Abstract
The adaptation of teak to acid soils was tested by studying physiological responses of teak clones to varying concentrations of Ca, H (pH) and Al in solution and soil culture. Nutrient solutions containing 0.05, 0.8 or 10 mM Ca were adjusted to pH 4, 5, 6, or 7. Overall, the greatest growth was obtained with nutrient solutions containing 0.8 mM Ca at pH 6. Foliar Ca deficiency symptoms developed at 0.05 mM Ca. Sensitivity of teak clones to Al was tested at pH 4 and 0, 50 or 300 µM Al by staining of roots. Roots that were stained with Chromezurol S (CAS) also stained with hematoxylin, but not necessarily with Eriochrome Cyanine R (ECR). On the other hand, roots that stained with hematoxylin did not necessarily stain with CAS or ECR. This indicates that hydrolysis of Al (as detected by CAS) leads to membrane damage which is detected by hematoxylin. A selection of clones were grown in split pots containing either limed or non-limed soil, and Al activity in the soil solution related to root biomass. Five clones were identified as Al resistant in the solution screening, and two clones were Al resistant in the soil screen, with two clones resisting low pH and Al toxicity in soil and solution screens. This study revealed that some teak clones tolerate low pH soils and moderately toxic 25 µM {Al3+} activity, and may be suitable for poor soils, but soils used in Australia for teak plantations are often too hostile and would require amelioration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Teak (Tectona grandis Linn. f.) originated in tropical and subtropical South and South-East Asia, and is widely planted elsewhere in the tropics and subtropics for its high value timber. Teak has a requirement for well drained and deep soils, preferably derived from shale, limestone or igneous material (Kaosa-ard 1998; Tanaka et al. 1998). These soils tend to be rich in base cations, especially Ca, Mg and K, and are acidic to slightly alkaline (Tanaka et al. 1998). Teak growth is poor in acidic topsoils (pH < 4.3) and low Ca saturation (Fernandez-Moya et al. 2015; Zech and Drechsel 1991). It has been suggested that teak requires >pH 4.7 (measured in 0.01 M CaCl2) for optimum growth (Craven et al. 2007; Drechsel and Zech 1994; Zech and Drechsel 1991). In addition, teak responds well to ample supplies of P, B and N (Craven et al. 2007; Drechsel and Zech 1991, 1994; Zhou et al. 2012). The response of teak to acid soils is similar to the response of Populus sp. which is also impaired at low pH due to possible Ca deficiency and Al toxicity (Hjelm and Rytter 2016).
Teak forestry plantations in northern Australia, and elsewhere, are often established on poorly fertile land because it is cheap to acquire. While it would be better to select species better adapted to these soil conditions, the market demand results in teak being planted in poorly-fertile soils, requiring the selection of clones that can tolerate the limitations present in these soils. In the area around Ingham and Tully, northern Queensland, virgin soils range from pH 3.8 to pH 5.1 in the topsoil (Congdon and Herbohn 1993; Gillman 1984). These soils are potentially affected by Ca-deficiency and Al-toxicity due to dissolution of Al-minerals at low pH (von Uexküll and Mutert 1995). Since trivalent aluminium (Al3+) is highly rhizotoxic, root growth may be decreased (Horst et al. 2010), leading to poor water and nutrient utilization in the field. Often subsoils may be acidic and this prevents the establishment of an extensive and deep root system which is required in forest trees for anchorage and water uptake during the dry season. The toxic effect of protons and Al can be partially alleviated by Ca2+ ions which stabilise the plasma membrane and the cell wall, and act as an intracellular secondary messenger (Kinraide 2003; White and Broadley 2003). A deficiency of Ca ions generally leads to leaf necrosis and die-back of the apical and root meristems (White and Broadley 2003), and increases susceptibility to low pH stress.
Several indicators of Al toxicity risk have been proposed, such as Al saturation, Al concentration or activity (van Schöll et al. 2004), and the Ca/Al ratio (Cronan and Grigal 1995; Lu and Sucoff 2003; Vanguelova et al. 2007). For instance, virgin soils in the Tully and Ingham areas in Northern Queensland have charge-based Ca/Al ratios ranging from 0.1 to 1.0 in the topsoil, and Al saturations ranging from 10 to 75% (Congdon and Herbohn 1993; Gillman 1984).
Acid soil infertility can be overcome by increasing the Ca status of soil or increasing pH. This can be achieved either by applying lime which raises pH and Ca concentration, and lowers Al solubility, or by adding soluble Ca forms (Ca sulfate, Ca chloride) to overcome Ca deficiency in roots (Carvalho and van Raij 1997). As an alternative or complimentary approach, clones can be selected which are more tolerant towards acidic soils low in Ca and potentially high in soluble Al (Smith et al. 2011). The availability of clones allows assessment of the influence of various environmental parameters on growth characteristics in the absence of any unexpected genotypic interference (Monteuuis and Goh 1999).
Identification of more acid-soil tolerant clones is often achieved by either measuring root growth in response to various Ca, H and Al concentrations, or by determining changes in biomass accumulation over time (Blamey et al. 2005; Narasimhamoorty et al. 2007). In this study, we determined the biomass, physiological, and histological responses of teak clones to ranges of Ca, H and Al concentrations in nutrient solutions and in soils under controlled environmental conditions. This study tested the hypothesis that there are genetic differences among teak clones to conditions of low pH, low Ca, and high soluble Al encountered in leached tropical soils.
Materials and methods
Tissue-culture propagated bare-rooted teak plantlets were obtained from Thai Orchid Labs Co Ltd. (Bangkok, Thailand). These clones represent superior trees identified in the field in Thailand, and subsequently introduced into tissue culture. The plantlets were transferred to aerated nutrient solution (1/7-strength Murashige-Skoog medium) and gradually hardened-off in a controlled temperature glasshouse (28 °C day, 23 °C night) under 70% shadecloth until plants showed signs of resuming growth. Thereafter, the plantlets were transferred to higher temperature (33 °C day, 28 °C night) under ambient light, until eventually, 14 clones (labelled clone A to clone N) were available in sufficient numbers for experimental work.
The first experiment investigated the response of teak clones to pH and Ca in solution culture. Twelve treatments were applied, consisting of three Ca concentrations (0.05, 0.8 and 10 mM CaCl2) and four pH values (pH 4.0, 5.0, 6.0 and 7.0). Between 5 and 10 plants (10–18 cm tall) of each clone were grown per treatment in 7 L aerated nutrient solutions containing the following macronutrients (concentrations expressed in mM): NH4 + (2.1), NO3 − (3.9), PO4 3− (0.14), SO4 2− (1.1), K+ (2.3), and Mg2+ (0.16). The micronutrients (concentrations in µM) consisted of Zn (2.5), B (20.0), Co (0.01), Cu (0.1), Mn (15.0), Mo (0.15) and Fe (13.0). The nutrient solution also contained 1 mM MES buffer to minimise pH fluctuations. The pH of the complete nutrient solutions was adjusted with HCl or Ca(OH)2 every second day and the nutrient solutions were replaced weekly. Plants were randomly assigned to the 12 treatments and each tub with nutrient solution contained an equal number of plants of each clone. Treatments were arranged in a complete randomized block design and tubs were randomly re-arranged in the glasshouse on a weekly basis.
The fresh weight of each individual plant was recorded at the beginning (day 0) and end (day 25) of the experiment. The relative growth rate of plants was calculated from the log-transformed weights (Hoffmann and Poorter 2002). The experiments were repeated twice. Data were analysed by PROC GLM using SAS V7.1 (2015). Main effects (Ca, pH, and clones) and their interactions were tested and treatment means were compared with Student’s t test with a probability level of P < 0.05. Residual analyses were performed to confirm the adequacy of the statistical model and adherence to the assumptions of the ANOVA.
At the conclusion of the experiment, plants were transferred to tubs with fresh complete nutrient solutions and grown for 10 month before plants were re-used for the second experiment.
In the second experiment, the effect of three Al concentrations on root injury of teak clones was investigated in solution culture. Teak clones were grown in 40 L solutions containing 1 mM CaCl2 and 5 µM H3BO3 since both Ca and B are required for meristem function. The aerated solutions were adjusted to pH 4.0 ± 0.1 with HCl or NaOH (no MES buffer was added). Following pH adjustment, three Al treatments (0, 50, and 300 µM as AlCl3) were added (addition of Al solutions did not change the solution pH). Thereafter, the plants were transferred to the treatment solutions which were replaced every second day. Each tub contained the same number of plants of each genotype and plants were of similar size.
At each of the sampling days (1, 3 and 7 days after transfer), the pH of the fresh and spent treatment solutions was measured and the total ions in solution determined after filtering to 0.22 µM by inductively coupled plasma optical emission spectroscopy. Changes in nutrient concentrations (µmol L−1 day−1) were summed over the 7 days (the duration of the trial) and subjected to an ANOVA (PROC GLM in SAS V7.1) with changes in Aluminium, Calcium, Potassium and Phosphate as main effects. Treatment means were compared with Student’s t test and differences at P > 0.05 were considered significant. The nutrient concentrations in the Al-free control were subtracted from the nutrient concentrations in the Al solutions, and the experiment was repeated twice.
Lateral roots (up to 20 mm long) from each plant of each clone and treatment were collected at day 1, day 3 and day 7. The roots were soaked in deionised water for 12–24 h at 4 °C to desorb weakly bound Al and divided into three batches containing 4–6 roots. The roots in each batch were stained for 24 h at 4 °C with either Eriochrome Cyanine R (ECR) (0.05%), Chromezurol S (CAS) (0.05%) or hematoxylin (0.2%), and de-stained for 1–2 days with deionised water at 4 °C. These dyes specifically react with Al in plant roots (more detail in “Discussion” section). Previous experiments had indicated that the staining was stable using this procedure. Roots were evaluated for the extent and severity of staining with each stain, and possible root abnormalities (e.g. kinks or ruptures), under 40× magnification with a microscope with both light and dark-field illumination. Scores were given based on extent of staining (1: 0–2 mm region stained; 2: 0–10 mm region stained) and severity of staining (0: no staining; 1: weak staining; 2: strong staining) for each stain and a composite score was calculated by multiplying the score for “extent of staining” by the score for “severity of staining”. Thus, roots could attain composite scores of 0–4, with a higher score indicating greater staining response and thus greater Al sensitivity. The overall score for each clone was then calculated by averaging the composite score for all stains and replicates for each time and concentration datum point. The staining score was evaluated by one operator to minimize subjectivity in evaluation. Furthermore, by using limited categories (no staining, weak staining and strong staining) the likelihood of ambiguous classification was avoided. The experiment was repeated twice. Average scores were analysed by PROC GLM of SAS and the least significant differences calculated for comparisons at the 5% level of probability.
The activity of Al3+ in the 45 and 300 µM [Al] solution in the presence of 1 mM Ca was calculated as 26 and 145 µM, respectively, using Phreeqci (Parkhurst and Appelo 1999). The calculated speciation of Al at both activities was 96% Al3+ and 7% AlOH2+. The timed ferron assay (Parker and Bertsch 1992) was used to establish that polymeric Al species corresponding to Al13 were not formed in the treatment solutions.
In the third experiment, a selection of clones (based on uniformity of plant material and relative sensitivity towards Al in solution) was used for a split pot experiment to test if the results observed in solution culture could be confirmed in soil culture. Plants were grown with half the roots in ameliorated soil (control) and the other half of the root system in high Al soil. To achieve this, teak plants were removed from solution culture, and potted in soil into plastic tubes (15 cm internal diameter by 17 cm length), whose bottom was temporarily closed with an acrylic plastic sheet. The soil was a Ferrosol (Oxisol) (Table 1) amended with a complete fertilizer (Miracle Grow All Purpose; Scotts Company USA), and incubated at field water capacity for 14 d before use. Split pots were prepared by placing a plastic septum (15 cm × 17 cm) vertically down the middle of another piece of plastic tube. One half of the tube was filled with the Ferrosol amended with Al2(SO4)3.16 H2O to pH 4.1 at −1 kPa suction (pH 3.7 in 1:5 water), whereas the other half contained the same AlSO4-amended Ferrosol limed with Ca(OH)2 to pH 5.1 at −1 kPa (pH 4.8 in 1:5 water) (Table 1). The limed soil had a low cation exchange capacity (3 cmol kg−1) with 71% Ca saturation and 5.6% Al saturation, whereas the non-limed soil had 29% Ca saturation and 27% Al saturation. The bottom of the pot was enclosed in fine nylon mesh to prevent loss of the soil. Hollow-fibre solution samplers (Menzies and Guppy 2000) were inserted in the middle of each half of the split-pot. This permitted non-destructive sampling of the soil solution at realistic soil water content.
After establishment of the plants in the original plastic tubes (6 weeks), the tubes with plants were placed on top of the split pots. The stacks of the two tubes were then placed randomly on a constant water-table (suction of −1 kPa at the height of the solution samplers) in a controlled temperature glasshouse at 33 °C. The composition of the soil solution extracted from each half of the bottom pot was determined by inductively coupled plasma optical emission spectroscopy and the activity of Al ({Al3+}) calculated with Phreeqci (Parkhurst and Appelo 1999). After growth for 5 weeks, the top tube was separated from the bottom tube with a knife. Each half of the bottom tube was removed, and the roots washed free from soil on a stainless steel screen, dried and weighed.
Results
Growth response of teak to pH and Ca
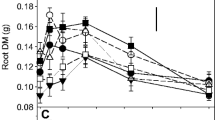
Averaged across all clones, there was a significant Ca × pH interaction (ANOVA, P < 0.0001). At pH 6, maximal growth of 0.027 g g−1 day−1 was observed with 0.8 mM Ca, decreasing significantly to 0.018 g g−1 day−1 with 0.05 mM Ca, and to 0.011 g g−1 day−1 with 10 mM Ca (Fig. 1). At pH 4 with 0.05 and 0.8 mM Ca, the growth rate ranged from 0.012 to 0.014 g g−1 day−1, but 10 mM Ca significantly decreased the growth rate to 0.008 g g−1 day−1 (Fig. 1). The growth rate at pH 5 ranged from 0.009 to 0.013 g g−1 day−1 and was not significantly affected by the Ca concentration. At pH 7, the growth rate with 0.05 mM and 0.8 mM Ca concentration were similar (0.017–0.018 g g−1 day−1) and growth was lower at 10 mM Ca (0.011 g g−1 day−1) but differences were not significant.
There were clonal differences in response to the Ca and proton concentrations (Fig. 2). At 0.05 mM Ca and pH 4, clones B, M and N grew poorly (0.005–0.007 g g−1 day−1), whereas clones C, D, J and L grew three times faster (~0.017 g g−1 day−1) (Fig. 2a). At pH 5 and 0.05 mM Ca, clones B and N grew poorly (0.004–0.006 g g−1 day−1), whereas clones C, D, F and M grew 3–4 times faster (0.016–0.020 g g−1 day−1). At pH 6, clones G and N grew poorly (0.015 g g−1 day−1), while clone B grew best (0.03 g g−1 day−1). At pH 7, clone I grew poorly (0.008 g g−1 day−1), but clones B, C, F and M grew well (~0.025 g g−1 day−1). At 0.05 mM Ca, the youngest fully expanded leaves developed interveinal chlorosis which progressed to necrosis (Fig. 3), however, within in the timeframe of the trial (6 weeks), no death of the shoot tip meristem was observed, but roots turned brown and stopped growing.
Mean relative growth rate of whole plants of 14 commercial teak (Tectona grandis) clones in nutrient solutions adjusted to pH 4, 5, 6 or 7, and containing a 0.05 mM Ca, b 0.8 mM Ca or c 10 mM Ca grown in a glasshouse. Mean values are based on at least five plants, with the standard error of the mean shown, if not obscured
At 0.8 mM Ca and pH 4, clones C and K grew poorly (0.006–0.008 g g−1 day−1), whereas clones E, I and N grew three times faster (~0.019 g g−1 day−1) (Fig. 2b). At pH 5, clones A, H and N grew poorly (0.005–0.007 g g−1 day−1), and clones C and I grew well (0.020 g g−1 day−1). At pH 6, clones G, H, and N grew poorly (0.020 g g−1 day−1), whereas most clones grew well, especially clone C (0.053 g g−1 day−1). At pH 7, clone A grew poorly (0.008 g g−1 day−1) but clones C and D grew well (~0.024 g g−1 day−1) (Fig. 2b). At 0.8 mM Ca, there were no visible symptoms of nutrient imbalances observed on the foliage over the tested pH range.
With 10 mM Ca in solution, the growth rates averaged across clones were significantly lower than with 0.8 mM Ca (compare Fig. 2b, c) possibly due to leaf drop of the oldest leaves which resulted in lower recorded fresh weights. At pH 4, clones A, K, and N grew poorly (0.005 g g−1 day−1), whereas clones B, F, and I grew three times faster (~0.017 g g−1 day−1) (Fig. 2c). At pH 5, clones A, G and J grew poorly (~0.008 g g−1 day−1) and clones B, F and M grew twice as fast (0.016 g g−1 day−1). At pH 6, clones J, K and L performed poorly (0.010 g g−1 day−1), but clones C, F and M were growing well (0.035 g g−1 day−1). At pH 7, clones G and K grew poorly (0.010 g g−1 day−1), whereas clones B, F, I and M grew well (~0.03 g g−1 day−1).
Response of teak roots to aluminium in solution
Exposing roots of intact teak plants to solutions containing 26 and 145 µM {Al3+} for up to 7 days resulted in an average decrease of Al in the solution by 19 ± 2 µM (equivalent to 11 µM {Al3+}). Addition of 26 or 145 µM {Al3+} to solutions containing 1 mM Ca and 5 µM B induced average release of 73 ± 15 µM Ca, 62 ± 19 µM K and 13 ± 3 µM P over 7 days from the roots into the solution (other ions were not measured here). Interestingly, the amount of Ca, K and P released from roots did not differ significantly between 26 µM Al and 145 µM Al additions. While root growth is a sensitive measure of Al sensitivity, it is difficult to measure in species with highly branched root systems. Therefore, we used staining to evaluate Al sensitivity of the teak clones.
Small primary and secondary lateral roots (10–20 mm long) collected from plants exposed for 3 and 7 days to the Al solutions were stained with the Al−specific dyes hematoxylin, CAS and ECR. Roots treated with Al stained purple with CAS and hematoxylin, and magenta with ECR. Untreated roots (control) did not stain (results not shown). An increase in solution Al concentration resulted in more clones staining and the staining response becoming more severe (Table 2). The root tips (0–2 mm) most often developed a colour reaction with stains, and in severely intoxicated roots, the whole apical part (0–10 mm) developed colour. We did not observe any morphological abnormalities (e.g. kinks or cracks) on the roots in response to the Al treatment.
In solution culture, clones with least staining were clones A, B, I, N and K (Table 2), therefore, these clones were considered to be resistant to 26 µM {Al3+}. The most Al-sensitive clones were C, E, H, and M, which gave a strong staining reaction in response to 26 µM {Al3+}. Clones G and H were sensitive to 26 µM {Al3+} within the first day, but developed resistance from day 3 onwards. Clones D, F, G, H, and I were more resistant to 145 µM {Al3+} but they all stained with the dye, albeit at a lower level than sensitive clones (Table 2). Clones A, B and K were sensitive to 145 µM {Al3+} but these clones were resistant to 26 µM {Al3+}.
Screening of teak in acid soil
A selection of clones with similar size and identified as either Al-sensitive or Al-resistant in solution culture were re-screened in split pots containing limed and non-limed soil. The calculated activity of Al in the soil solution of the acidic soil (non-limed) increased with decreasing soil pH (Fig. 4), and ranged from 20 to 25 µM {Al3+}, similar to the concentration used in the solution screening (26 µM {Al3+}), but the Al activity of 145 µM {Al3+} used in the solution screening could not be attained in the soil screening. With increased Al activity in the soil solution, root biomass decreased from 1.4 g at 0 µM {Al3+} to 0.1 g at 30–40 µM {Al3+} (Fig. 5), but there were clear clonal differences in root growth. At 20–25 µM {Al3+} in soil solution, clones D and I increased or maintained root growth relative to the limed control (Fig. 6). At an Al activity of 9 µM, clones E and M grew poorly compared to the control. Poorest growth was observed with clone G but this clone also experienced the highest Al3+ activity (33 µM).
Effects of soil solution pH on calculated Al3+ activity in the split pots. Soil solution extracts at −1 kPa were collected with hollow fibre solution samplers and the Al activity calculated from the measured ion concentrations using the Phreeqci (Parkhurst and Appelo 1999) software (solid circles). The dashed line represents the Al activity calculated from the solubility of gibbsite (Ksp 10−34)
Root dry weight after 5 weeks of growth on limed and non-limed soil as function of the Al3+ activity of the soil. The Al activity was calculated with the Phreeqci software (Parkhurst and Appelo 1999) from the composition of the soil solution
Average relative root growth of six teak clones on limed and non-limed soil. Root growth (shaded bars) in the non-limed soil was expressed relative to growth in the limed soil (horizontal dashed line). The filled circles represent the calculated Al activity in the non-limed soil. Values are the average of 2–4 replicates with the standard errors shown
Discussion
Growth response of teak to pH and Ca
The pH and Ca requirement of teak was determined first because H and Ca can affect Al toxicity and interfere with screening (Kinraide 2003; Rangel et al. 2005). Best growth was observed at pH 6 and 0.8 mM Ca, with growth decreased by up to 40% at pH 4 and pH 7 (Fig. 1). A Ca concentration of 0.05 mM resulted in deficiency symptoms (interveinal chlorosis progressing to necrosis and root meristem death) (Fig. 3), in agreement with symptoms observed by Barroso et al. (2005). Therefore, teak requires at least 0.8 mM Ca in solution, which is a concentration suitable for many plants (Inoue et al. 1988; White and Broadley 2003). It can be concluded that teak has a moderate Ca requirement (0.8 mM) and requires slightly acidic pH (pH 6) for optimum growth, confirming results obtained by Tanaka et al. (1998).
There were clonal differences in Ca and pH requirement (Fig. 2). Clone C grew well at pH 5–7 and 0.8 mM Ca and had a maximum growth rate of 0.05 g g−1 day−1, but performed poorly at pH 4. Clones C, D, E and L were suitable for 0.05 mM Ca and a range of pH values. Therefore, selection of clones is an approach to overcome site-specific limitations. Optimally, the results from this glasshouse study need to be confirmed by field trials.
Decreased growth at low pH or high pH can be attributed to suppressed transpiration and stomatal conductance (Kang et al. 2011; Yan et al. 1992). Growth reduction with 10 mM Ca may be due to ionic stress, possibly resulting in accumulation of Ca-oxalate in leaves (White and Broadley 2003) and leaf drop. This possibility needs to be confirmed by further investigations in the glasshouse and in the field.
Response of teak roots to aluminium in solution
Roots were stained with the dyes CAS, ECR and hematoxylin to assess their response to Al in solution. Five clones (A, B, I, N, and K) gave very low staining in response to 25 µM {Al3+} (corresponding to 45 µM Al in solution), indicating that these clones are comparably Al-resistant, but no clones were resistant to 145 µM {Al3+}.
The three stains have different modes of action: Hematoxylin is widely used for measuring Al sensitivity of plants, and relies on the formation of an Al-phosphate-hematoxylin complex in the root. The phosphate required for complex formation is released from plant roots due to Al stress as the plasma membrane becomes permeable to phosphate (Ownby 1993). Thus, intensity of hematoxylin staining indicates the extent of membrane damage caused by Al, but not the concentration of Al bound. For instance, roots may have the same staining response but one species limits membrane damage despite binding a lot of Al in the cell wall, whereas another species has high membrane damage despite binding less Al in the cell wall. The dye ECR forms a coloured complex with Al ions and indicates the quantity of Al bound in the roots. The rationale of this dye is that plants with high Al sensitivity have more Al bound in roots, therefore Al sensitive plants are considered to stain more strongly with ECR (Wang et al. 2006). The dye CAS reacts with Al and hydrolytic Al species such as AlOH, and indicates the amount of Al bound and the extent of Al hydrolysis (Wehr et al. 2010a). Hydrolysis of Al in the cell wall affects ion homeostasis in the cell wall and cytoplasm (Garzon et al. 2011) and results in the formation of polymeric Al species such as Al13 7+ which is more toxic than Al3+.
Overall, we observed that if roots stain with CAS, they also stain with hematoxylin (data not shown). Likewise, if roots stain with ECR, they also stain with hematoxylin. Yet, if roots stain with hematoxylin they do not necessarily stain with ECR or CAS. This is due to the fact that the three stains detect different biochemical pathways of Al sensitivity in roots. The three stains differentiate between total Al bound (ECR), Al hydrolysis (CAS) and Al-induced membrane damage (hematoxylin). If roots bind high concentrations of Al (as detected by ECR), Al toxicity and membrane damage is likely (as detected by hematoxylin). On the other hand, low concentration of Al can cause membrane damage in sensitive species (reaction with hematoxylin), but the Al concentration bound to the cell wall is too low to be detected with ECR. If Al bound to cell walls undergoes hydrolysis (as detected by CAS), membrane damage is likely to occur (as detected by hematoxylin), but membrane damage does not necessarily lead to hydrolysis of Al in the cell wall, therefore roots may stain with hematoxylin, but not with CAS. Likewise, a high concentration of Al bound to cell walls (and thus detected by ECR) will lead to Al hydrolysis, which can be detected by CAS. But Al hydrolysis can occur at low Al concentrations (depending on the solution pH), which can be detected by CAS, but the sensitivity of ECR is not sufficient to detect low Al concentrations.
We consider hematoxylin to be the most suitable dye since it detects membrane damage which is the best predictor of root growth inhibition. Similarly, Choudhary et al. (2011) found that hematoxylin was the most suitable stain to screen Al-resistant genotypes and stated that there is good agreement between hematoxylin staining and plant growth in acid soil. The short term (1–3 days) effect of Al can be attributed to stress and activation of Al resistance mechanisms. The response of plants after 7 days is more representative of the situation encountered by field-grown plants which are exposed to Al stress throughout their life-cycle.
Addition of 26 or 145 µM {Al3+} to solutions with 1 mM Ca and 5 µM B at pH 4 resulted in a net release of 73 µM Ca, 62 µM K and 13 µM P from the roots over 7 days while the solution Al concentration decreased by 19 µM and pH decreased by 0.1–0.3 units (data not shown). Interestingly, the release of ions and adsorption of Al was not affected by the Al concentration in the solution. It appears that 26 µM {Al3+} is sufficient to cause membrane damage and leakage, and the damage did not increase appreciably with increasing Al concentration (Wehr et al. 2010b). Leakage of protons and K in response to low pH or Al was also observed in Arabidopsis (Bose et al. 2010a, b). We observed differences in Al sensitivity of teak clones based on the staining response of roots grown in Al solutions. Yet, total biomass accumulation in solution culture did not show clear treatment differences (data not shown). Thus, biomass can be misleading since water and nutrients are transported to roots by mass flow in the aerated solution. By contrast, Al toxicity in soil grown plants will decrease the size of the root system, which will profoundly affect water and nutrient uptake from the soil, and results in greater biomass decrease than those observed in solution culture.
Screening of teak in acid soil
Root biomass in acid soil decreased with increasing Al3+ activity due to increasing Al-toxicity (Fig. 5). There were genotypic differences in root biomass production in response to Al (Fig. 6). Clones D and I experienced similar Al activity in the non-limed soil (22–25 µM) and maintained their root biomass relative to the limed control. Hence, these clones can be considered resistant to 22–25 µM {Al3+}. In contrast, clones E and M are Al sensitive with 8–9 µM {Al3+} decreasing root growth by 25–45% compared to the limed soils. Clone B grew well in the non-limed soil but had only a low Al activity (8 µM), whereas clone G had a high Al activity (33 µM) and grew poorly in acid soil. Since the Al activity in soil solution could not be maintained at a constant level, it is more difficult to rank genotypes B, E, G, and M in the soil screening. Clones B, E, and M experienced less than 10 µM {Al3+}, and this resulted in good growth in clone B which appeared to grow better than clone D which experienced a fourfold greater Al activity. Clones E and M grew poorly despite the soil solution Al being 5–10 µM Al. Therefore, these two clones appear very Al sensitive. Clone G also grew poorly, but it experienced 35 µM {Al3+} which would have inhibited growth.
Screening both in solution and soil identified clones D and I as resistant up to 26 µM {Al3+}, whereas clones E and M where identified as Al sensitive. Discrepancies for the other clones are possibly due to the soil solution concentration not being maintained at the same levels as in solution screening. Furthermore, since the unstirred layer around a root cortex is likely to be greater in soil than in aerated solution, less organic acid exudation is necessary to neutralize Al ions in the soil-grown roots. Consequently, soil screening will identify more Al-resistant lines than solution screening. Yet, advantages of solution screening are that more plants can be tested, and the activity of Al ions in solutions can be readily maintained, permitting ranking of genotypes at a given Al activity. The correlation between results from solution screening and soil screening for Al resistance appears to be species dependent (de Wit et al. 2010; Scott et al. 2008). Soil strength does also play a role since high soil strength can trigger an increased organic acid exudation from the roots and the organic acids can indirectly complex Al ions and alleviate Al toxicity (Horst et al. 1990; Nguyen 2003). By contrast, Haling et al. (2011) suggested that Al-resistant lines are more Al sensitive in high-strength soils. Therefore, it is necessary to confirm results obtained from the solution screen in soil.
The molar Ca/Al ratio in the limed soil was 19.3, and 1.6 in the non-limed soil (Table 1), which resulted in Al toxicity. Indeed, Zhou et al. (2012) found that the molar ratio of exchangeable Ca/Al of <2.5–6.5 inhibited teak growth in their pot trial. Obviously, there will be differences when the ratio is expressed based on solution Ca/Al concentrations or exchangeable Ca/Al concentrations. Interestingly, the exchangeable Ca/Al ratios of a range of soils used for teak plantations in the greater Ingham area of North Queensland range from 0.6 to 5, suggesting that a number of the soils are unsuitable for teak plantations without extensive soil amelioration such as liming and/or gypsum application to lower the exchangeable Al concentration and increase the Ca concentration.
Conclusion
Teak clones differed in their growth response to pH and Ca in nutrient solutions. Six clones (E, G, I, J, L and N) could be identified which tolerated pH 4 and a moderate (0.8 mM) Ca concentration and five clones (C, D, E, J and L) tolerated pH 4 and low (0.05 mM) Ca. Overall, the 14 teak clones tested grew best at pH6 with 0.8 mM Ca. Screening of teak clones using Al staining, revealed five clones (A, B, I, N, and K) which resisted 25 µM {Al3+} (corresponding to a concentration of 45 µM Al in solution), but no clones were resistant to 145 µM {Al3+}. Further screening of clones in split pots containing Al-toxic and limed soil, indicated that clones D and I were tolerant to 20–25 µM {Al3+} in soil. Thus, clones D and I were most resistant to pH 4 and Al toxicity, as assessed by biomass, staining and root growth tests. Since plants are ultimately grown in the field in soil, we consider soil screening yielding definite results, whereas solution screens permit rapid screening of a large number of genotypes under controlled conditions and the most promising clones can then be further screened in split-pot trials and results confirmed in field trials. The applicability of screening has already been demonstrated by Moya and Marin (2011) for selection of teak clones with superior wood quality in response to soil and site factors.
Abbreviations
- CAS:
-
Chromeazurol S
- ECR:
-
Eriochrome cyanine R
References
Barroso DG, Figueiredo FAMMdA, Pereira RdC, Mendonca AVR, Silva LdC (2005) Macronutrient deficiency diagnosis in teak seedlings. Rev Arvore 29:671–679
Blamey FPC, Nishizawa NK, Yoshimura E (2005) Digital microscopy: a useful technique for measuring root elongation in solution. Soil Sci Plant Nutr 51:705–708
Bose J, Babourina O, Shabala S, Rengel Z (2010a) Aluminium-induced ion transport in Arabidopsis: the relationship between Al tolerance and root ion flux. J Exp Bot 61:3163–3175
Bose J, Babourina O, Shabala S, Rengel Z (2010b) Aluminum-dependent dynamics of ion transport in Arabidopsis: specificity of low pH and aluminum responses. Physiol Plant 139:401–412
Carvalho MCS, van Raij B (1997) Calcium sulphate, phosphogypsum and calcium carbonate in the amelioration of acid subsoils for root growth. Plant Soil 192:37–48
Choudhary AK, Singh D, Kumar J (2011) A comparative study of screening methods for tolerance to aluminum toxicity in pigeonpea Cajanus cajan (L.) Millspaugh. Aust J Crop Sci 5:1419–1426
Congdon RA, Herbohn JL (1993) Ecosystem dynamics of disturbed and undisturbed sites in north Queensland wet tropical rain forest. I. Floristic composition, climate and soil chemistry. J Trop Ecol 9:349–363
Craven D, Braden D, Ashton MS, Berlyn GP, Wishnie M, Dent D (2007) Between and within-site comparisons of structural and physiological characteristics and foliar nutrient content of 14 tree species at a wet, fertile site and a dry, infertile site in Panama. For Ecol Manag 238:335–346
Cronan CS, Grigal DF (1995) Use of calcium/aluminum ratios as indicators of stress in forest ecosystems. J Environ Qual 24:209–226
de Wit H, Eldhuset T, Mulder J (2010) Dissolved Al reduces Mg uptake in Norway spruce forest: results from a long-term field manipulation experiment in Norway. For Ecol Manag 259:2072–2082
Drechsel P, Zech W (1991) Foliar nutrient levels of broad-leaved tropical trees: a tabular review. Plant Soil 131:29–46
Drechsel P, Zech W (1994) DRIS evaluation of teak (Tectona grandis L.f.) mineral nutrition and effects of nutrition and site quality on teak growth in West Africa. For Ecol Manag 70:121–133
Fernandez-Moya J et al (2015) Soil fertility characterisation of teak (Tectona grandis L.f.) plantations in Central America. Soil Res 53:423–432
Garzon T, Gunse B, Moreno A, Tomos AD, Barcelo J, Poschenrieder C (2011) Aluminium-induced alteration of ion homeostasis in root tip vacuoles of two maize varieties differing in Al tolerance. Plant Sci 180:709–715
Gillman GP (1984) Using variable charge characteristics to understand the exchangeable cation status of oxic soils. Aust J Soil Res 22:71–80
Haling R, Simpson R, Culvenor R, Lanbers H, Richardson A (2011) Effect of soil acidity, soil strength and macropores on root growth and morphology of perennial grass species differing in acid-soil resistance. Plant, Cell Environ 34:444–456
Hjelm K, Rytter L (2016) The influence of soil conditions, with focus on soil acidity, on the establishment of poplar (Populus spp.). New For. doi:10.1007/s11056-016-9541-9
Hoffmann W, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 80:37–42
Horst WJ, Klotz F, Szulkiewicz P (1990) Mechanical impedance increases aluminum tolerance of soybean (Glycine max) roots. Plant nutrition—physiology and applications, vol. 41. Developments in plant and soil sciences, vol 41. Kluwer Academic Publ, Dordrecht, pp 351–355
Horst WJ, Wang Y, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot 106:185–197
Inoue K, Yokata H, Yamada Y (1988) Effect of Ca in the medium on root growth under low pH conditions. Soil Sci Plant Nutr 34:359–374
Kang Y, Park J, Kim S, Kang N, Park K, Lee S, Jeong B (2011) Effects of root zone pH and nutrient concentration on the growth and nutrient uptake of tomato seedlings. J Plant Nutr 34:640–652
Kaosa-ard A (1998) Overview of problems in teak plantation establishment. In: Kashio M, White K (eds) Teak for the future-proceedings of the second regional summary on teak. FAO Regional Office for Asia and the Pacific, Bangkok, p 249
Kinraide T (2003) Toxicity factors in acidic forest soils: attempts to evaluate separately the toxic effects of excessive Al3+ and H+ and insufficient Ca2+ and Mg2+ upon root elongation. Eur J Soil Sci 54:323–333
Lu E-Y, Sucoff EI (2003) Responses of quaking aspen seedlings to solution calcium and aluminum. J Plant Nutr 26:97–123
Menzies N, Guppy C (2000) In-situ soil solution extraction with polyacrylonitrile hollow-fibers. Commun Soil Sci Plant Anal 31:1875–1886
Monteuuis O, Goh DKS (1999) About the use of clones in teak. Bois For Trop 261:28–38
Moya R, Marin JD (2011) Grouping of Tectona grandis (L.f.) clones using wood color and stiffness. New For 42:329–345
Narasimhamoorty B, Blancaflor EB, Bouton JH, Payton ME, Sledge MK (2007) A comparison of hydroponics, soil, and root staining methods for evaluation of aluminum tolerance in Medicago truncatula (barrel medic) germplasm. Crop Sci 47:321–328
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23:375–396
Ownby JD (1993) Mechanisms of reaction of hematoxylin with aluminium-treated wheat roots. Physiol Plant 87:371–380
Parker DR, Bertsch PM (1992) Identification and quantification of the “Al13” tridecameric polycation using ferron. Environ Sci Technol 26:908–914
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2)—a computer program for speciation, batch reaction, one-dimensional transport, and inverse geochemical calculations. United States Geological Survey, Denver
Rangel AF, Mobin M, Rao IM, Horst WJ (2005) Proton toxicity interferes with the screening of common bean (Phaseolus vulgaris L.) genotypes for aluminium resistance in nutrient solution. J Plant Nutr Soil Sci 168:607–616
Scott BJ, Ewing MA, Williams R, Humphries AW, Coombes NE (2008) Tolerance of aluminium toxicity in annual Medicago species and lucerne. Aus J Exp Agric 48:499–511
Smith E, Naik D, Cumming JR (2011) Genotypic variation in aluminum resistance, cellular aluminum fractions, callose and pectin formation and organic acid accumulation in roots of Populus hybrids. Environ Exp Bot 72:182–193
Tanaka N, Hamazaki T, Vacharangkura T (1998) Distribution, growth and site requirements of teak. JARQ 32:65–77
van Schöll L, Keltjens WG, Hoffland E, van Breemen N (2004) Aluminium concentration versus the base cation to aluminium ratio as predictors for aluminium toxicity in Pinus sylvestris and Picea abies seedlings. For Ecol Manag 195:301–309
Vanguelova EI et al (2007) Tree fine root Ca/Al molar ratio—indicator of Al and acidity stress. Plant Biosyst 141:460–480
von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. In: Date RA, Grundon NJ, Rayment GE, Probert ME (eds) Plant soil interactions at low pH. Kluwer Academic, Dordrecht, pp 5–19
Wang J, Raman H, Read B, Zhou M, Mendham N, Venkatanagappa S (2006) Validation of an Alt locus for aluminium tolerance scored with eriochrome cyanine R staining method in barley cultivar Honen (Hordeum vulgare). Aust J Agric Res 57:113–118
Wehr JB, Blamey FPC, Hanna JV, Kopittke PM, Kerven GL, Menzies NW (2010a) Hydrolysis and speciation of Al bound to pectin and plant cell wall material and its reaction with the dye chrome azurol S. J Agric Food Chem 58:553–5560
Wehr JB, Smith T, Blamey FPC, Menzies NW (2010) Aluminum sensitivity and optimum Ca and pH requirement of teak (Tectona grandis Linn. f.) clones used for forestry plantations in Australia. In: Gilkes RJ, Prakongkep N (eds) 19th world congress of soil science: soil solutions for a changing world, Brisbane, 1–6 Aug 2010b. IUSS, pp 18–21
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Yan F, Schubert S, Mengel K (1992) Effect of low root medium pH on net proton release, root respiration, and root-growth of corn (Zea mays L.) and Broad bean (Vicia faba L.). Plant Physiol 99:415–421
Zech W, Drechsel P (1991) Relationships between growth, mineral nutrition and site factors of Teak (Tectona grandis) plantations in the rain forest zone of Liberia. For Ecol Manag 41:221–235
Zhou ZZ, Liang KN, Xu DP, Zhang YC, Huang GH, Ma HM (2012) Effects of calcium, boron and nitrogen fertilization on the growth of teak (Tectona grandis) seedlings and chemical property of acidic soil substrate. New For 43:231–243
Acknowledgements
Part of the research was supported under the Australian Research Council’s Discovery Projects funding scheme (project number DP 0665467). We thank Elders Forestry Ltd for the supply of plant material and partial funding for this study. We thank Anna Sheldon, Brigid McKenna, Sarah Vaughan and Sally Collins for help with the set-up of the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wehr, J.B., Blamey, F.P.C., Smith, T.E. et al. Growth and physiological responses of teak (Tectona grandis Linn. f.) clones to Ca, H and Al stresses in solution and acid soils. New Forests 48, 137–152 (2017). https://doi.org/10.1007/s11056-016-9560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-016-9560-6