect measurement of soil water potential (ΨSoil) on days 5 (WW and −5 + RW) and 9 (WW and −9 + RW). For this, we selected fully expanded leaves of 3 plants per clone and per treatment at the end of the differential irrigation period (n = 3), we covered them with nylon bags to prevent leaf transpiration, and measured ΨL PD ~ 2 h before dawn with a pressure chamber (2.5 + 10 Model, Bio-Control, Buenos Aires, Argentina) based on Scholander (1965).
The mean midday photosynthetic photon flux density (PPFD, 400–700 nm) inside the greenhouse during the experiment was ≈ 920 μmol m−2 s−1. PPFD was measured using a Skye SKP 215 hemispherical sensor attached to a SpectroSense + 2 (Skye Instruments Ltd, Powys, UK). Air temperature and relative humidity (RH) were recorded inside the greenhouse every 30 min by Hygro Button loggers (iButton DS 1923; https://www.maximintegrated.com/en.html). We used the air temperature and RH measurements to calculate the vapor pressure deficit (VPD, kPa) (Ewers and Oren 2000).
Morphological and physiological measurements
Plant growth was measured recording plant height (H, mm; n = 10) and total leaf area per plant (LA; n = 10). Plant height was measured with a metric tape. Total leaf area per plant was calculated by adding up the individual areas of all fully expanded green leaves. Individual leaf area (Individual LA) was estimated using an equation relating leaf area to leaf width. Exponential equations were adjusted (R2 = 0.96, p < 0.05) using data of leaf blade width and individual leaf area (n = 100) measured with a portable LI-3100C Area Meter (LI-COR Biosciences, Lincoln, Nebraska, USA).
As the different clones presented different growth rates, they started the experiment (day 0) with different H and LA. Then, the H and LA growth of the different genotypes under the different treatments was calculated on a relative basis to allow comparison. Relative variations in H (ΔH), and in LA (ΔLA) were calculated as:
At the end of the experimental period, we evaluated the total number of leaves by counting all the leaves in each experimental unit, and individual LA, by measuring the area of 10 leaves per experimental unit (n = 10). Sensitivity to drought was calculated as the average difference in ΔH between the WW treatment and the −5 + RW and −9 + RW treatments for those clones that significantly decreased ΔH in the water-shortage treatments. Sensitivity was considered null for clones that did not reduce ΔH under −5 + RW and −9 + RW treatments.
We measured stomatal conductance (gs, mmol of water vapor m−2 s−1) with a steady-state diffusion porometer (SC-1, Decagon Devices, Pullman, WA, USA). To achieve a large number of measurements in a short time, gs was measured only on the abaxial leaf surface of fully expanded leaves (which presented the highest values of stomatal conductance) of 4 experimental units per clone and treatment (n = 4). Measurements were done around mid-morning on sunny days (~ 10:30 am) given it was the moment of maximum stomatal opening of the day (Fig. S3), based on daily gs recordings on one plant per clone that we had taken previous to the experiment. Stomatal conductance measurements were done on days 0, 5, 9, 15, and 28. To determine the stomatal response of plants to water deficit, it is necessary to compare gs under the water deficit treatments and gs under the WW treatment. However, as stomatal conductance depends on DPV, and in our experiment the DPV on days 0 (plants without water stress), 5 and 9 were very different (Fig. 1) which hampered the comparison between different days. So, we decided to calculate the relative magnitude of stomatal closure (|Δgs|) by comparing the gs of the plants on the days of maximum stress for each of the treatments (days 5 and 9) and the gs of the well-watered plants on those same days. We calculated |Δgs| as follows:
were |Δgs| is the absolute value of the subtraction between the gs value recorded at day 5 or day 9 in either water deficit treatment (gs WD) and the average value of gs recorded in the WW treatment (average gs WW) on days 5 or 9 respectively, divided by the average gs WW.
Once the gs values of the stressed plants reached the values of the plants from the WW treatment, we decided to end the experiment (Fig. 7).
Statistical analysis
All statistical tests were done using Infostat 2018 software (Di Rienzo et al. 2018). ΔH, ΔLA, |Δgs| individual LA and ΨL PD were analysed by fitting linear models, considering clones (clone) and treatments (treat) as fixed factors, also testing the significance of their interaction. We used linear mixed models for repeated measures in time to analyse the SWC and gs partitioned by clone. In the analysis of gs we considered treatments (treat) and days (day) as additional fixed factors of the models, also testing the significance of their interaction (treat × day), while in SWC analysis we considered clone (clone), treatments (treat), and days (day) as additional fixed factors of the model, also testing the significance of their interactions (clone × treat, clone × day, treat × day and clone × treat × day). In both linear mixed models for repeated measures in time we used the experimental unit as a random effect. Selected models were tested for homoscedasticity and normality of residuals by visual assessment of plots. The correct variance structure used in the fitted models was determined by comparison of Akaike's and Bayesian's Information Criterion. Post-hoc comparison of means was done with DGC multiple-comparisons test (Di Rienzo et al. 2002). Linear correlation between Individual LA and total number of leaves were assessed with Pearson’s correlation coefficient. To determine the variables most correlated with ΔH, we performed a Pearson Correlation Matrix for each water-shortage treatment using the average values of ΔH, ΔLA, individual LA, |Δgs| of each water shortage treatment, ΔH under the WW treatment (ΔH under WW) and sensitivity to drought stress. The average values of each variable were used because we wanted to evaluate the sensitivity to water stress, which was obtained through the difference between the growth of the clones under the water shortage treatments with respect to the growth under the WW treatment, and result in a single value for clone. Thus, due the low sample size (N = 8) we consider the significance of Pearson correlations at p ≤ 0.05 (**) and 0.05 < p ≤ 0.1 (*).
Results
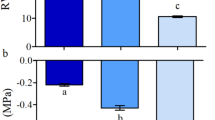
The progression of the soil water content (SWC) changed with time accordingly to the treatment (Fig. 1). The effects of clone and the interaction between clone and treatment was not significant. In the WW treatment, SWC was kept around 20% during the whole experimental period. Irrigation withdrawal for 5 and 9 days resulted in a decrease in SWC to ~ 15% and ~ 8%, respectively (Fig. 1). Once irrigation was resumed, soil water content increased to values similar to those of the control treatment (WW) on days 9 and 15 respectively (Figs. 1, S4). Considering the SWC of the days of maximum stress for each treatment (days 5 and 9) only significant effect for treatment was detected, being SWC not different between clones (Fig. S4). In line with SWC results, pre-dawn leaf water potential (ΨLPD that is a proxy for soil water potential) varied due to treatments but did not vary between genotypes; the longer the days of suspension of irrigation, the higher the water stress (Fig. 2).
Pre-dawn leaf water potential of plants grown under: a WW and −5 + RW treatments on day 5 and; b WW and −9 + RW treatments on day 9. Bars represent the mean ± SE (n = 3). Data were analysed using a lineal model followed by a DGC post-test. Letters indicate significant differences between the means among treatments
The ΔH of the clones was differentially affected by the irrigation treatments (Fig. 3). In the WW treatment, three groups were ranked from the highest to the lowest ΔH. ‘Stoneville 67’, ‘Australiano 129/60’, ‘Catfish 2’ and ‘Guardi’ showed the highest height growth, which were followed by ‘Dvina’ with intermediate height growth, and then by ‘I45/51’, ‘Conti 12’ and ‘I-214’ clones, which were the least productive under the WW treatment (Fig. 3). All clones showing intermediate and low ΔH with high water availability (‘Dvina’, ‘I45/51’, ‘Conti 12’ and ‘I-214’), did not decrease their height growth after water-shortage periods (Fig. 3). These clones did not reduce their ΔH under water-shortage treatments; they grew poorly under high-water availability and temporary water-shortage. The clones that were sensitive to both water-shortage treatments showed the highest height growth in WW, and were ‘Stoneville 67’, ‘Catfish 2’, and ‘Guardi’ (Fig. 3). The only clone with a high height growth under the WW treatment that was not affected by temporary water-shortage treatment in our experiment was ‘Australiano 129/60’ (Fig. 3). In consequence, the clones can be separated into three groups of ΔH under the water-shortage treatments: ‘Australiano 129/60’ that showed the highest height growth in both temporary water-shortage treatments, followed by ‘Guardi’, ‘Stoneville 67’, ‘Catfish 2’ and ‘Dvina’ with intermediate height growth, and by ‘I45/51’, ‘I-214’ and ‘Conti 12’ with the lowest height growth.
Height growth variation (∆H) at the end of the experimental period of Populus clones grown under different water shortage treatments (WW: Well-watered; −5 + RW: 5 days of irrigation withdrawal + re-watering; −9 + RW: 9 days of irrigation withdrawal + re-watering). Horizontal lines correspond to the mean (n = 10) and dots represent the values of each experimental unit. Data were analysed using lineal model followed by a DGC post-test. Letters indicate significant differences between the means among clone × treatment groups
The variation in total plant leaf area (ΔLA) of the clones was differentially affected by the water-shortage treatments (Fig. 4). Under the WW treatment, the clones were separated into three groups according to their ΔLA. Those that increased their LA the most during the experimental period were ‘Australiano 129/60’ and ‘Stoneville 67’, followed by ‘Conti 12’, ‘Catfish 2’ and ‘Guardi’. In opposition, ‘Dvina’, ‘I45/51’ and ‘I-214’ showed the least variation in LA in well-watered conditions (Fig. 4). From the comparison of Figs. 3 and 4, we observed that 'Conti 12', despite being one of clones showing highest ΔLA, it was not among those with highest ΔH. Regarding the investment on leaf area during a period affected by water-shortage (−5 + RW, −9 + RW), the clones ‘Guardi’, ‘Dvina’, ‘I-214’, and ‘I45/51’, maintained the same ΔLA in all of the treatments. On the contrary, ‘Australiano 129/60’, ‘Stoneville 67’, and ‘Catfish 2’ presented a decrease in their ΔLA under both water-shortage treatments, being ‘Stoneville 67’ the most sensitive in this variable (Fig. 4). ‘Conti 12’ only presented a decrease in its ΔLA in the most severe water-shortage treatment. ‘Australiano 129/60’ and ‘Guardi’ were the clones with the highest ΔLA in both water-shortage treatments, despite decreasing it in the case of ‘Australiano 129/60’ under −5 + RW and −9 + RW.
Leaf area growth variation (∆LA) at the end of the experimental period of Populus clones grown under different water shortage treatments (WW: Well-watered; −5 + RW: 5 days of irrigation withdrawal + re-watering; −9 + RW: 9 days of irrigation withdrawal + re-watering). Horizontal lines correspond to the mean (n = 10) and dots to the values of each experimental unit. Data were analysed using lineal model followed by a DGC post-test. Letters indicate significant differences between the means among clone × treatment groups
Leaf area variation depends on both the total number of leaves and leaf size. Individual leaf area (individual LA) varied among the clones (Fig. 5). Under conditions of high-water availability, ‘Dvina’, ‘I45/51’, ‘Stoneville 67’, ‘Guardi’ and ‘Catfish 2’ had the largest leaf sizes; and were followed by ‘I-214’, with an intermediate leaf size, whereas ‘Conti 12’ and ‘Australiano 129/60’ had leaves with the lowest individual LA (Fig. 5). Water-shortage treatments affected the leaf size of ‘Australiano 129/60’, ‘Catfish 2’, ‘I45/51’ and ‘Dvina’ in relation to the WW treatment. −9 + RW treatment induced the reduction in leaf expansion of ‘Australiano 129/60’ (39%) and ‘Catfish 2’ (31%). Whereas −5 + RW treatment only induced the reduction of the individual LA of ‘Catfish 2’ (15%). On the other hand, −5 + RW treatment favoured the increase in leaf size of ‘I45/51’ (20%) and −9 + RW treatment, of ‘Dvina’ (20%). Individual LA showed a negative correlation with total leaf number in all treatments, indicating that the larger the leaf size, the more difficult to produce it (Fig. S5).
Individual leaf area (individual LA) at the end of the experimental period of Populus clones grown under different water shortage treatments (WW: Well-watered; −5 + RW: 5 days of irrigation withdrawal + re-watering; −9 + RW: 9 days of irrigation withdrawal + re-watering). Horizontal lines correspond to the mean (n = 10) and dots represent the values of each experimental unit. Data were analysed using lineal model followed by a DGC post-test. Letters indicate significant differences between the means among clone × treatment groups
Clones showed different stomatal responses to the water-shortage treatments (Fig. 6). Under −5 + RW, ‘Australiano 129/60’ showed the highest stomatal closure (|Δgs|) on day 5, followed by ‘Guardi’, ‘I214’, ‘Stoneville 67’, ‘Catfish 2’ and ‘Dvina’. ‘Conti 12’ and ‘I45-51’ showed the least stomatal control in this treatment as they had the lowest |Δgs| values. All the clones apart from ‘Catfish 2’, showed a tight and similar stomatal closure in the −9 + RW treatment. ‘Catfish 2’ was the clone that closed the stomata the least in this treatment maintaining a similar |Δgs| to the −5 + RW treatment (Fig. 6).
Magnitude of stomatal closure (|Δgs|) of Populus clones in the moment of maximum water stress of each water-shortage treatments (−5 + RW: 5 days of irrigation withdrawal + re-watering; −9 + RW: 9 days of irrigation withdrawal + re-watering). Bars correspond to the mean ± SE (n = 4). Data were analysed using lineal model followed by a DGC post-test. Letters indicate significant differences between the means among clone × treatment groups
To understand the stomatal behaviour of each clone, we also analysed the gs profile under the three water treatments throughout the experiment (Fig. 7). Through this partitioning it was possible to observe that the clones showed different stomatal behaviours in relation to the water treatments. Four different patterns of stomatal response can be distinguished. One type of stomata response was displayed by ‘Conti 12’ and ‘I45/51’, which did not close their stomata under moderate water shortage, but only under the more intense water-shortage treatment (Fig. 7a, d). On the other hand, ‘Guardi’, ‘Stoneville 67’ and ‘Dvina’ closed their stomata gradually in response to both water shortage treatments: stomatal closure increased according to the level of water stress (Fig. 7b, f, h). Conversely, ‘I-214’ and ‘Australiano 129/60’ presented the same type of stomatal behaviour in which the gs values reached by each clone on day 5 were similar to those reached on day 9 (Fig. 7c, e). Finally, ‘Catfish 2’ that presented a similar gs values on day 5 and 9, but did not recover gs rapidly after moderate water stress, since on day 9 (4 days after irrigation was resumed in the −5 + RW treatment) it remained with its stomata closed despite the rewatering of the plants of the −5 + RW treatment (Fig. 7g).
Progression of stomatal conductance (gs) of Populus clones grown under different water irrigation treatments (WW: Well-watered; −5 + RW: 5 days of irrigation withdrawal + re-watering; −9 + RW: 9 days of irrigation withdrawal + re-watering. a) ‘Conti 12’; b) ‘Guardi’; c) ‘I-214’; d) ‘I45/51’; e) ‘Australiano 129/60’; f) ‘Stoneville 67’; g) ‘Catfish 2’; h) ‘Dvina’. Arrows indicate the moment when irrigation was reassumed in −5 + RW and −9 + RW treatments. Dots represent the mean ± SE (n = 4). Letters indicate significant differences between the means among day × treatment groups
To understand how the measured variables correlated with the growth of plants, we performed a Pearson correlation matrix analysis for each water-shortage treatment, considering the following variables: ΔH, ΔLA, |Δgs|, individual LA of water-shortage treatments, ΔH under WW, and sensitivity (Tables 2, 3). Under the −5 + RW treatment, we found a significant and positive correlation between growth measured as ΔH and ΔLA, with |Δgs|, indicating that the ability to maintain high growth in height and leaf area is related to greater stomatal control (Table 2). In addition, ΔLA was also negatively correlated with individual LA, indicating that a high leaf area growth under moderate water stress was sustained by smaller leaves (Table 2). Additionally, plants with a higher ΔH under WW, showed the highest ΔH in moderate water stress despite showing the highest sensitivity (Table 2). In the −9 + RW treatment, relationships between measured variables changed. In this treatment, the most productive plants in WW were still the most sensitive to water stress. However, growth measured as ΔH and ΔLA did not correlate with stomatal adjustment, possibly because in this treatment there was no longer a gradient of stomatal closure as in the −5 + RW treatment, but all clones showed a similar maximum closure (Fig. 6).
Discussion
In consonance with our hypothesis, the P. deltoides and P. × canadensis clones studied here showed differences in their ∆H under short-term water stress (Fig. 3). In general terms, clones with the highest ∆H under the WW treatment presented the highest sensitivity to water-shortage treatments. The ability to maintain height and leaf area growth under short-term water stress was associated with a rapid reduction in stomatal conductance (at day 5). Although Populus is considered one of the fast-growing forest species most vulnerable to drought, we found differences in the degree of drought resistance in our study. ‘Australiano 129/60’ proved to be a fast-growing and drought resistant clone with the greatest height growth under both water-shortage treatments. These characteristics point to ‘Australiano 129/60’ as a genotype potentially suitable for forest production under irrigated conditions in arid environments, which merits its selection for field establishment and productivity studies.
Under optimal conditions of water availability, the clones showed different height growth, the most productive clone (‘Stoneville 67’) showed a height growth almost 90% higher than the least productive clone (‘Conti 12’). In both water-shortage treatments, sensitive clones (P. deltoides ‘Stoneville 67’, P. deltoides ‘Catfish 2’ and P. × canadensis ‘Guardi’) presented reductions in ΔH up to 16% with respect to the WW treatment. Previous studies reported that under a minimum Ψsoil of − 0.60 MPa, P. × canadensis clones reduced their height by 15% in field conditions (Monclus et al. 2005). On the other hand, in a pot experiment, plants of P. × canadensis cultivated at 30% of field capacity reduced their height by 30% (Guo et al. 2010). Also, 3-months-old P. deltoides clones cultivated in pots showed a reduction of ~ 40% in height when subjected to water stress with Ψsoil between − 1.25 and − 1.80 MPa (Guarnaschelli et al. 2011). Although the reductions reported in pot experiments are greater than those found in our experiment, in those studies the plants were subjected to more intense water stress and a longer water restriction period. Under both treatments ‘Australiano 129/60’ showed the highest height growth, while ‘Conti 12’, ‘I45/51’ and ‘I-214’ showed the lowest height increase.
In the most severe water stress (day 9), where stomatal adjustment was maximal for most of the clones, those with the highest height growth were the ones that maintained high leaf area growth. This is in line with our hypothesis. Strong stomatal closure contributes to keeping high leaf water potential, thus maintaining leaf expansion, conserving water and avoiding defoliation (Chen et al. 1997). This is positive in the short term, as long as the stress is transient, because over longer periods the stomatal closure can lead to a reduction in growth due to carbon starvation (McDowell 2011). In the case of our study, the clones that grew the most under transient water stress showed these responses.
The different clones showed variable leaf sizes with a negative correlation with the total number of leaves. The presence of small leaves, as shown by ‘Australiano 129/60’ and ‘Conti 12, is generally associated with higher major vein density and leaf conductivity, which may better provide irrigation for access of isolated leaf sectors (McKown et al. 2010; Attia et al. 2015). This characteristic could explain the aptitude of ‘Australiano 129/60’ to further increase the total leaf area per plant, especially under water stress. Furthermore, Scoffoni et al. (2011) related that a major vein density and small leaf size reduce leaf hydraulic vulnerability improving whole-plant drought resistance. This was demonstrated with a positive correlation between leaf size and vulnerability to xylem cavitation found in a study with five hybrids of Populus (Schreiber et al. 2016). Also, small leaves minimizes the resistance of the boundary layer, facilitating energy dissipation providing additional advantages for growth under drought (Pallardy 2008; Scoffoni et al. 2011). Thus, a high total leaf area formed by small leaves could be an adaptive and acclimated response to xeric environments (Dunlap et al. 1995; Pearce et al. 2005). In addition, the rapid acclimation response to water-shortage of ‘Australiano 129/60’ and ‘Catfish 2’ by reducing leaf size highlights their plasticity in leaf traits in response to water deficit. These findings are consistent with those reported in P. deltoides, P. trichocarpa, P. × canadensis, P. deltoides × trichocarpa and P. nigra clones which showed a reduction in their leaf size when implanted in more arid regions (Roden et al. 1990; Marron et al. 2002; Pallardy 2008; Viger et al. 2016). However, in this experiment large-leaved plants of P. × canadensis ‘I45/51’ under −5 + RW and P. deltoides ‘Dvina’ under −9 + RW, increased their leaf size. Both clones presented low ∆LA values, so the water content could have been enough to maintain the few leaves in a state of turgor that made it possible to keep leaf expansion. In the case of 'I45/51’, which presented the effect only in the mildest water-shortage treatment, we hypothesized that the −9 + RW treatment was too intense for this clone, which led to an interruption in leaf development. Despite unusual, the increase in leaf size has been reported for P. × canadensis clones exposed to a period of water restriction followed by the re-establishment of irrigation in a field experiment (Marron et al. 2003).
In terms of stomatal conductance, under the −5 + RW treatment, |Δgs| was positively correlated with ∆H, so we can support our hypothesis, since plants with a high growth in height tended to present a tight stomatal control. The clones that showed strong stomatal closure at the beginning of the water restriction period were able to maintain height growth throughout the experiment. Stomatal control is an adaptive response of plants exposed to drought stress that results in a reduction of the negative impacts of water deficit on plants through the regulation of leaf-level water loss to the atmosphere (Franks et al. 2007). The limitation of CO2 supply by stomatal closure can result in an increase in water use efficiency of plants, which in turn will result in high yield potential and high yield (Blum 2005). Furthermore, |Δgs| was also positively associated with ΔLA and negatively associated with individual LA, possibly meaning that when water was limited, clones with a high leaf area composed of small leaves displayed tighter stomatal control to protect their tissues from dehydration and allow plants to avoid other costly processes, e.g. defoliation (Chen et al. 1997). Thus, once gs levels recovered after water-shortage period, photosynthesis was not limited by photosynthetic area, resulting in a higher photosynthetic rate and higher growth capacity. When water was limiting, ‘Australiano 129/60’ rapidly closed its stomata, probably maintaining the xylem tension within the hydraulic safety margin. This behaviour avoids the formation of cavitation, acting to preserve the integrity of the xylem (Fichot et al. 2010, 2015). On the other hand, ‘Catfish 2’ presented a similar and moderate level of stomatal closure for −5 + RW and −9 + RW in the moment of maximum stress of each treatment. However, their stomata were not totally closed under both water-shortage treatments, which could be associated with the timing and pattern of ABA production in response to beginning of water shortage. Variation in patterns of ABA production were associated with different stomatal responses induced by water stress in Populus genotypes (Chen et al. 1997). In addition, ‘Catfish 2’ presented a slow recovery of gs after the water shortage period, maybe due to the formation of embolisms in its hydraulic system in response to the increased water deficit.
Conclusion
High growth rate and high biomass yield are among the main characteristics that made the genus Populus one of the most widely cultivated in the world. In our experiment, the studied clones showed different height growth. Water-shortage treatments led to a differential decrease in the growth of some clones, indicating differences in drought resistance. In general terms, we found that the sensitive clones tended to be those with higher height growth. These clones were P. deltoides ‘Stoneville 67’ and ‘Catfish 2’, and P. × canadensis ‘Guardi. Oppositely, the clones with lower height growth under the WW treatment were the most resistant to water stress. However, ‘Australiano 129/60’ was shown to be a fast-growing and drought-resistant clone, as it did not reduce its height growth under the water-shortage treatments. Under decreased soil water content, this clone rapidly reduced stomatal conductance and was able to maintain the height and leaf area growth throughout the experimental period. These drought coping mechanisms and the smaller leaf size of this clone appear to have contributed to overcoming the short-term water scarcity event without affecting its growth. In addition, its high growth in height may provide a competitive advantage over weeds during planting and the establishment of a forest plantation. However, further studies are needed to determine whether this clone can be recommended for a commercial plantation, as studies related to silvicultural management, pest risks and the quality of its wood products are still lacking. Our results and the need for drought-resistant clones to establish more sustainable plantations highlight the need for further research and consideration of alternative poplar clones.
Data availability
The datasets generated during and/or analyzed during the current study are available in the OSF Repository.
Code availability
References
Arneth A, Denton F, Agus F (2019) Framing and context. Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. Intergovernmental Panel on Climate Change (IPCC), Geneva, pp 1–98
Attia Z, Domec JC, Oren R, Way DA, Moshelion M (2015) Growth and physiological responses of isohydric and anisohydric poplars to drought. J Exp Bot 66:4373–4381. https://doi.org/10.1093/jxb/erv195
Blum A (2005) Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust J Agric Res 56(11):1159–1168
Bogeat-Triboulot MB, Brosché M, Renaut J, Jouve L, Le Thiec D, Fayyaz P, Vinocur B, Witters E, Laukens K, Teichmann T, Altman A, Hausman JF, Polle A, Kangasjärvi J, Dreyer E (2007) Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol 143:876–892. https://doi.org/10.1104/pp.106.088708
Braatne JH, Hinckley TM, Stettler RF (1992) Influence of soil water on the physiological and morphological components of plant water balance in Populus trichocarpa, Populus deltoides and their F1 hybrids. Tree Physiol 11:325–339. https://doi.org/10.1093/treephys/11.4.325
Calderón AD (2010) Forestación con álamos para la obtención de madera de calidad. Cátedra de Dasonomía—Facultad de Ciencias Agrarias, UNCuyo, Mendoza.
Chen S, Wang S, Altman A, Hüttermann A (1997) Genotypic variation in drought tolerance of poplar in relation to abscisic acid. Tree Physiol 17:797–803. https://doi.org/10.1093/treephys/17.12.797
Di Rienzo JA, Guzmán AW, Casanoves F (2002) A multiple-comparisons method based on the distribution of the root node distance of a binary tree. J Agric Biol Environ Stat 7:129–142. https://doi.org/10.1198/10857110260141193
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2018) Infostat versión 2018. Centro de Transferencia InfoSat, FCA, Universidad Nacional de Córdoba, Córdoba
Dunlap JM, Stettler RF, Heilman PE (1995) Genetic variation and productivity of Populus trichocarpa and its hybrids. VIII. Leaf and crown morphology of native P. trichocarpa clones from four river valleys in Washington. Can J for Res 25:1710–1724. https://doi.org/10.1139/x95-185
Ewers BE, Oren R (2000) Analyses of assumptions and errors in the calculation of stomatal conductance from sap flux measurements. Tree Physiol 20:579–589. https://doi.org/10.1093/treephys/20.9.579
Fichot R, Barigah TS, Chamaillard S, Le Thiec D, Laurans F, Cochard H, Brignoolas F (2010) Common trade-offs between xylem resistance to cavitation and other physiological traits do not hold among unrelated Populus deltoides × Populus nigra hybrids. Plant Cell Environ 33:1553–1568. https://doi.org/10.1111/j.1365-3040.2010.02164.x
Fichot R, Brignolas F, Cochard H, Ceulemans R (2015) Vulnerability to drought-induced cavitation in poplars: synthesis and future opportunities. Plant Cell Environ 38:1233–1251. https://doi.org/10.1111/pce.12491
Franks PJ, Drake PL, Froend RH (2007) Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ 30(1):19–30
Giovannelli A, Deslauriers A, Fragnelli G, Scaletti L, Castro G, Rossi S, Crivellaro A (2007) Evaluation of drought response of two poplar clones (Populus x canadensis Mönch “I-214” and P. deltoides Marsh. ’Dvina’) through high resolution analysis of stem growth. J Exp Bot 58:2673–2683. https://doi.org/10.1093/jxb/erm117
Guarnaschelli B, Garau M, Cortizo S, Alvares J, Lemcoff JH (2011) Respuestas diferenciales a la sequía en clones de Populus deltoides cultivados en el Delta del Paraná. In: Terc Congr Int Salicaceas en Argentina. p 9
Guo XY, Zhang XS, Huang ZY (2010) Drought tolerance in three hybrid poplar clones submitted to different watering regimes. J Plant Ecol 3:79–87. https://doi.org/10.1093/jpe/rtq007
Levitt J (1980) Responses of plants to environmental stresses. Vol. II. Water, radiation, salt, other stresses, 2nd edn. Academic Press Inc., New York, p 607
Marlats RM, Senisterra GE, Marquina JL, Ciocchini GR (2009) Populus spp.: Supervivencia y crecimiento en clones implantados en Buenos Aires, Argentina. Revista de La Facultad de Ciencias Agrarias 41(1):77–84
Marron N, Delay D, Petit JM, Dreyer W, Kahlem G, Delmotte FM, Brignolas F (2002) Physiological traits of two Populus x euramericana clones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiol 22:849–858. https://doi.org/10.1093/treephys/22.12.849
Marron N, Dreyer E, Boudouresque E, Delay D, Petit JM, Delmotte FM, Brignolas F (2003) Impact of successive drought and re-watering cycles on growth and specific leaf area of two Populus x canadensis (Moench) clones, “Dorskamp” and “Luisa_Avanzo.” Tree Physiol 23:1225–1235. https://doi.org/10.1093/treephys/23.18.1225
Marron N, Villar M, Dreyer E, Delay D, Boudouresque E, Petit JM, Delmotte FM, Guehl JM, Brignolas F (2005) Diversity of leaf traits related to productivity in 31 Populus deltoides x Populus nigra clones. Tree Physiol 25:425–435. https://doi.org/10.1093/treephys/25.4.425
McDowell NG (2011) Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol 155(3):1051–1059
McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
McKown AD, Cochard H, Sack L (2010) Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. Am Nat 175:447–460. https://doi.org/10.1086/650721
Monclus R, Dreyer E, Delmotte FM, Villar M, Delay D, Boudouresque R, Petit JM, Marron N, Bréchet C, Brignolas F (2005) Productivity, leaf traits and carbon isotope discrimination in 29 Populus deltoides x P. nigra clones. New Phytol 167:53–62. https://doi.org/10.1111/j.1469-8137.2005.01407.x
Monclus R, Dreyer E, Villar M, Delmotte FM, Delay D, Petit JM, Barbaroux C, Le Thiec D, Bréchet C, Brignolas F (2006) Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides x Populus nigra. New Phytol 169(4):765–777. https://doi.org/10.1111/j.1469-8137.2005.01630.x
Pallardy SG (2008) Physiology of woody plants. Elsevier, Amsterdam
Pearce DW, Millard S, Bray DF, Rood SB (2005) Stomatal characteristics of riparian poplar species in a semi-arid environment. Tree Physiol 26:211–218. https://doi.org/10.1093/treephys/26.2.211
Raven JA (2002) Selection pressures on stomatal evolution. New Phytol 153:371
Roden J, Van Volkenburgh E, Hinckley TM (1990) Cellular basis for limitation of poplar leaf growth by water deficit. Tree Physiol 6:211–219. https://doi.org/10.1093/treephys/6.2.211
Rood SB, Patiño S, Coombs K, Tyree MT (2000) Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees 14:248–257
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346. https://doi.org/10.1126/science.148.3668.339
Schreiber SG, Hacke UG, Chamberland S, Lowe CW, Kamelchuk D, Bräutigam K, Campbell MM, ThomasBR, (2016) Leaf size serves as a proxy for xylem vulnerability to cavitation in plantation trees. Plant Cell Environ 39:272–281. https://doi.org/10.1111/pce.12611
Scoffoni C, Rawls M, Mckown A, Cochard H, Sack L (2011) Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol 156:832–843. https://doi.org/10.1104/pp.111.173856
Silim S, Nash R, Reynard D, White B, Schroeder W (2009) Leaf gas exchange and water potential responses to drought in nine poplar (Populus spp.) clones with contrasting drought tolerance. Trees 23:959–969. https://doi.org/10.1007/s00468-009-0338-8
Sparks JP, Black RA (1999) Regulation of water loss in populations of Populus trichocarpa: the role of stomatal control in preventing xylem cavitation. Tree Physiol 19(7):453–459
Tschaplinski TJ, Tuskan GA, Gunderson CA (1994) Water-stress of black and eastern cottonwood clones and four hybrid progeny. I. Growth, water relations, and gas exchange. Can J for Res 24:364–371. https://doi.org/10.1139/x94-049
Viger M, Smith HK, Cohen D, Dewoody J, Trewin H, Steenackers M, Bastien C, Taylor G (2016) Adaptive mechanisms and genomic plasticity for drought tolerance identifed in European black poplar (Populus nigra L.). Tree Physiol. https://doi.org/10.1093/treephys/tpw017
Acknowledgements
The authors are very grateful to Hugo DeBandi, Gualberto Zalazar, Nicolás Giuffre and Gabriel Zalazar for their invaluable help in maintaining the assay and carrying out the measurements.
Funding
This research received funding from Ministerio de Agricultura, Ganadería y Pesca of Argentina under Grant Agreement No PIA 14004 to I.A.M.
Author information
Authors and Affiliations
Contributions
Conceptualization: CVG, CVG and IAM; Methodology: EAR, AG and LNB; Formal analysis and investigation: EAR and AG; Writing—original draft preparation: EAR and CVG; Writing—review and editing: IAM, CVG, AG and CVG; Funding acquisition: CVG, CVG and IAM; Resources: IAM, CVG; Supervision: CVG, CVG and IAM.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
'Not applicable'.
Consent to participate
'Not applicable'.
Consent for publication
All authors give their informed consent to this publication and its content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rovida Kojima, E.A., Gonzalez, C.V., Mundo, I.A. et al. Differential responses of Populus deltoides and Populus × canadensis clones to short-term water deficit. New Forests 54, 421–437 (2023). https://doi.org/10.1007/s11056-022-09929-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-022-09929-7