Abstract
Although areas planted with eucalyptus have increased under the subtropical conditions of southern Brazil, fertilization has followed practices from nearby tropical regions. The objective of this study was to determine the response of Eucalyptus urograndis to N, P, and K in two regions of Parana State (Jaguariaíva and Ventania) on a sandy oxisol following two harvest cycles of Pinus taeda. Site preparation prior to the new experiment included the application of rock phosphate (26.2 kg ha−1 of P) by in-row subsoiling. Five rates of N and P (0, 13, 26, 52, and 105 kg ha−1) and K (0, 29, 58, 116, and 232 kg ha−1) were separately tested. Treatment response was evaluated by determining maximum technical efficiency, height, and diameter at breast height (DBH) at 3, 6, 9, 12, 18, 24, 30, and 36 months after planting, and volume at 36 months. At all assessments, N additions did not influence growth parameters indicating that N demands were met by organic matter/litter mineralization. Phosphorus enhanced growth until the 18th month, with maximum gains at months 9 and 6 for Jaguariaíva and Ventania (56 and 59% of DBH for the 105 kg ha−1 P rate, respectively). Potassium improved growth only after the 24th month; maximum rate occurred at 166 kg ha−1 of K with a volume of 163 m3 ha−1 at the Jaguariaíva site at 36 months. In conclusion, findings indicated that Eucalyptus urograndis grown in southern Brazil could be responsive to P and K fertilization, but not to the addition of N fertilizer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eucalyptus is the most planted forest species in Brazil, and plantation acreage continues to expand. In 2015, ~ 5.6 million hectares were cultivated on a great diversity of soils. Since eucalyptus management and selection favored growth under subtropical climate, the 162,181 ha increase in cultivated area between 2006 and 2015 resulted in the state of Parana ranking 7th in planted area (280,000 ha) in Brazil (IBA 2016). However, this rapid increase did not incorporate new developments in fertility practices that have occurred in other locations where eucalyptus was more traditional.
In the subtropical region of east-central and northeast Parana State, expanding eucalyptus cultivation has displaced pine tree plantations. In other regions of Brazil, eucalyptus has been used in the renovation of old plantations or to replace pastures. One unique aspect of planting after pine cultivation is the accumulated litter (25–90 Mg ha−1) and stocks of nitrogen (117–612 kg N ha−1), phosphorus (9–19 kg P ha−1), and potassium (58–280 kg K ha−1) in these regions (Bizon 2005). Given these nutrient stocks, there is uncertainty how eucalyptus will respond to fertilizer application since it is considered an efficient species concerning nutrient use (Gonçalves et al. 2004).
Response to fertilizer application can vary among nutrients, soils, and weather conditions (Barreto et al. 2012; Maeda and Bognola 2012; Dias et al. 2015; Sampaio et al. 2016). The response to N is the most variable and uncertain among macronutrients due to litter/soil organic matter stocks and associated immobilization/mineralization rates (Gama-Rodrigues et al. 2005; Pulito et al. 2015). Positive response to N additions have usually been observed where the process of soil N mineralization is low due to either high C:N or low organic matter quantity (Gonçalves et al. 2004; Santana et al. 2008; Pulrolnik et al. 2009).
Responses to P application have been more frequent and to a higher degree for eucalyptus since Brazilian soils generally have low P availability and high adsorption capacity. These characteristics are related to the high level of soil weathering (Barros and Novais 1996; Novais et al. 2007). In addition, since P fertilizers applied in forests can vary in form from low reactive (sedimentary rock phosphate) to highly reactive (super phosphate), the method of application is very important (Maeda and Bognola 2011; Dias et al. 2015). Thus, applying P near the roots during the first month of plantation establishment has promoted meaningful increases in tree growth (Fernandez et al. 2000; Gonçalves et al. 2004; Stahl 2009; Dias et al. 2015). Powdered reactive rock phosphate has been applied during subsoiling in order to create an enrichened vertical soil zone to complement soluble P fertilizer at a lower cost (Dias et al. 2015). Phosphate fertilization has also been important in reducing the need for replanting (Rocha et al. 2013). Furthermore, growth acceleration from P applications can decrease needed cultural treatments and time until harvest, which can increase the number of harvest cycles over time.
Low K availability associated with low soil moisture may increase eucalyptus response to K fertilization (Barros et al. 2014; Sampaio et al. 2016). On one hand, the introduction of eucalyptus in subtropical climates without a dry season could diminish the response to K application. On the other hand, when eucalyptus replaces pines in subtropical conditions without fertilizer, the depletion of K from pine growth (Motta et al. 2014) may cause eucalyptus to respond to K fertilization. For this reason, K restitution is necessary to guarantee sustained plant growth (Gava 1997; Faria et al. 2002; Silva et al. 2012; Biagiotti et al. 2017).
There is a paucity of information regarding eucalyptus fertilization practices in subtropical regions and on the use of traditional fertilizers in reforestation efforts. This paper aims to study the effects of N, P, and K applications on the growth of the Eucalyptus urograndis hybrid in two regions (Jaguariaíva and Ventania) of the Parana state of Brazil.
Materials and methods
Experimental sites were established on December 2013 at Jaguariaíva (24°15′12″ S and 49°40′40″ W; 926 m altitude) and April 2014 at Ventania (24°01′17.25″ S and 50°15′39.62″ W; 718 m altitude) in the state of Parana, Brazil. The Jaguariaíva site was characterized by gently undulating terrain, and the soil was classified as a Dystrophic Oxisol with a sandy loam texture from the Furnas sandstone geological formation of the Parana group. The Ventania site varied from gently undulating to undulating, and the soil was classified as a Dystrophic Oxisol with a sandy loam texture from an undifferentiated sandstone (Rio do Sul, Mafra e Campo do Tenente) of the Itararé group.
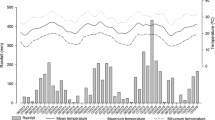
According to Alvares et al. (2013), both study sites were characterized as humid subtropical in climatic zone C. The Jaguariaíva site was a Cfb type having milder temperatures during summer, while the Ventania site was in a transition region (between Cfb and Cfa) with summers characterized as having hotter temperatures. During the 3 year research span, the daily mean air temperature for Ventania and Jaguariaíva was 22.9 and 21.8 °C, respectively. The daily mean temperatures for the hottest and the coldest days were 26.5 and 14.6 °C for Ventania compared to 23.6 and 14.2 °C for Jaguariaíva, respectively. The accumulated precipitation for 3 years was 6131 and 8895 mm for Ventania and Jaguariaíva, respectively. Temperature and precipitation data for the study sites are shown in Fig. 1.
The two experimental areas were approximately three hectares each and were previously planted twice with Pinus taeda at plant spacings of 3 m × 2 m. These pine plantations were subjected to a shallow cut with log and branch extraction at the Jaguariaíva experimental site and shallow cut and branches and leaves peeling (slash peeling) at the Ventania site. Following pine harvest at each experimental site, soil samples (20 samples per site) were composited in 20 cm increments to a depth of 100 cm for chemical characterization (Table 1) using the methodology described by the Brazilian Company of Agricultural Research (EMBRAPA 2009). Samples were subjected to hydrofluoric acid extraction using microwave digestion, and total K was determined by flame spectrophotometery.
Prior to planting, study sites were subsoiled (45 cm depth) in the middle of lines of pine stumps. During this same operation, 200 kg ha−1 of rock phosphate (RP; 12.5% of P total and 6% in citric acid) was applied to a depth of 25 cm. The source of rock phosphate used was a sedimentary based phosphate from Peru (Bayovar) which has higher solubility than igneous sources. After subsoiling, 2000 kg ha−1 of dolomitic limestone (28% CaO + 14.5% MgO) was broadcast on the soil surface without incorporation.
Eucalyptus urograndis hybrid seedlings (Eucalyptus urophylla × Eucalyptus grandis, AEC 224 clone) were planted on 3 m × 3 m spacings using manual equipment (seedling planter) along the subsoiling line. After planting, 565 m2 plots were delineated (8 lines with 8 plants each) and assembled in random blocks with four replications. A visual inspection was conducted each week for 1 month in order to detect and replace dead seedlings.
For the two locations, three independent experiments were conducted side by side; each experiment consisted of five treatments. Based on fertilization practices used by the regional timber company (i.e., 80, 32.5, and 134 kg ha−1 for N, P, and K respectively), treatments were defined to be below and above these regional rates. For N fertilization urea was applied at five rates (0, 30, 60, 120, and 240 kg ha−1 of N). For P fertilization, triple super phosphate (TSP) was applied at 0, 13, 26, 52, and 105 kg ha−1 of P. For K fertilization, rates of 0, 29, 58, 116 and 232 kg ha−1 of K were used. The amount of N, P, and K was divided into four equal parts that were applied at 0, 90, 270, and 365 days after planting (Fig. 1). During each application, fertilizer was further divided into two equal parts with half being applied beside the seedling and the other half being applied 15 cm away from the seedling along the subsoiling line.
For each nutrient tested, fixed rates of other nutrients were applied at planting based on regional timber company practices: (1) for the N experiment, 22 kg ha−1 of P, 14 kg ha−1 of K, and 0.8 kg ha−1 of B were applied at planting in all treatments, and three additional side-dress applications were performed at 3, 9, and 12 months after planting using 3.5, 40, and 0.8 kg ha−1 of P, K, and B, respectively; (2) for the P experiment, 8.5 kg ha−1 of N, 14 kg ha−1 of K, and 0.8 kg ha−1 of B were applied at planting in all treatments, and three additional side-dress applications were performed at 3, 9, and 12 months after planting using 24, 40, and 0.8 kg ha−1 of N, K, and B, respectively; and (3) for the K experiment, 8.5 kg ha−1 of N, 22 kg ha−1 of P, and 0.8 kg ha−1 of B were applied at planting in all treatments, and three additional side-dress applications were performed at 3, 9, and 12 months after planting using 24, 3.5, and 0.8 kg ha−1 of N, P, and B, respectively. In all cases, the fertilizer sources were urea (46% N), TSP (19% P), potassium chlorate (52% K), and boric acid (17% B).
In the third month, collar diameter (CD) and total height (H) were measured. At 6, 9, 12, 18, 24, 30, and 36 months after planting, diameter at breast height (DBH) and total height (H) were measured on the 36 central trees within each plot. Diameters were measured with a tape and heights with an Haglof electronic hypsometer.
Based on the Hohenadl method, tree trunk volume at 36 months was estimated using equations of Spurr (1952) and Meyer (1953) at Jaguariaíva and Ventania. To validate equations, the adjusted coefficients of determination, standard errors of estimate, coefficients of variation, and graphical analysis of waste were evaluated (Figueiredo-Filho et al. 2014). The mean annual increment (MAI; m3 ha−1 year−1) was determined by dividing the volume at 36 months by 3 years with an assumed 4% total mortality over the 3-year period.
Dendometric data were submitted to analysis of variance and considered significant at ρ < 0.01 or 0.01 < ρ < 0.05. The rate of maximum technical efficiency (MTE), representing the amount of nutrients necessary for best growth, was calculated through the first derivative (dy/dx = 0) of the parabolic regression equation presenting downward-facing concavities (y = − ax2 + bx + c). After obtaining the first derivative (MTE), this value enters the equation in place of x (fertilizer rate) to obtain the maximum growth of the dendrometric variable (y). Coefficients of determination (r2) were determined for these regressions. The programs R (3.4.1), Microsoft Excel® 2016 and SigmaPlot 10.0. were utilized.
Results
By the third year, overall plant size (Table 2) showed regular growth rates comparable to the national average. The Ventania site had slightly higher growth rates than the Jaguariaíva site as reflected by measured and calculated growth parameters. Although greater growth occurred at Ventania (compared to Jaguariaíva), foliar concentration data showed low difference between the two sites at 36 months; overall mean concentrations at the Jaguariaíva site were 20.6, 1.12, and 5.29 g kg−1 for N, P, and K while corresponding values for Ventania were 19.9, 1.16, 7.05 g kg−1 for N, P, and K, respectively (Bassaco 2018).
Size responses in terms of collar diameter (CD), diameter at breast height (DBH), and height (H) are shown in Fig. 2, while volume (V) changes can be seen in Fig. 3 for the Jaguariaíva and Ventania sites. During the first evaluation at Jaguariaíva, the highest N rate resulted in the lowest growth due to almost total seedling mortality from N toxicity by NH4+ (high urea amount of ~ 246 g seedling−1) (Fig. 2a). Despite little difference in seedling age, rapid growth in the first month resulted in large growth differences between original and replacement seedlings. This mortality event was unexpected since N was divided and applied at distances recommended for commercial plantations. Differences due to replanting decreased over time and generally reached equivalent heights at 30 months and equivalent DBH at 36 months (Fig. 2d).
Growth response of Eucalyptus urograndis according to applied rates of N (0, 30, 60, 120, and 240 kg ha−1), P (0, 13, 26, 52, and 105 kg ha−1) and K (0, 29, 58, 116, and 232 kg ha−1) in terms of collar diameter (CD), diameter at breast height (DBH), and height (H) for 3–12 months (a, b, c) and 18–36 months (d, e, f) at Jaguariaíva and Ventania counties, Paraná State—Brazil. Within a given variable, *significant at 5% probability, **significant at 1% probability, and ns not significant
Enhancement of diameter and height were observed with P addition at both sites until the 24th month (Fig. 2b, e). The growth difference among treatments diminished with tree age, suggesting that a lack of P compromised initial growth at both locations. In the first 6 months, it was noted that trees in the 0 kg ha−1 P treatment were similar in size to border trees that received no fertilization (visual observation). The response to P was higher during initial growth and followed a parabolic response pattern thereafter; MTE varied from 56.1–109 to 40.9–98.0 kg ha−1 of P at Jaguariaíva and Ventania, respectively, for the different periods analyzed. There was a DBH gain of up to 56% (compared to control) at the 9th month (Fig. 2b).
In contrast to P, the K application effect was directly proportional to tree age. Most differences in size occurred after 24 months, and there were responses to almost all the evaluated variables at Jaguariaíva after 36 months (Fig. 2f and Table 3). Due to high soil weathering, the very low available K was expected to limit initial growth. Furthermore, K deficiency symptoms were observed where K was not applied and with the 29.1 kg ha−1 rate of K. Since these symptoms were noted at 6 months and tree height was similar across all treatments, the K application effect appears to be more directly related to tree age than tree size. The occurrence and intensity of symptoms were concentrated in leaves closer to the tree trunk in the lower part of the canopy. Additionally, leaves with symptoms suffered premature senescence. The effect of K at the Jaguariaíva site was manifested in the 18th month for the parameter height (i.e., MTE of 61.2 kg ha−1 of K) and increased MTE to 166 kg ha−1 K by the 36th month; this represented a relative gain of 45% (163 m3 ha−1) compared to the control (Table 3 and Fig. 3).
Discussion
In our study, the mean annual increment (MAI) varied from 46 to 63 m3 ha−1 year−1; this exceeds the national average of 35 m3 ha−1 year−1 for eucalyptus (IBA 2016) and indicates a high growth potential. Obtained MAI are considered comparable to sites of good performance that have displayed increments varying from 41.5 to 54 m3 ha−1 year−1 under fertilization (Santana et al. 2008; Pulito et al. 2015; Melo et al. 2016).
Eucalyptus displayed better growth at the Ventania site even though measured soil conditions were similar to the Jaguariaíva site. This response may be due to the slightly higher air temperature at Ventania compared to Jaguariaíva since eucalyptus has generally shown higher growth potential under tropical conditions (Stape et al. 2010). Hybridization of Eucalyptus urophylla may have resulted in the selection of factors in a clone that make it more suited for growth in this climate (Junior and Garcia 2003). However more studies should be conducted to identify which resource(s) favors optimal growth in this region.
Application of N did not significantly increase growth at both sites at 3 years. Eucalyptus growth enhancement due to N application was found at 7 of 11 sites in the first years after planting, but the lack of effect at harvest (Pulito et al. 2015) suggest a decrease in response with time. The high demand for N occurs in the first 2 or 3 years during canopy formation. Santana et al. (2008) reported allocations of N in trees ranging between 130 and 330 kg ha−1 for different Brazilian climates, soil conditions, and eucalyptus species (4.5–8.5 years old). Others have reported values of 100 kg ha−1 in Eucalyptus nitens (Smethurst et al. 2004) and 89 kg ha−1 in Eucalyptus urograndis (Barreto et al. 2012) for the first 2 years. These findings highlight the importance of a sufficient N supply in the first years of growth.
The absence of an N response in our study suggests that the initial high demand for N was met by the large pool of available N (i.e., soil and litter residue) from the previous pine plantations. The soils at the study sites had large quantities of organic matter; the total organic C (23.6 and 21.0 g dm−3 in Jaguariaíva and Ventania, respectively) suggests a high reservoir of N (Table 1). Using eleven soils under eucalyptus forest with average of organic C of 13 g kg−1, Pulito et al. (2015) found in situ N mineralization ranging between 100 and 200 kg ha−1 year−1 for the 0–20 cm depth.
This highlights the importance of soil organic matter mineralization for supplying available N to trees as reported by others (Gonçalves et al. 2004; Gama-Rodrigues et al. 2005; Barreto et al. 2012; Pulito et al. 2015). Additionally, the application of limestone, implementation of subsoiling, and the abundance of rain may have maintained high microbial activity, and the consequent mineralization of N from soil organic matter limited the N response at both sites. A survey of Pinus taeda experimental areas near our site indicated that the amount N in litter varied from 111 to 175 kg ha−1 (Bizon 2005). The initial amount of litter at our site was substantial, and visual observations indicated that most of the litter decomposed during the first year, which suggests that fast decay of Pinus litter could result in an important N source.
As mentioned previously, the N response at the Jaguariaíva site was affected by plant mortality during establishment that required replacement seedlings. The lack of a N response occurred in the period when eucalyptus has a higher N demand (first 24 months) due to greater biomass production. Previous work supports this contention and suggests that the biogeochemical cycle can intensify in the third year and the response to application of N can diminish (Santana et al. 2008; Laclau et al. 2010; Melo et al. 2016). On the other hand, in later cycles during nutrient exportation, response to N application in sandy soils can result in high eucalyptus growth (Pulito et al. 2015).
The greater percentage increase observed in the first months after planting (Fig. 2b) illustrates the importance of P in the initial phase of eucalyptus growth (Gonçalves et al. 2004; Maeda and Bognola 2011, 2012; Stahl et al. 2013; Dias et al. 2014). The elevated response to P application was expected due to the very low availability of this nutrient throughout the entire soil profile (Table 1). In addition, Oxisols have an elevated capacity for P adsorption, especially under acid conditions as observed at the study sites (m% > 80%).
It is important to highlight that the control treatment received ~ 26 kg ha−1 of P as RP. On one hand, the RP did not release enough P to supply the initial needs for eucalyptus growth. Similar responses were observed with Eucalyptus dunnii, where the partial substitution of soluble phosphate for the natural source was not efficient in the first year after planting (Dias et al. 2014). On the other hand, it is possible that RP started to release more P over time, leading to a boost in growth and differences among treatments. The slow solubility of RP is well known (Caione et al. 2013), even under the acid conditions of our soils. Additionally, an inverse relation between age and response to P application has been reported in eucalyptus (Xu et al. 2005; Melo et al. 2016). These authors suggest that higher soil exploration and lower P demand after initial growth may be causes for this inverse relationship. Soil exploration aided by mycorrhizae growth likely helped meet P demands. Although seedlings were not inoculated with mycorrhizae at planting, natural inoculation was likely since mycorrhizae were visually observed in litter at our study site. In addition, root growth increases (depth and length) may have reached more of the P from the RP applied during subsoiling. Our deep soils were well suited for good rooting and other have reported rooting depths of 10 m in 2 years with fine and medium roots tending to concentrate within 2 m (both depth and lateral distance) of tree base (Pinheiro et al. 2016; Bouillet et al. 2002).
The amount of additional P necessary to reach maximum growth varied by parameters and study site. At the Jaguariaíva site, 109 kg ha−1 of P was required to reach the maximum DBH at 24 months, while approximately 93.3 kg ha−1 of P was needed at the Ventania site to obtain maximum DBH at 9 months. Although short term evaluations suggested that P fertilization could be important for initial tree growth, this could be problematic in recommending rates for the whole life cycle. Others have reported on fertility levels required for maximum growth in soils with low P availability and clay content between 24 and 43% (Fernandez et al. 2000; Dias et al. 2014; Xu et al. 2002, 2005). Values of P higher than our study were found by Fernandez et al. (2000) and Dias et al. (2014) who reported values of 145 (clay = 43% and available soil P < 1 mg dm−3) and 131 (clay = 24% and available soil P = 51 mg dm−3) kg ha−1 of P, respectively. In comparison, Xu et al. (2002, 2005) reported that respective values of 208 (clay = 39% and available soil P = 1.81 mg kg−1) and 200 (clay = 26% and soil P = 1.53 mg kg−1) kg ha−1 of P were required for maximum growth. Thus, it can be inferred from findings of others and the current effort that a lower clay content may equate to lower amounts of P that could hinder maximum growth response.
Trees planted with no K were able to maintain growth until 24 months. The abundance and regular distribution of rain could be a major factor explaining the absence of a K growth response since greater initial responses have been reported under water deficit conditions (Faria et al. 2002; Silva et al. 2012; Sampaio et al. 2016; Biagiotti et al. 2017) due to better stomatal control (hydric regulation) (Hawkesford et al. 2012). The low precipitation in August 2014 (61 and 26 mm at Jaguariaíva and Ventania, respectively; Fig. 1) could have restricted metabolic activity. In addition, it is possible that the abundance of K supplied to seedlings at nurseries resulted in a K content that met initial growth requirements, but deficiency was observed as of the sixth month. It is well known that K has a high capacity for internal redistribution (40–75%) to meet plant demands (Laclau et al. 2010). This could explain why K deficiency symptoms were only observed on old leaves close to the trunk. Thus, external canopy leaves maintained enough K reserves for internal redistribution such that plant growth was not affected at the beginning. However, others reported an increase in K demand with forest age (Almeida et al. 2010; Battie-Laclau et al. 2014). In these studies, it is possible that increased demand could not be met by K uptake and plant K internal redistribution. This can be observed by the significant variation in foliar concentration between treatments after the 24th month (i.e., 0 kg ha−1 K2O = 4.76 g kg−1 K and 232 kg ha−1 K2O = 7.41 g kg−1 K) (Bassaco 2018). In contrast, Silva et al. (2012) and Biagiotti et al. (2017) found a diminishing K response over time with the control treatment always having a lower growth response compared to fertilized treatments. Melo et al. (2016) noted that eucalyptus trees have the ability to extract K from low soluble forms. These authors working on 8 eucalyptus sites found that K extracted from soil with boiling nitric acid proved to be a better predictor of foliar K concentration than Mehlich 1 extractable K. The importance of low soluble K forms could increase with time due to root expansion and exhaustion of easily soluble forms. The levels of extractable and total K were both very low in our soils which limited further supply from low soluble sources during plant growth.
Sampaio et al. (2016) worked with different clones of Eucalyptus to select those most adapted to hydric deficit and to adjust the response curve for fertilization. For the clone used in our study, they found an MTE of 28.8, 80.7, and 32.7 kg ha−1 of K for DBH, H, and volume, respectively. In the 36th month of our study with this clone, higher results were observed (164–166 kg ha−1 of K).
Frequently eucalyptus studies under water deficit conditions have been conducted to evaluate the effect of K under water stress conditions. However, this element can also be limiting in environments without water restrictions where soils have low rates of nutrient absorption, are susceptible to leaching, and have K levels below 0.1 cmolc dm−3. For these reasons, studies evaluating conditions of low fertility in sandy soils have been conducted (Silva et al. 2012; Biagiotti et al. 2017). Silva et al. (2012) suggested a single K application in the 3rd month after planting for sandy soils. However, it is necessary to ensure that plants absorb sufficient amounts of K since deficiency symptom may appear later resulting in loss of production. Thus, a nutritional follow-up assessment of a reforestation site in the first 12 months is essential to determine if maintenance fertilization is required.
Conclusion
The growth of the clone Eucalyptus urograndis was above the national average, indicating a good potential for development in the Parana region. Soils with high levels of organic matter and residue litter were able to supply adequate amounts of N required to maintain high growth rates. Our findings complement other studies which reported that N fertilization was not required for a large number of soils in the neighboring state of São Paulo. The requirement for a soluble P source was demonstrated during the initial phase of growth and decreased with time. Phosphorus no longer influenced growth after 2 years in both areas; this may be due to P from RP becoming more available and more extensive exploration of the soil volume. Although additional RP during subsoiling may optimize growth in the 2nd year at a lower cost, more research is required to determine the proper combination of soluble P and RP to insure good growth. Positive response to K fertilization occurred at 18 months in terms of tree height at Jaguariaíva (MTE = 61 kg ha−1 of K). By the 36th month, all variables were influenced by K with an MTE of 166 kg ha−1 of K and volume of 163.1 m3 ha−1. Since soil profiles at both sites had very low levels of K, it is likely that K translocations within trees occurred to meet demand requirements. Considering the low K reserve in these soils, split applications of K fertilizer may be an efficient alternative practice for this region of Brazil.
Overall, our results showed that Eucalyptus urograndis growth was influenced by P and K fertilization but not by N fertilization in the Parana state of Brazil. Results were obtained in years experiencing elevated precipitation patterns, and longer-term evaluations may be required to capture the effects of varying precipitation levels.
References
Almeida JCR, Laclau JP, Goncalves JLD, Ranger J, Saint-Andre L (2010) A positive growth response to NaCl applications in Eucalyptus plantations established on K-deficient soils. For Ecol Manag 259:1786–1795. https://doi.org/10.1016/j.foreco.2009.08.032
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Barreto PAB, Gama-Rodrigues AC, Gama-Rodrigues EF, Barros NF (2012) Nitrogen balance in soil under eucalyptus plantations. R Bras Ci Solo 36:1239–1248. https://doi.org/10.1590/S0100-06832012000400018
Barros NF, Novais RF (1996) Eucalypt nutrition and fertilizer regimes in Brazil. In: Attiwill PM, Adams MA (eds) Nutrition of eucalypts. CISRO, Collingwood, pp 335–356
Barros NF, Neves JCL, Novais RF (2014) Nutrição e adubação mineral do eucalipto. In: Vale AB, Machado CC, Pires JMM, Vilar MB, Costa CB, Nacif AP (eds) Eucaliptocultura no Brasil: silvicultura, manejo e ambiência. SIF, Viçosa, MG, pp 187–208
Bassaco MVM (2018) Nitrogênio, fósforo e potássio no crescimento e nutrição de Eucalyptus urophylla × Eucalyptus grandis em dois sítios no estado do Paraná, Brasil. Dissertation, Federal University of Parana
Battie-Laclau P, Laclau JP, Domec JC et al (2014) Effects of potassium and sodium supply on drought-adaptive mechanisms in Eucalyptus grandis plantations. New Phytol 203:401–413. https://doi.org/10.1016/j.foreco.2016.01.004
Biagiotti G, Valeri SV, Cruz MCP, Vasconcelos RT (2017) Potassium fertilization in plantation of Corymbia citriodora (Hook.) K.D. Hill & L.A.S. Jonhson. Sci For 113:129–137. https://doi.org/10.18671/scifor.v45n113.12
Bizon JMC (2005) Avaliação da sustentabilidade nutricional de plantios de Pinus taeda L. usando um balanço de entrada e saída de nutrientes. Dissertação, Escola Superior de Agricultura Luis de Queiroz (ESALQ/USP)
Bouillet JP, Laclau JP, Arnaud M, M’Bou AT, Saint-André L, Jourdan C (2002) Changes with age in the spatial distribution of roots of Eucalyptus clone in Congo: impact on water and nutrient uptake. For Ecol Manage 171:43–57. https://doi.org/10.1016/S0378-1127(02)00460-7
Caione G, Fernandes FM, Lange A (2013) Residual effect of phosphorus sources in soil chemical properties, nutrition and biomass productivity of sugarcane. Agrária 8:189–196. https://doi.org/10.5039/agraria.v8i2a2016
Dias LPR, Gatiboni LC, Ernani PR, Miquelluti DJ, Chaves DM, Brunetto G (2014) Partial substitution of soluble phosphate by rock phosphate in the planting of Eucalyptus benthamii and Eucalyptus dunnii in southern Brazil. R Bras Ci Solo 38(2):516–523. https://doi.org/10.1590/S0100-06832014000200016
Dias LPR, Gatiboni LC, Brunetto G, Simonete MA, Bicaratto B (2015) Relative efficiency of rock phosphates in fertilization of planting seedlings Eucalyptus dunnii Maiden and Eucalyptus benthamii Maiden et Cambage in soil with and without liming. Ci Flor 25(1):37–48. https://doi.org/10.5902/1980509817443
Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) (2009) Manual de análises químicas de solos, plantas e fertilizantes, 2nd edn. Informação Tecnológica, Rio de Janeiro, p 628
Faria GE, Barros NF, Novais RF, Lima JC, Teixeira JL (2002) Yield and nutritional status of coppiced Eucalyptus stands as affected by potassium applied at planting time. R Árvore 26(5):577–584. https://doi.org/10.1590/S0100-67622002000500008
Fernandez JQP, Dias LE, Barros NF, Novais RF, Moraes EJ (2000) Productivity of Eucalyptus camaldulensis affected by rate and placement of two phosphorus fertilizers to a Brazilian oxisol. For Ecol Manag 127:93–102. https://doi.org/10.1016/S0378-1127(99)00121-8
Figueiredo-Filho A, Machado SA, Miranda ROV, Retslaff F (2014) Compêndio de equações de volume e de afilamento de espécies florestais plantadas e nativas para as regiões geográficas do Brasil. Editora UFV
Gama-Rodrigues EF, Barros NF, Gama-Rodrigues AC, Santos GA (2005) Carbon, nitrogen and activity of microbial biomass in soil under eucalypt plantations. R Bras Ci Solo 29:893–901. https://doi.org/10.1590/S0100-06832005000600007
Gava JL (1997) Efeito da adubação potássica em plantios de E. grandis conduzidos em segunda rotação em solos com diferentes teores de potássio trocável, vol 30. Instituto de Pesquisa Florestal, Piracicaba, pp 89–94
Gonçalves JLM, Stape JL, Laclau JP, Smethurst P, Gava JL (2004) Silvicultural effects on the productivity and wood quality of eucalypt plantations. For Ecol Manag 193:45–61. https://doi.org/10.1016/j.foreco.2004.01.022
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Moller SI, White P (2012) Functions of macronutrients. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, p 651
Industria Brasileira de Árvores (IBÁ) (2016) Relatório indústria brasileira de árvores. Brasília
Junior LS, Garcia JN (2003) Potencial de melhoramento genético em Eucalyptus urophylla procedente da Ilha Flores, vol 64. Scientia Forestalis (IPEF), Piracicaba, pp 23–32
Laclau JP, Ranger J, Goncalves JLD, Maquere V et al (2010) Biogeochemical cycles of nutrients in tropical Eucalyptus plantations main features shown by intensive monitoring in Congo and Brazil. For Ecol Manag 259:1771–1785. https://doi.org/10.1016/j.foreco.2009.06.010
Maeda S, Bognola IA (2011) Effect of liming, Gafsa rock phosphate and triple superphosphate in early growth and phosphorus uptake in Eucalyptus dunnii. Pesq Flor Bras 31(68):355–361. https://doi.org/10.4336/2011.pfb.31.68.355
Maeda S, Bognola IA (2012) Influence of lime and phosphorus in initial growth of eucalyptus and in P critical level. Pesq Flor Bras 32(72):401–407. https://doi.org/10.4336/2012.pfb.32.72.401
Melo EASC, Gonçalves JLM, Rocha JHT et al (2016) Responses of clonal eucalypt plantations to N, P and K fertilizer application in different edaphoclimatic conditions. Forests 7(1):1–10. https://doi.org/10.3390/f7010002
Meyer HA (1953) Forest mensuration. Penns Valley Publisher Inc, State College, p 357
Motta ACV, Barbosa JZ, Consalter R, Reissmann CB (2014) Nutrição e adubação da cultura do pinus. In: Renato de Mello Prado. (Org.). Nutrição e adubação de espécies florestais e palmeiras, 1st edn, vol 1. Gráfica e Editora Santa Terezinha, Jaboticabal, pp 383–425
Novais RF, Smyth TJ, Nunes FN (2007) Fósforo. In: Novais RF, Alvarez VVH, Barros NF, Fontes RLF, Cantarutti RB, Neves JCL (eds) Fertilidade do solo. Sociedade Brasileira de Ciência do Solo, Viçosa, pp 471–537
Pinheiro RC, Deus JC, Nouvellon Y, Campoe OC, Stape JL, Aló LL, Guerrini IA, Jourdan C, Laclau JP (2016) A fast exploration of very deep soil layers by Eucalyptus seedlings and clones in Brazil. For Ecol Manage 366:143–152. https://doi.org/10.1016/j.foreco.2016.02.012
Pulito A, Gonçalves JHT, Smethurst P et al (2015) Available nitrogen and responses to nitrogen fertilizer in brazilian eucalypt plantations on soils of contrasting texture. Forests 6(4):973–991. https://doi.org/10.3390/f6040973
Pulrolnik K, Barros NF, Silva IR, Novais RF, Brandani CB (2009) Carbon and nitrogen pools in soil organic matter under eucalypt, pasture and savanna vegetation in Brazil. R Bras Ci Solo 33(5):1125–1136. https://doi.org/10.1590/S0100-06832009000500006
Rocha JHT, Pietro MR, Borelli KBC, Neves MB (2013) Production and development of Eucalyptus seedlings in function of doses of phosphorus. R. Cerne 19(4):535–543. https://doi.org/10.1590/S0104-77602013000400002
Sampaio TF, Dalcin TE, Bogiani JC, Mori ES, Guerrini IA (2016) Selection of eucalyptus clones and adjustment of potassium doses for extended drought in Bahia Savanna. R Árvore 40(6):1031–1039. https://doi.org/10.1590/0100-67622016000600008
Santana RC, Barros NF, Novais RF, Leite HG, Comerford NB (2008) Nutrient allocation in eucalypt plantations in Brazil. R Bras Ci Solo 32:2723–2733. https://doi.org/10.1590/S0100-06832008000700016
Silva PHM, Pogiani F, Libardi PL, Gonçalves AN (2012) Fertilizer management of eucalypt plantations on sandy soil in Brazil: initial growth and nutrient cycling. For Ecol Manage 30:67–78. https://doi.org/10.1016/j.foreco.2012.10.033
Smethurst P, Holza G, Moronia M, Baillie C (2004) Nitrogen management in Eucalyptus nitens plantations. For Ecol Manage 193:63–80. https://doi.org/10.1016/j.foreco.2004.01.023
Spurr SH (1952) Forest inventory. Ronald Press, New York, p 476
Stahl J (2009) Resposta inicial de Eucalyptus spp. à adubação fosfatada e potássica no Planalto Sul Catarinense. Dissertação, Centro de Ciências Agroveterinária, Universidade Estadual de Santa Catarina (UFSC)
Stahl J, Ernani PR, Gatiboni LC, Chaves DM, Neves CU (2013) Dry matter yield and nutritional efficiency of Eucalyptus benthamii and Eucalyptus dunnii clones due to addition of phosphorus rates to the soil. Ci Florest 23(2):287–295. https://doi.org/10.5902/198050989275
Stape JL, Binkley D, Ryan MG, Fonseca S et al (2010) The Brazil Eucalyptus potential productivity project: influence of water, nutrients and stand uniformity on wood production. For Ecol Manag 259:1684–1694. https://doi.org/10.1016/j.foreco.2010.01.012
Xu D, Dell B, Malajczuk N, Gong M (2002) Effects of P fertilisation on productivity and nutrient accumulation in a E. grandis × urophylla plantation in southern China. For Ecol Manag 16:100–189. https://doi.org/10.1016/S0378-1127(01)00485-6
Xu D, Dell B, Yang Z, Malajczuk N, Gong M (2005) Effects of phosphorus application on productivity and nutrient accumulation of a Eucalyptus urophylla plantation. J Trop For Sci 17:447–461. https://doi.org/10.1016/S0378-1127(01)00485-6
Acknowledgements
Funding was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (BR), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Florestal Vale do Corisco.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bassaco, M.V.M., Motta, A.C.V., Pauletti, V. et al. Nitrogen, phosphorus, and potassium requirements for Eucalyptus urograndis plantations in southern Brazil. New Forests 49, 681–697 (2018). https://doi.org/10.1007/s11056-018-9658-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-018-9658-0