Abstract
Climate change is predicted to cause northward migration of boreal tree species. However, the success of such a migration will be determined by trees’ ability to acclimate to the environmental conditions at the potential new site. This study was aimed to assess the interactive effects of changes in photoperiod associated with northward migration and soil warming on the ecophysiological traits of jack pine (Pinus banksiana Lamb.) under different carbon dioxide concentrations [CO2]. We examined the responses of foliar gas exchange and seedling cold hardiness after exposing 1-year old seedlings to two [CO2] (400 and 950 µmol mol−1), two soil temperatures (growing season average at seed origin and 5 °C warmer) and three photoperiod regimes (seed origin, and 5° and 10° further north) for one growing season. We found that the CO2 elevation significantly increased photosynthetic rate measured at treatment CO2, photosynthetic water use efficiency, maximum rate of carboxylation and triose phosphate utilization. Some of the effects were modified by photoperiod. Furthermore, the CO2 elevation and soil warming both reduced seedling cold hardiness, as indicated by increases in injury index. The lack of interactions between photoperiod and other treatments (elevated [CO2] and soil temperature) suggests that young jack pine seedlings may have the ability to acclimate to the new photoperiod regimes at higher latitudes north of the seed origin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate is changing rapidly. Other than increasing CO2 and temperature levels, precipitation patterns and weather extremes are also expected to change (Cao and Caldeira 2010; IPCC 2013; Luthi et al. 2008). These changes will occur in a relatively short period of time so trees may have to rapidly acclimate or adapt to new conditions, or migrate to new locations, or a combination of both in order to preserve their populations (Aitken et al. 2008). Trees live longer than other plant forms and, therefore, their physiological plasticity to acclimate to the changing environment of their current location or the different environment of a new location is critical (Lambers and Oliveira 2019).
Elevated atmospheric [CO2] generally stimulates photosynthesis in C3 plants with a concomitant increase in biomass (Eamus and Ceulemans 2001) but such a positive effect is often not sustained due to down regulation of photosynthetic capacity (Cui and Nobel 1994; Lambers and Oliveira 2019). In the meantime, stomatal conductance typically decreases under elevated [CO2] (Bunce 2004; Gunderson et al. 2002; Leakey et al. 2006; Marchi et al. 2004; Medlyn et al. 2001; Morgan et al. 2004).
Soil temperature is likely to increase under the predicted changes of climate in boreal forests where photosynthesis and tree growth are generally restricted by low soil temperatures (Aphalo et al. 2006; Ensminger et al. 2008). Changes in soil temperature influence morphological and physiological traits (Cai and Dang 2002; Newaz et al. 2016), such as stomatal conductance and water uptake (Alvarez-Uria and Körner 2007; Ambebe et al. 2010; Frechette et al. 2011; Zhang and Dang 2007), and development of cold hardiness (Dalen and Johnsen 2004; Lutze et al. 1998). Low soil temperature restricts nutrient and water uptake (Grossnickle 2000; Peng and Dang 2003; Stathers and Spittlehouse 1990) and retards establishment and early growth of newly planted seedlings (Grossnickle 2000; Smith 1985). Soil temperature can also interact with air temperature in affecting the ecophysiological traits of trees, for example, the beneficial effects of increased soil temperature may be offset by increases in vapor pressure deficit and transpiration rate associated with a concurrent increase in air temperature (Domec et al. 2009).
In response to changing climate, trees are expected to migrate northward (Bunnell and Kremsater 2012), either naturally or with human assistance. As the predicted shifts of climate envelopes are substantial for many North American species (McKenney et al. 2007, 2011), trees would face quite different environments from their current habitats on their way north, for example, photoperiod getting longer in the summer and shorter in the winter and a faster transition between seasons. This photoperiod change may have significant implications for plant phenology and physiological processes (Lambers and Oliveira 2019; Pothier and Prevost 2002). A possible consequence may include the desynchronization in seasonal cold hardiness and bud phenology development, leading to increasing risks of cold damage (Augspurger 2013; Gu et al. 2008; Li et al. 2015; Man et al. 2009).
The effects of individual environmental factors on boreal trees, such as temperature and photoperiod, are well documented (DeLucia and Smith 1987; Fraser 1962; Li et al. 2015; Peng and Dang 2003; Soolanayakanahally et al. 2013; Way and Sage 2008a, b). Comparatively, interactive effects are less studied but can be quite different from individual effects (Curtis et al. 2000; Nowak et al. 2004). For example, the stimulation of elevated [CO2] to photosynthesis can decrease with decreasing light (Allen et al. 1990) but the relative stimulation to biomass may increase with decreases in light condition (Marfo and Dang 2009) and the ecophysiological mechanisms of response to elevated CO2 change with light conditions (Dang et al. 2020a, b). The ecophysiological response to the interactions of elevated CO2 and nutrient supply varies with species (Dang et al. 2020b). Elevated [CO2] reduces the negative impact of drought stress (Mishra et al. 1999) and the response can be altered by photoperiod (Inoue et al. 2020).
Jack pine (Pinus banksiana Lamb.) is a conifer tree species of great ecological and economic importance and the second most planted tree species in Canada (Forestry Canada 1992). Jack pine represents about 37% of the total annual softwood harvest volume in Ontario (OMNR 1991). It grows in a wide range of climatic conditions (Rudolf 1965). Despite of its high levels of genetic diversity, the distribution and productivity of jack pine will be affected by climate change (Colombo et al. 2007). However, our knowledge how jack pine may respond to climate change and changes in environmental conditions associated with a potential northward migration is still limited. Because of its great ecological and commercial values and sensitivity to climate change (Colombo et al. 2007; Newaz et al. 2016; Rudolf 1965), such knowledge is particularly important for the development of proper adaptive strategies to sustain the distribution and productivity in the future. In this study, we assessed the physiological responses of jack pine to elevated [CO2] under different scenarios of soil warming and northward migration. Although the interactive effects of [CO2] and soil temperature on jack pine have been examined previously (Zhang and Dang 2005, 2007), how the responses may vary with photoperiod are unknown. Since warmer soil temperature, elevated [CO2] and longer photoperiod all can increase photosynthesis, we hypothesize that the combination of warmer soil temperature, longer growing season photoperiod, and elevated [CO2] would enhance the physiological performance of jack pine; but a faster rate of decline in photoperiod during the cold hardening phase in the fall would hinder the proper development of cold hardiness because the trees would have less time to complete the cold hardening process.
Materials and methods
Plant materials
One-year old jack pine seedlings were obtained from the Boreal Tree Seedling Nursery near Thunder Bay. The seedlings were raised from seeds collected from Kakabeka region (48° 57′ N & 90° 44′ W). A total of 608 seedlings, relatively uniform in height and diameter (mean height 13.05 cm; mean root collar diameter 0.21 cm), were planted in pots of 15 cm in height and 13 cm in diameter filled up with a mixture of premium grade vermiculite and peat moss in a ratio of 1:1 (v/v).
Experimental design
The experiment was conducted in four green houses at Lakehead University’s Thunder Bay campus following a split-split-plot design, with [CO2] as the whole plot, soil temperature as the sub-plot and photoperiod as the sub-sub plot. The treatments included two levels of [CO2] (400 and 950 µmol mol−1), two soil temperatures (soil temperature at seed origin, and 5° C warmer) and three photoperiod regimes (photoperiod at seed origin, and 5° and 10° further north). Two levels of [CO2] were randomly assigned to four independent greenhouses with two replicates of each. The two levels of soil temperature were set up within each level of [CO2] and three levels of photoperiod were assigned within each soil temperature.
Environmental controls
The experiment was conducted between November 20, 2013 and May 31, 2014. The photoperiods in all the greenhouses were set the same that simulated the seasonal course from April 16 to October 31 for the location of the longest photoperiod treatment. The photoperiods for the shorter day treatments were achieved by manual shading at both ends of the day (Newaz et al. 2016). The temperatures in all the greenhouses simulated the seasonal course of the seed origin. The dates referred to hereafter are the simulated dates. The [CO2] in the greenhouses was elevated using electronic ignition natural gas CO2 generators (model GEN-2E, Custom Automated Products Inc, Riverside, CA). Circulation fans were used to distribute CO2 evenly throughout each of the greenhouses. The [CO2] in each greenhouse was monitored and controlled automatically with an Argus CO2 Control System (Argus, Vancouver, BC, Canada). The levels of CO2 in each treatment varied within ± 10 µmol mol−1 of the set point. The soil temperature of seed origin simulated the biweekly mean soil temperatures measured in 2012 and 2013 at the seed origin (Kakabeka region, Newaz et al. 2016). The soil temperatures were the averages of field measurements at 5 cm and 20 cm depth. Soil temperatures were controlled using the soil temperature control system designed by Cheng et al. (2000) and the settings were adjusted in synchronization with the adjustment of photoperiods. Bud setting time was defined as the number of days counted from September 16 (simulated) to the day when the setting of the terminal bud was completed. The simulated day length and day/night air temperature on September 16 were 12 h and 14 °C/8 °C, respectively. Photoperiods were extended using high-pressure sodium lamps when the natural day lengths were shorter than the set values or shortened through manual shading when the natural day lengths were longer than the set values. Seedlings were fertilized biweekly with 7.14 mmol N, 0.56 mmol P and 2.12 mmol K per litre of water during the rapid growth phase and 1.78 mmol N, 2.20 mmol P and 4.64 mmol K per liter of water during the hardening phase (Scarratt 1986). The soil moisture level was monitored using a Delta-T ML2x probe and HH2 moisture meter (Delta-T Devices, Cambridge, UK) and maintained at 60–70% of the field water capacity. In all greenhouses relative humidity was maintained at 55–60% during the rapid growth phase and 45–50% during the hardening phase. The day and night air temperatures were controlled to mimic the mean biweekly day and night air temperatures at the seed origin. Relative humidity and air temperature were controlled automatically using the Argus Control System referred to previously.
Gas exchange measurements
Foliar gas exchange was measured on three randomly selected seedlings of each treatment combination in the middle of the simulated summer (102th–112th day of treatment) when the seedlings were in the rapid growing stage (Newaz et al. 2016). All measurements were made between 09:30 A.M. and 2:30 P.M. The measurements were made on current foliage using a PP-Systems CIRAS-3 open gas exchange system (PP System Inc., Amesbury, MA, USA). Photosynthetic responses to CO2 concentration (A/Ci curves) were measured at 50, 150, 250, 400, 550, 730, 900 and 1200 μmol mol−1 CO2, at photosynthetically active radiation of 800 μmol m−2 s−1, 25 °C air temperature and 50% relative humidity. The net rate of carbon dioxide assimilation (An), stomatal conductance (gs) and transpiration rate (E) were calculated according to Farquhar et al. (1980). Water use efficiency (WUE) was calculated as An/E for each measurement. The A/Ci response curves were analyzed to estimate the maximum rate of carboxylation (Vcmax), maximum rate of electron transport (Jmax), triose phosphate utilization (TPU) and other relevant parameters according to Sharkey et al. (2007). The light saturated (PAR 800 μmol m−2s−1) An measured at the growth [CO2] (400 and 950 µmol mol−1) and at the ambient [CO2] for both CO2 treatments (An-400) were derived from the A/Ci curves. gs, E and WUE measured at the corresponding growth [CO2] were used in data analyses. The needles used in foliar gas exchange measurements were collected and the projected leaf area was determined using the Regent WinSeedle system (Regent Instruments Inc, Quebec, Canada). To avoid the impact of needle removal for leaf area measurement on growth and physiology, seedlings used in foliar gas exchange measurement were not used in any other measurements.
Electrical conductivity and index of injury
The cold hardening phase was started in mid-April (mimicking the environment of mid-September) with day length and day and night air temperature at 12 h and 14 °C and 8 °C, respectively, and continued until the end of May (mimicking end of October) with day length and day and night air temperature of 10 h and 7 °C and 2 °C, respectively. The cold hardiness of shoot tips was assessed when growth had stopped and buds were set (Newaz et al. 2016) following the method by Colombo et al. (1989). Terminal shoot tips of 3 cm in length were collected from nine seedlings randomly selected from each treatment combination and divided among three testing temperatures (− 5, − 15 and − 30 °C). Each shoot tip was rinsed with distilled water and placed in a test tube containing 15 ml distilled water and allowed to incubate at room temperature (20–25 °C) for 24 h. After a vigorous shaking of the test tubes, the control electrical conductivity (ECC) of the bathing solution was determined using a Fisher Accumet AR 29 electrical conductivity meter (Fisher Scientific, Ottawa, Canada). The bathing solution was then separated and the wet shoot tips in stoppered test tubes were cooled down to each testing temperature at a rate of 5 °C per hour using a programmable freezer (Foster Refrigeration Ltd., King’s Lynn Norfolk, U.K.). After 70 min at the testing temperature, the temperature inside the freezer was gradually raised (5 °C per hour) to room temperature and the samples were transferred to an insulated box at 5 °C and left overnight. The previously separated bathing solution was returned to the corresponding test tubes, which were incubated for 24 h at room temperature. At this stage, electrical conductivity of the bathing solutions was measured again as the freezing electrical conductivity (ECF). The samples were then placed in a drying oven at 80 °C for 2 h to kill the tissues and left in room temperature for another 24 h. The electrical conductivity of the post-killing bathing solution was measured (ECK).
The index of injury (Ii), which is inversely related to cold hardiness (Flint et al. 1967), was calculated as follows (Colombo et al. 1989):
where Ii = index of injury (%), ECC = control electrical conductivity, ECF = electrical conductivity of the bathing solution measured following freezing of the shoot tips, and ECK = electrical conductivity of the bathing solution measured after killing the shoot tips.
Statistical analysis
All data were subjected to Analysis of Variance (ANOVA). Shapiro–Wilk and Bartlett tests were conducted to check for normality of distribution and homogeneity of variance, respectively. The Jmax, TPU and Rd data were transformed using natural logarithm function, gs using square root and WUE using reciprocal of square root to meet the ANOVA assumptions. Tukey’s HSD Post-hoc comparisons were carried out when ANOVA tests showed significant treatment effects or interactions (P ≤ 0.05). In the analyses, [CO2], soil temperature and photoperiod were treated as fixed variables. All analyses were done using R 3.5.0 (R Development Core Team 2018).
Results
Gas exchange
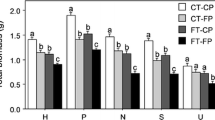
While the net CO2 assimilation rate at ambient [CO2] (An-400) was not affected by any of the treatments or their interactions (Table 1), the net CO2 assimilation rate at growth [CO2] (An-growth) was significantly higher at elevated [CO2] (Fig. 1a). The photosynthetic water-use efficiency (WUE) was significantly higher at elevated [CO2] (Fig. 1b). The maximum rate of carboxylation (Vcmax) tended to increase with increasing photoperiod under the ambient [CO2] but the differences between different photoperiod treatments were not statistically significantly; under elevated [CO2], in contrast, Vcmax was highest at the intermediate photoperiod but the differences between photoperiods were again not statistically significant (Fig. 1c, Table 1). Vcmax was not significantly different between the two CO2 treatments under the same photoperiod regime despite the significant interaction between CO2 and photoperiod (Fig. 1c, Table 1). Although the triose phosphate utilization (TPU) was generally greater with elevated [CO2], the differences varied with soil temperature and photoperiod regime (significant 3-way interaction) (Fig. 1d, Table 1). However, the treatments or their interactions had no significant effect either on stomatal conductance (gs) or on transpiration rate (E) (Table 1). Elevated [CO2] marginally increased the light saturated rate of electron transport (Jmax) (P = 0.079, Table 1).
Mean (± SE) net photosynthetic rate at growth CO2 (An-growth), photosynthetic water-use efficiency (WUE), maximum rate of carboxylation (Vcmax) and triose phosphate utilization (TPU) in jack pine seedlings grown under two levels of [CO2] (Ca = 400 and Ce = 950 µmol mol−1), two soil temperatures (Ts = seed origin and Te = 5 °C warmer) and three photoperiod regimes (Ps = seed origin, and Pnm1, Pnm2 = 5° and 10° further north). Means with different letters within the same graph were significantly different from each other (p ≤ 0.05)
Index of injury
The index of injury (Ii) was significantly higher with elevated [CO2] at all three testing temperatures (Fig. 2a–c). Ii increased with elevated soil temperature at testing temperatures − 15 and − 30 °C (Fig. 2d, e) but was not affected by photoperiod or any treatment interaction (Table 1).
Index of injury (Ii, mean ± SE) at − 5, − 15 and − 30 °C in jack pine seedlings. Seedlings were grown under two levels of [CO2] (Ca = 400 and Ce = 950 µmol mol−1), two soil temperatures (Ts = seed origin and Te = 5 °C warmer) and three photoperiod regimes (Ps = seed origin, and Pnm1, Pnm2 = 5° and 10° further north). For each graph, means with different letters were significantly different from each other (p ≤ 0.05)
Discussion
The greater net photosynthetic rate (An-growth) under elevated atmospheric [CO2] (‘CO2 fertilization effect’) is consistent with the findings of Zhang and Dang (2005) on jack pine and white birch seedlings, Danyagri and Dang (2014a) on mountain maple, DeLucia and Thomas (2000) on four hardwood species and Tjoelker et al. (1998) on five boreal tree species including jack pine. The increase is probably due to the increased availability of substrate (CO2) and enhanced photosynthetic enzyme activity (Zhang and Dang 2006), as the CO2 assimilation in C3 plants is not CO2-saturated at current CO2 level (Lambers and Oliveira 2019). The seedlings in all treatment combinations were well-fertilized and therefore the effects of elevated [CO2] were unlikely limited by nutrient availability. The improved photosynthetic water-use efficiency (WUE) under elevated [CO2] was also consistent with the results of other studies (Keenan et al. 2013; Long et al. 2004; Zhang and Dang 2005) and was likely primarily resulted from reduced stomatal conductance (Bunce 2004; Gunderson et al. 2002; Leakey et al. 2006; Medlyn et al. 2001) as reductions in stomatal conductance have greater negative impact on transpiration than on photosynthesis, leading to an increase of the photosynthesis–transpiration ratio (Lambers and Oliveira 2019). However, decreases in leaf area to root mass ratio or leaf mass ratio may also be a contributing factor (Norby and O’Neill 1991; Poorter 1999; Newaz et al. 2017).
The lack of photosynthetic down-regulation in this study is consistent with the observations by Osborne et al. (1997), Liang et al. (2001), Zhang and Dang (2006), Darbah et al. (2010), and Danyagri and Dang (2014b), but different from those by Ellsworth et al. (2004), Lewis et al. (2004), Nowak et al. (2004), Tissue and Lewis (2010) and Watanabe et al. (2011). Photosynthetic down-regulations are usually associated with nutrient restriction or reduced sink strength (Jach and Ceulemans 1999; Lambers and Oliveira 2019). In this study, jack pine seedlings were well fertilized and had a strong sink as shown by high TPU under elevated [CO2] (Hicklenton and Jolliffe 1980; Koch et al. 1986; Peet et al. 1986; Porter and Grodzinski 1984). The stimulated photosynthesis at elevated [CO2], however, may not be maintained for long due to feedback within plants and ecosystem (Luo and Reynolds 1999), as well as the change of sink activity with developmental stage and leaf age (Long et al. 2004).
Elevated [CO2] and warmer soil temperature both reduced the cold hardiness of jack pine seedlings, which is consistent with the findings of other studies (Barker et al. 2005; Guak et al. 1998; Lutze et al. 1998; Repo et al. 1996). The cold hardiness in all the treatments was between − 15 °C and − 30 °C, as indicated by the index of injury values (Colombo et al. 1989). Our previous study has shown that elevated [CO2] and warmer soil temperature both increased the growth of those seedlings (Newaz et al. 2016). The trade off between increased growth and reduced cold hardiness is also reported for other species (Dalen and Johnsen 2004). The reduction in cold hardiness may be related to reduced carbohydrate availability (Ögren et al. 1997) due to higher rates of root respiration under warmer soil temperatures (Ögren et al. 1997; Repo et al. 2004), or higher temperatures for ice nucleation under elevated [CO2] (Lutze et al. 1998). Soil temperature and [CO2] levels also affect membrane fluidity, calcium influx to the cytosol, xylem sap pH, and ABA concentration, which would all influence cold hardiness development (Hwei-Hwang et al. 1983; Monroy and Dhindsa 1995). The lack of photoperiod and interaction effects on cold hardiness in jack pine seedlings may suggest that the earlier and faster decline in photoperiod at higher latitudes may have promoted both growth cessation and bud set (Newaz et al. 2016). Our results suggest that there may be considerable risks associated with transferring jack pine seeds to higher latitudes according to the projected shifts of its climate envelope or niche because trees may suffer substantial frost damages due to reductions in cold hardiness. However, our results shed no light on the effects of the rate of air temperature declines in the fall on the development of cold hardiness (Bigras et al. 2001) because all the seedlings in the study were exposed to the same air temperature regime. Synchronized changes in air temperature and photoperiod and their interactions with other environmental factors may play a critical role in regulating the process of cold hardiness development. Therefore, studies to better understand the interacting effects of soil temperature, air temperature, photoperiod regime and [CO2] are imperative before any reliable adaptive policy or strategy can be made.
The findings of this study suggest that elevated atmospheric [CO2] accelerated physiological processes, however, both elevated [CO2] and warmer soil temperature retarded cold hardiness in young jack pine seedlings. The lack of significant photoperiod effects or significant interactions with [CO2] or soil temperature indicates that the effects of elevated [CO2] and soil temperature on physiological processes and cold hardiness may not change with changes in photoperiod regimes associated with northward migration under predicted responses to climate change, which does not support our hypothesis on possible changes in cold hardiness due to photoperiod. The significant negative effects of elevated [CO2] and warmer soil temperature on cold hardiness found in this study suggest that forest managers should pay special attention to the cold hardiness of jack pine even when managing the species at its current location, particularly when transferring jack pine seeds to higher latitudes. Given the long rotation time of trees and the continued increases in atmospheric [CO2] and temperature, our results suggest that trees may become more vulnerable to frost damage in the future. However, this study was carried out on 1-year-old tree seedlings under controlled environmental conditions. The responses of trees tend to change with age and size and other environmental conditions. Therefore, investigations on older trees and under field conditions are warranted for the development of proper adaptive strategies in forest management.
References
Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S (2008) Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol Appl 1:95–111
Allen SG, Idso SB, Kimball BA (1990) Interactive effects of CO2 and environment on net photosynthesis of Water-Lily. Agric Ecosyst Environ 30:81–88
Alvarez-Uria P, Körner C (2007) Low temperature limits of root growth in deciduous and evergreen temperate tree species. Funct Ecol 21:211–218
Ambebe TF, Dang QL, Li JL (2010) Low soil temperature inhibits the effect of high nutrient supply on photosynthetic response to elevated carbon dioxide concentration in white birch seedlings. Tree Physiol 30:234–243
Aphalo PJ, Lahti M, Lehto T, Repo T, Rummukainen A, Mannerkoski H, Finér L (2006) Responses of silver birch saplings to low soil temperature. Silva Fennica 40:429–442
Augspurger CK (2013) Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage risk is increasing. Ecology 94:41–50
Barker DH, Loveys BR, Egerton JJG, Gorton H, Williams WE, Ball MC (2005) CO2 enrichment predisposes foliage of a eucalypt to freezing injury and reduces spring growth. Plant Cell Environ 28:1506–1515
Bigras FJ, Ryyppo A, Lindstrom A, Stattin E (2001) Cold acclimation and deacclimation of shoots and roots of conifer seedlings. In: Bigras FJ, Colombo SJ (eds) Conifer cold hardiness. Kluwer Academic Publishers, Dordrecht, pp 57–88
Bunce J (2004) Carbon dioxide effects on stomatal responses to the environment and water use by crops under field conditions. Oecologia 140:1–10
Bunnell F, Kremsater LL (2012) Migrating like a herd of cats: climate change and emerging forests in British Columbia. J Ecosyst Manage 13:1–24
Cai TB, Dang QL (2002) Effects of soil temperature on parameters of a coupled photosynthesis-stomatal conductance model. Tree Physiol 22:819–828
Cao L, Caldeira K (2010) Atmospheric carbon dioxide removal: long-term consequences and commitment. Environ Res Lett 5:024011
Cheng S, Dang QL, Cai TB (2000) A soil temperature control system for ecological research in greenhouses. J For Res 5:205–208
Colombo SJ, Glerum C, Webb DP (1989) Winter hardening in first-year black spruce (Picea mariana) seedlings. Physiol Plantarum 76:1–9
Colombo SJ, McKenney DW, Lawrence KM, Gray PA (2007) Climate change projections for ontario: practical information for policymakers and planners. -39. 2007. Sault St. Marie, Ontario Forest Research Institute
Cui M, Nobel PS (1994) Gas exchange and growth responses to elevated CO2 and light levels in the CAM species Opuntia ficus-indica. Plant Cell Environ 17:935–944
Curtis PS, Vogel CS, Wang X, Pregitzer KS, Zak DR, Lussenhop J, Kubiske M, Teeri JA (2000) Gas exchange, leaf nitrogen, and growth efficiency of Populus tremuloides in a CO2-enriched atmosphere. Ecol Appl 10:3–17
Dalen LS, Johnsen Ø (2004) CO2 enrichment, nitrogen fertilization and development of freezing tolerance in Norway spruce. Trees 18:10–18
Dang QL, Marfo J, Du FG, Man R, Inoue S (2020a) CO2 stimulation to boreal conifers is greater at lower light but response mechanism varies with light level. J Plant Ecol (in press)
Dang QL, Marfo J, Du FG, Newaz MS (2020b) Nutrient supply and belowground interaction alter responses to CO2 elevation in black spruce and white spruce. For Ecol Manage. https://doi.org/10.1016/j.foreco.2020.118271
Danyagri G, Dang QL (2014a) Soil temperature and phosphorus supply interactively affect physiological responses of white birch to CO2 elevation. Am J Plant Sci 5:219–229
Danyagri G, Dang QL (2014b) Effects of elevated carbon dioxide concentration and soil temperature on the growth and biomass responses of mountain maple (Acer spicatum L.) seedlings to light availability. J Plant Ecol 7:535–543
Darbah JNT, Sharkey TD, Calfapietra C, Karnosky DF (2010) Differential response of aspen and birch trees to heat stress under elevated carbon dioxide. Environ Pollut 158:1008–1014
DeLucia EH, Smith WK (1987) Air and soil temperature limitations on photosynthesis in Engelmann spruce during summer. Can J For Res 17:527–533
DeLucia EH, Thomas RB (2000) Photosynthetic responses to CO2 enrichment of four hardwood species in a forest understory. Oecologia 122:11–19
Domec JC, Noormets A, King JS, Sun G, McNulty SG, Gavazzi MJ, Boggs JL, Treasure EA (2009) Decoupling the influence of leaf and root hydraulic conductances on stomatal conductance and its sensitivity to vapour pressure deficit as soil dries in a drained loblolly pine plantation. Plant Cell Environ 32:980–991
Eamus D, Ceulemans R (2001) Effects of greenhouses gases on the gas exchange of forest trees. In: Karnosky DF, Ceulemans R, Scarascia-Mugnozza GE, Innes JL (eds) The impact of carbon dioxide and other greenhouse gases on forest ecosystems. CAB International, Wallingford, pp 17–56
Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD (2004) Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated CO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob Change Biol 10:2121–2138
Ensminger I, Schmidt L, Lloyd J (2008) Soil temperature and intermittent frost modulate the rate of recovery of photosynthesis in Scots pine under simulated spring conditions. New Phytol 177:428–442
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Flint HL, Boyce BR, Beattie DJ (1967) Index of injury: a useful expression of freezing injury to plant tissues as determined by the electrolytic method. Can J Plant Sci 47:229–230
Forestry Canada (1991). Selected forestry statistics Canada. Information Report E-X-46, -231. 1992. Ottawa, Forestry Canada, Policy and Economics Directorate
Fraser DA (1962) Growth of spruce seedlings under long photoperiods. Technical Note No. 114. Canada Department of Forestry, Forest Research Branch
Frechette E, Ensminger I, Bergeron Y, Gessler A, Berninger F (2011) Will changes in root-zone temperature in boreal spring affect recovery of photosynthesis in Picea mariana and Populus tremuloides in a future climate? Tree Physiol 31:1204–1216
Grossnickle SC (2000) Ecophysiology of northern spruce species: the performance of planted seedlings. NRC Research Press, Ottawa
Gu L, Hanson PJ, Post WM, Kaiser DP, Yang B, Nemani R, Pallardy SG, Meyers T (2008) The 2007 eastern US spring freeze: increased cold damage in a warming world? Bioscience 58:253–262
Guak S, Olszyk DM, Fuchigami LH, Tingey DT (1998) Effects of elevated CO2 and temperature on cold hardiness and spring bud burst and growth in Douglas-fir (Pseudotsuga menziesii). Tree Physiol 18:671–679
Gunderson CA, Sholtis JD, Wullschleger SD, Tissue DT, Hanson PJ, Norby RJ (2002) Environmental and stomatal control of photosynthetic enhancement in the canopy of a sweetgum (Liquidambar styraciflua L.) plantation during 3 years of CO2 enrichment. Plant Cell Environ 25:379–393
Hicklenton PR, Jolliffe PA (1980) Alterations in the physiology of CO2 exchange in tomato plants grown in CO2-enriched atmospheres. Can J Bot 58:2181–2189
Hwei-Hwang C, Li PH, Brenner ML (1983) Involvement of abscisic acid in potato cold acclimation. Plant Physiol 71:362–365
Inoue S, Dang QL, Man R, Tedla B (2020) Photoperiod, [CO2] and soil moisture interactively affect the phenology and growing season length of trembling aspen: a perspective for climate change-induced tree migration. Environ Exp Bot 180(2020):104269. https://doi.org/10.1016/j.envexpbot.2020.104269
IPCC (2013) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, USA
Jach ME, Ceulemans R (1999) Effects of elevated atmospheric CO2 on phenology, growth and crown structure of Scots pine (Pinus sylvestris) seedlings after two years of exposure in the field. Tree Physiol 19:289–300
Keenan TF, Hollinger DY, Bohrer G, Dragoni D, Munger JW, Schmid HP, Richardson AD (2013) Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499:324–327
Koch KE, Jones PH, Avigne WT, Allen LH (1986) Growth, dry matter partitioning, and diurnal activities of RuBP carboxylase in citrus seedlings maintained at two levels of CO2. Physiol Plantarum 67:477–484
Lambers H, Oliveira RS (2019) Plant physiological ecology 3rd. Springer, New York. p 736, ISBN-13: 978-3030296384
Leakey ADB, Bernacchi CJ, Ort DR, Long SP (2006) Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open-air conditions. Plant Cell Environ 29:1794–1800
Lewis JD, Lucash M, Olszyk DM, Tingey DT (2004) Relationships between needle nitrogen concentration and photosynthetic responses of Douglas-fir seedlings to elevated CO2 and temperature. New Phytol 162:355–364
Li J, Dang QL, Man R (2015) Photoperiod and nitrogen supply limit the scope of northward migration and seed transfer of black spruce in a future climate associated with doubled atmospheric CO2 concentration. Am J Plant Sci 6:189–200
Liang N, Tang Y, Okuda T (2001) Is elevation of carbon dioxide concentration beneficial to seedling photosynthesis in the understory of tropical rain forests? Tree Physiol 21:1047–1055
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Luo Y, Reynolds JF (1999) Validity of extrapolating field CO2 experiments to predict carbon sequestration in natural ecosystems. Ecology 80:1568–1583
Luthi D, Le Floch M, Bereiter B, Blunier T, Barnola JM, Siegenthaler U, Raynaud D, Jouzel J, Fischer H, Kawamura K, Stocker TF (2008) High-resolution carbon dioxide concentration record 650,000-800,000 years before present. Nature 453:379–382
Lutze JL, Roden JS, Holly CJ, Wolfe J, Egerton JJG, Ball MC (1998) Elevated atmospheric [CO2] promotes frost damage in evergreen tree seedlings. Plant Cell Environ 21:631–635
Man R, Kayahara GJ, Dang QL, Rice JA (2009) A case of severe frost damage prior to budbreak in young conifers in northeastern Ontario: Consequence of climate change? For Chron 85:453–462
Marchi S, Tognetti R, Vaccari FP, Lanini M, Kaligarič M, Miglietta F, Raschi A (2004) Physiological and morphological responses of grassland species to elevated atmospheric CO2 concentrations in FACE-systems and natural CO2 springs. Funct Plant Biol 31:181–194
Marfo J, Dang QL (2009) Interactive effects of carbon dioxide concentration and light on the morphological and biomass characteristics of black spruce and white spruce seedlings. Botany 87:67–77
McKenney DW, Pedlar JH, Lawrence K, Campbell K, Hutchinson MF (2007) Potential impacts of climate change on the distribution of North American trees. Bioscience 57:939–948
McKenney DW, Pedlar JH, Rood RB, Price D (2011) Revisiting projected shifts in the climate envelopes of North American trees using updated general circulation models. Glob Change Biol 17:2720–2730
Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomäki S, Laitat E, Rey A, Roberntz P, Sigurdsson BD, Strassemeyer J, Wang K, Curtis PS, Jarvis PG (2001) Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol 149:247–264
Mishra RS, Abdin MZ, Uprety DC (1999) Interactive effects of elevated CO2 and moisture stress on the photosynthesis, water relation and growth of Brassica species. J Agron Crop Sci 182:223–230
Monroy AF, Dhindsa RS (1995) Low-temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25 degrees C. Plant Cell 7:321–331
Morgan JA, Pataki DE, Körner C, Clark H, Del Grosso SJ, Grünzweig JM, Knapp AK, Mosier AR, Newton PCD, Niklaus PA, Nippert JB, Nowak RS, Parton WJ, Polley HW, Shaw MR (2004) Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140:11–25
Newaz MS, Dang QL, Man R (2016) Morphological response of jack pine to the interactive effects of carbon dioxide, soil temperature and photoperiod. Am J Plant Sci 7:879–893
Newaz MS, Dang QL, Man R (2017) Eco-physiological potential of jack pine (Pinus banksiana) for assisted northward migration: interactions among photoperiod, [CO2] and moisture stress. Nordic J Bot 35:633–643
Norby RJ, O’Neill EG (1991) Leaf area compensation and nutrient interactions in CO2-enriched seedlings of yellow-poplar (Liriodendron tulipifera L.). New Phytol 117:515–528
Nowak RS, Ellsworth DS, Smith SD (2004) Functional responses of plants to elevated atmospheric CO2: Do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol 162:253–280
Ögren E, Nilsson T, Sundblad L-G (1997) Relationship between respiratory depletion of sugars and loss of cold hardiness in coniferous seedlings over-wintering at raised temperatures: indications of different sensitivities of spruce and pine. Plant Cell Environ 20:247–253
OMNR (1991) Statistics 1989–1990. Queen’s Printer for Ontario, Toronto, p 106
Osborne CP, Drake BG, LaRoche J, Long SP (1997) Does long-term elevation of CO2 concentration increase photosynthesis in forest floor vegetation? (Indiana strawberry in a Maryland forest). Plant Physiol 114:337–344
Peet MM, Huber SC, Patterson DT (1986) Acclimation to high CO2 in monoecious cucumbers: II. Carbon exchange rates, enzyme activities, and starch and nutrient concentrations. Plant Physiol 80:63–67
Peng YY, Dang QL (2003) Effects of soil temperature on biomass production and allocation in seedlings of four boreal tree species. For Ecol Manage 180:1–9
Poorter L (1999) Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct Ecol 13:396–410
Porter MA, Grodzinski B (1984) Acclimation to high CO2 in bean: carbonic anhydrase and ribulose bisphosphate carboxylase. Plant Physiol 74:413–416
Pothier D, Prevost M (2002) Photosynthetic light response and growth analysis of competitive regeneration after partial cutting in a boreal mixed stand. Trees 16:365–373
Repo T, Hanninen H, Kellomaki S (1996) The effects of long-term elevation of air temperature and CO2 on the frost hardiness of Scots pine. Plant Cell Environ 19:209–216
Repo T, Leinonen I, Ryyppö A, Finér L (2004) The effect of soil temperature on the bud phenology, chlorophyll fluorescence, carbohydrate content and cold hardiness of Norway spruce seedlings. Physiol Plantarum 121:93–100
Rudolf PO (1965) Jack pine (Pinus banksiana Lamb.). In: Fowells HA (ed) Silvics of forest trees of the United States, Agriculture Handbook 271. US Department of Agriculture, Washington DC, pp 338–354
Scarratt JB (1986) An evaluation of some commercial soluble fertilizers for culture of jack pine container stock. Informatio Report O-X-377. Sault Ste. Marie, Ontario, Canadian Forestry Service, Great Lakes Forestry Centre: 21
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30:1035–1040
Smith WK (1985) Environmental limitations on leaf conductance in Central Rocky Mountain conifers. Turner H.and Tranquillini W. 3rd IUFRO Workshop. 270, Eidg. Anst. Forstel, Versuch (Berl.): 95–101
Soolanayakanahally RY, Guy RD, Silim SN, Song M (2013) Timing of photoperiodic competency causes phenological mismatch in balsam poplar (Populus balsamifera L.). Plant Cell Environ 36:116–127
Stathers RJ, Spittlehouse DL (1990) Forest Soil Temperature Manual. FRDA Report No. 130. British Columbia Ministry of Forests. Victoria, BC
Tissue DT, Lewis JD (2010) Photosynthetic responses of cottonwood seedlings grown in glacial through future atmospheric [CO2] vary with phosphorus supply. Tree Physiol 30:1361–1372
Tjoelker MG, Oleksyn J, Reich PB (1998) Seedlings of five boreal tree species differ in acclimation of net photosynthesis to elevated CO2 and temperature. Tree Physiol 18:715–726
Watanabe M, Watanabe Y, Kitaoka S, Utsugi H, Kita K, Koike T (2011) Growth and photosynthetic traits of hybrid larch F1 (Larix gmelinii var. japonica x L. kaempferi) under elevated CO2 concentration with low nutrient availability. Tree Physiol 31:965–975
Way DA, Sage RF (2008a) Elevated growth temperatures reduce the carbon gain of black spruce [Picea mariana (Mill.) B.S.P.]. Glob Change Biol 14:624–636
Way DA, Sage RF (2008b) Thermal acclimation of photosynthesis in black spruce [Picea mariana (Mill.) B.S.P.]. Plant Cell Environ 31:1250–1262
Zhang S, Dang QL (2005) Effects of soil temperature and elevated atmospheric CO2 concentration on gas exchange, in vivo carboxylation and chlorophyll fluorescence in jack pine and white birch seedlings. Tree Physiol 25:523–531
Zhang S, Dang QL (2006) Effects of carbon dioxide concentration and nutrition on photosynthetic functions of white birch seedlings. Tree Physiol 26:1457–1467
Zhang S, Dang QL (2007) Interactive effects of soil temperature and CO2 on morphological and biomass traits in seedlings of four boreal tree species. For Sci 53:453–460
Acknowledgements
The authors thank Drs. C. Shahi for advice on statistical analysis and D. Morris for providing soil temperature data. We also acknowledge J. Lee and E. Laura for their laboratory assistance. The research was funded by an NSERC Discovery Grant to Q.L. Dang (Project No. 203198-2013-RGPIN) and Lakehead University Graduate Assistantship to Md Newaz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Newaz, S.M., Dang, QL. & Man, R. CO2 elevation and soil warming reduce cold hardiness of jack pine under photoperiods of seed origin and latitudes of potential migration. New Forests 52, 777–790 (2021). https://doi.org/10.1007/s11056-020-09831-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-020-09831-0