Abstract
Restoration of degraded pasture lands in the tropics through afforestation is widely supported. The greatest obstacle to afforestation, however, is the long delay before initial financial returns from wood harvesting are realized. Interplanting young trees with food or energy crops has been proposed as a strategy to help overcome this obstacle. We investigated the impact of this practice on the survival and growth performance of young tropical tree seedlings in Panama. Five native timber tree species and the exotic species Tectona grandis were interplanted with four different crop rotations and monitored over 2 years. Survival of young tree seedlings was up to eight times higher when planted in association with Manihot esculenta. Only during the first 3 months after maize sowing was a significant negative effect of intercropping on tree seedling survival found. Here, survival rate of tree seedlings was up to four times lower than in the pure plantation. Tree growth was not adversely affected by crops. In fact, Astronium graveolens, Cedrela odorata and Terminalia amazonia showed significantly superior growth performance in association with both Zea mays and Cajanus cajan. When combined with the latter, the height increment of these tree species was up to four times that achieved in pure plantations. We conclude that intercropping can be an important silvicultural practice to facilitate forest restoration. Multi-purpose shrubby crop species with cropping cycles of more than 6 months are particularly beneficial, as they quickly shade out grasses, thus reducing the need for herbicides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conversion of tropical rainforest to agricultural land is recognized as one of the major drivers of deforestation (FAO 2010; Goers et al. 2012). Once forest is converted to agricultural land, crop and livestock yields often decline rapidly due to unsustainable land-use practices and resulting losses in soil fertility (Ashton et al. 2001; Fischer et al. 2011). It is, therefore, widely acknowledged that the restoration of these degraded and unproductive lands is necessary in order to recover their ecological, as well as their economic value, improve the livelihoods of the local people and avoid further deforestation (Lamb et al. 2005; Knoke et al. 2013). However, humid tropical forests are often characterized by low levels of resilience, particularly where repeated fires or cattle grazing have been part of the land-use history (Holl 2007; Palomeque 2012). In these areas, natural succession is often hindered by the absence of a viable seed source (Günter et al. 2007), competition with aggressive and often, exotic grasses (Kuusipalo et al. 1995; Griscom et al. 2009), unfavorable microclimate, soil limitations (Moran et al. 2000) and fires (Aide and Cavelier 1994; Zimmermann 2002). Emerging secondary forest ecosystems usually contain relatively few valuable hardwoods, and hence, have a relatively low economic value (Aide et al. 2000; Günter et al. 2008). Active reforestation with fast-growing tropical hardwoods has, therefore, been suggested as an economically superior alternative to natural succession (Lamb et al. 2005; Knoke and Huth 2011). However, the greatest obstacle to the implementation of timber plantations remains the long-term investments of land, labor and other farm resources they require (Garen et al. 2009; Vieira et al. 2009), as tropical tree plantations do not typically provide economic returns during the first 5–10 years (Onyekwelu et al. 2011).
In order to overcome this problem, the sequential intercropping of juvenile tree plantations with food and energy crops, also known as the “Taungya” agroforestry practice has been suggested (Jordan 1992; Schlönvoigt and Beer 2001). The majority of scientific studies that have investigated such timber-based agroforestry systems have—as is commonly the case in agroforestry research—focused either on the production levels of the agricultural component (Khybri et al. 1992; Prasad et al. 2010) or on the economic performance of the entire system in comparison to pure cropping systems (Current 1995; Paul et al. 2014). However, scant attention has been dedicated to the fact that intercropping might also have substantial silvicultural advantages. These might include, for example, improved microclimate, shading out of competing grasses, reduced soil evaporation and improved soil fertility (Vieira et al. 2009). In contrast, competition for light, water and soil nutrients might also serve to inhibit tree growth. Potential allelopathic effects or attracted pests could harm tree seedlings (Rao et al. 1997).
Studies in Asia (Martin and van Noordwijk 2009; Bertomeu 2012), Africa (Chamshama et al. 1992; Kiriinya 1994; Norgrove and Hauser 2002; Imo 2009; Ehiagbonare 2006) and Latin America (Kapp and Beer 1995; Somarriba et al. 2001; Schlönvoigt and Beer 2001; Haggar et al. 2003; Ceccon 2008) have demonstrated that tree growth in intercropping systems is not necessarily negatively influenced by interplanting with short-term crops. Most of these studies, however, have only addressed the reforestation activities of small-scale landowners. Thus, the potential for the use of intercropping in commercial reforestation projects has, so far, been widely disregarded.
The present study aims to evaluate the suitability of such sequential intercropping systems for the production of both timber and food resources in tropical hardwood plantations. The main hypothesis addressed is:
Intercropping tree seedlings with annual crops does not negatively influence survival and initial growth of commercial timber species.
This hypothesis was tested in the eastern part of the Republic of Panama. This particular study site was chosen because, although such systems are frequently applied by small-holders in the region, systematic investigations of timber-based agroforestry systems in this area are still lacking (Fischer and Vasseur 2000; Garen et al. 2011). The region is also known for intensive, large-scale reforestation activities. Since 2000, 3900 ha of commercial hardwood plantations have been established annually in Panama, mostly in projects financed by foreign investors (Sloan 2008). Of this area, 85 % has been planted with the exotic tree species Tectona grandis (ANAM 2010), mainly due to lack of experience with native tree species (Oelmann et al. 2010). The present study, therefore, focused on the use of native tree species in order to identify suitable methods for their use in reforestation activities.
Materials and methods
Study site
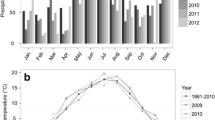
The study site is located at the border of the provinces of Panama and Darién in eastern Panama (8°54′15″N 78°19′55″W). This region is often referred to as the agricultural frontier of the country (Sloan 2008; Peterson St-Laurent et al. 2013), as deforestation began in earnest only in the last three to four decades. Today, 8 % of the agricultural area in the township of Tortí has already been abandoned (INEC 2011). The trial for this study—as is the case for most other reforestation activities in the area (Sloan 2008)—was established on abandoned pasture land. The site is situated at an elevation of 119 m asl, has a mean annual temperature of 26.4 °C and mean annual precipitation of 1910 mm (ETESA 2011b). The climate is characterized by a 3-month dry period from January to March during which the area receives less than 30 mm of monthly rainfall (ETESA 2011b). The natural vegetation zone has been classified as “Humid Tropical Forest” (Holdridge 1967). In 2009, the year the plantation was established, Panama suffered from the “El Niño” phenomenon, which led to very low rainfall levels during the rainy season in 2009 and a long subsequent dry period until the end of May 2010 (ETESA 2011a).
The soils in the study site developed from sedimentary rock, including tertiary limestone, Arenite and Lutite (ANAM 2011), and are classified as Vertisols (in the classification of the World Reference Base for Soil Resources). These soils have a high clay content of more than 60 % (clayey soil texture) and contain “Gilgai” formations, which are mounds and depressions that develop due to the shrinking and swelling of the soils that results from alternating wet and dry periods (Mermut et al. 1996).
Selection of tree and crop species
Five native (Astronium graveolens Jacq., Cedrela odorata L., Dalbergia retusa Hemsl., Hieronyma alchorneoides Allemao, Terminalia amazonia (J.F.Gmel.) Exell) and one exotic tree species (Tectona grandis L.f.) were selected for this trial. All of them have been identified as suitable commercial hardwood species for this area (see references in Online Resource (OR) 1 Table S1). Only crops that can be harvested within 1 year and are frequently planted as staple food crops in the region were selected and combined to create four different crop rotations:
-
1.
Maize (Zea mays L.)—Beans (Phaseolus vulgaris L.)—Maize–Maize (M-B-M-M)
-
2.
Ginger (Zingiber officinale Roscoe)—Pigeon pea (Cajanus cajan (L.) Huth) (G-P)
-
3.
Beans (P. vulgaris)—Rice (Oryza sativa L.)—Rice (B-R-R)
-
4.
Cassava (Manihot esculenta Crantz)—Soybeans (Glycine max (L.) Merr.) (C-S)
Trial design
Survival and growth performance of the six tree species was to be evaluated in each of four different intercropping rotations as well as in pure forest plantations. For this purpose, twenty plots of 1210 m2 (27 × 44.8 m) each were set up within an area of 3.7 ha. One of the five treatments—namely the four intercropping treatments (M-B-M-M, G-P, B-R-R, C-S) and the pure forest plantation treatment (FP)—was randomly allocated to each plot. The plots themselves were then further divided into six subplots (of 201.6 m2 each), each of which contained 16 individuals of one of the six tree species, corresponding to a planting density of 3 × 4 m. Hence, each plot of 1210 m2 contained one crop rotation and each of the six different tree species. Consequently, each tree species—crop rotation combination had four replications and a total tree number of 64 trees. The agroforestry plots were located adjacent to one another, while the pure forest plantation plots were situated approximately 200 m away from the agroforestry trials. This separation was done in order to avoid potential effects of the crops on the pure forest plantation plots (as proposed by Langton 1990). A detailed description of the experimental design can be found in Paul (2014). The entire trial is contained within a larger reforestation project of 72 ha which is owned and managed by the Forest Finance Group.
Trial management
The trial and surrounding plantation were both established in August 2009. The seeds for T. grandis and A. graveolens originated from the forest seed bank of the Tropical Agricultural Research and Higher Education Centre (CATIE) in Costa Rica, while the seeds used to produce the seedlings of all other tree species were collected from at least 5 mother trees per species from an area within an 80 km radius of the trial site. Prior to planting, the entire area was mechanically and chemically cleared of tall grasses and woody successional vegetation using a tractor and the herbicide Glyphosate (4 L ha−1). This method of field preparation was adopted as it is representative of common agricultural practices in the area. The containerized seedlings, which had been grown in the project’s nursery, were then planted and shortly thereafter, fertilized using 20 g of 15-30-8 N-P-K fertilizer per tree. Competing vegetation in the pure forest plantation plots was cleared manually and chemically once every 4 months, while both manual and chemical weed control in the agroforestry plots were carried out prior to each crop sowing. Any tree seedlings that did not survive during the first 12 months after planting were replaced during this period.
Crop cultivation began 2 weeks after tree planting, with the exception of the B-R-R treatment (Fig. 1). Sowing and subsequent management of crops were carried out in accordance with local practices (Schuchmann 2011). Thus, all crops were sown and harvested manually and, with the exception of the first and third maize rotations, no fertilizer was applied. In order to meet the certification standards of the Forest Stewardship Council (FSC), no insecticides were used. Seeds (or in the case of cassava, stem cuttings) were obtained from local farmers. In the M-B-M-M treatment, maize was first sown 2 weeks after tree establishment, again after 9 months and finally, after 12 months, with a cultivation length of 10 weeks for each rotation. Beans were planted between the first two maize rotations in order to help maintain soil fertility. Due to seasonally low levels of precipitation, no new crops were planted during the period from December to April in any of the treatments (Fig. 1). In the B-R-R treatment, there was a fallow period of 8 weeks after tree planting before bean cultivation began. Beans were harvested after 3 months and followed by two rotations of rice, the first of which was planted 9 months after tree planting. Rice (planted in the B-R-R treatment) and soybeans (planted in the C-S treatment) had a rotation period of 3–4 months (Fig. 1). The two shrubby crops—cassava (C-S treatment) and pigeon pea (G-P treatment)—had longer rotation periods of 12 and 9 months, respectively (Fig. 1). Pigeon pea was first sown 9 months after tree planting. As the previously planted ginger in this treatment (G-P) did not germinate, this effectively meant a fallow period of 9 months in this treatment. Planting distances for maize, beans, rice and soybeans were 30 cm within rows and 90, 70, 30 and 70 cm between rows, respectively. Pigeon pea and cassava were both planted at 1 × 1 m intervals. A radius of 50 cm around each tree was left free of crops in all treatments.
Data collection
Root collar diameter (measured at 5 cm above soil level) (from here also referred to simply as “diameter”) and height were selected as the main indicators for tree growth. During the time of intercropping from August 2009 to March 2011 (i.e. a total of 18 months) these parameters were measured every 3 months. In order to capture possible mid-term effects of intercropping, the tree growth performance parameters were again measured after 24 months (i.e. 6 months after the final crop harvest).
Although the trial was established on a plain, the presence of gilgais resulted in heterogeneity in the microrelief which was observed to have strong effects on the growth and survival rates of tree seedlings. Therefore, the relative planting position of each tree was also recorded, using 1 as the code for trees planted in a relative depression 0 for all other planting positions (as e.g. on the flank or top of a mound).
Data analysis
The primary goal of this study was the assessment of the effects of the various agricultural treatments on the survival and growth rates of each of the various tree species used here. Thus, all analyses were carried out separately for each tree species to prevent treatment effects from being masked by potentially stronger species effects.
A Cox proportional hazard model was used to compare tree survival rates among the various agricultural treatments. This semi-parametric regression model not only takes into account the chronological distribution of mortality over the observation period, but also addresses features typical for survival data, such as positive skewness and the fact that these data are often censored (Collett 2003). In this context we refer to “right censoring” which means that the event of interest (in this case, the death of a tree) may occur beyond the end of the follow-up period (Clark et al. 2003). Consequently, the length of the period of survival is unknown. Among the various semi-parametric models that can handle such censored data, the Cox proportional hazard model was chosen in order to allow for the incorporation of multiple covariates (Bradburn et al. 2003). Similar to the odds ratio derived using logistic regression, the Cox regression gives the hazard ratio (HR), which represents the change in the hazard—or probability of death—between any given treatment and another treatment. The hypothesis to be tested here was that the HR between any given pair of treatments was significantly different from 1 when controlling for the effect of microrelief. To test this hypothesis, the Wald statistic was used (Quinn and Keough 2002). The analysis was carried out for all 16 trees per tree species and plot using the “COXREG” command in SPSS 20.
Following the “top height tree collective” concept commonly applied in forest science (Pretzsch 2009) only the five most vital trees of each tree species per plot (“target trees”), and hence, 20 trees per treatment were selected as sample population. As the focus of this study was on stands established for commercial timber production (rather than for protection or other ecological purposes) this seems an appropriate method in this instance. In this context, height growth performance after 24 months was used as the primary indicator of vitality (Evans and Turnbull 2004; Aguirre 2007). In young timber plantations, tree height is an adequate indicator for future growth expectations, as trees that show good development in height growth usually develop larger crowns, thus allowing them to intercept more light. Consequently, these trees usually have a competitive advantage over others, and thus, the highest probability of reaching maturity (Aguirre 2007). Trees which are inferior at the time of canopy closure usually do not succeed in reaching a dominant position (Evans and Turnbull 2004). Less vital trees are likely to either die or be removed in thinning operations in order to further stimulate the growth of the target trees (Pretzsch 2009).
By using this approach, we also intended to standardize sample size and avoid potential differences in means that could be caused by the high differences in survival rates found in this study. This is because a higher survival rate for a particular species in a given treatment inherently results in a wider range of heights and diameters compared to a species (and/or) treatment with a low survival rate.Footnote 1 Furthermore, as the most vital trees with the highest genetic adaption for a particular site are the most likely survivors, tree growth in treatments with low survival rates could be overestimated compared to that in treatments with higher survival rates. In order to separate between effects of mortality and growth analysis we focused on the target trees. Statistical results were, however, not affected by using these different sample populations. For comparison, we have provided growth data for all trees in the Online Supplementary Material (OR1 Table S4).
We applied a linear mixed model to test for statistical differences among the five treatments in the two dependent growth variables (G)—height (h) and diameter status (rcd)—at the end of the observation period. This approach allowed us to include a random intercept for each plot which served to account for the clustered nature of trees within subplots (Piepho et al. 2003). The intercropping treatment and the binary microrelief variable were included in the model as fixed factors. As previously discussed, all analyses for each of the dependent growth variables were carried out separately for each tree species.
with b0 being the intercept, b1 the fixed effect of treatment, b2 the fixed effect of microrelief, u0j the variability of intercepts and εij the model error. Subscript i represents the individual trees and j the plots. No other soil variables apart from the microrelief position were included in the model; as, due to the relative homogeneity of the soils across all plots, their inclusion did not significantly improve the model (OR1 Table S2).
A simple variance component matrix was used to include the random plot effect. To estimate the covariance parameters, the residual maximum likelihood estimation (REML) approach was used. Type III sum of squares was applied to estimate fixed effects, and where significant effects were detected, pair-wise comparisons using least significant differences (LSD) were run. Error probabilities below 5 % (p < 0.05) were accepted as statistically significant. This analysis was carried out using the “MIXED” command in the statistical software package SPSS 20. To achieve normality, the dependent variables root collar diameter and height were transformed using the natural logarithm.
The same model was applied to test for significant differences in the height and diameter increments during different time periods for each tree species. The alternative approach of using a longitudinal linear mixed model to include a random time effect was rejected, as nonlinear growth trajectories were observed which led to non-normally distributed residuals and clearly nonlinear patterns in the residual plots. By analyzing the growth increments within different time periods, the model fit was improved and the allocation of effects to specific crops due to differences in their planting and harvesting periods was simplified.
Four time intervals were selected—(1) months 0–3, (2) months 0–9, (3) months 9–18 and (4) months 18–24. The first and last of these periods were chosen to reflect the initial phase (months 0–3) and the phase after enrichment plantings had stopped (18–24 months), while the two 9-month periods correspond to the main sowing and harvesting periods for all crops with the exception of cassava (Fig. 1). The division between the two nine-month periods corresponds to the end of the first dry season, which is a critical phase in the establishment of forest plantations in such climates. The growth increment in the height and diameter of each tree for each period was calculated as an absolute growth rate (AGR) following Kikvidze and Armas (2010) using the formula
with h being the heights (or diameters) at times t1 and t2, and t2-1 the length of the time period between t1 and t2 (expressed in months). All increment parameters were subsequently transformed using the natural logarithm.
Results
Survival
Survival rates were generally highest for A. graveolens and C. odorata, with a total survival rate of 90 and 97 % respectively after 2 years. In contrast, the survival rate for H. alchorneoides was only 37 % across all treatments. Figure 2 reveals that mortality of all species was particularly high between 3 and 6 months after planting—the period corresponding to the end of the rainy season and the middle of the first dry season.
For the majority of tree species, the lowest survival rate was found in the M-B-M-M treatment (Fig. 2; Table 1). The Cox regression, which compares the relative hazard for mortality between treatments confirmed these strong differences: For instance, the hazard of mortality of T. amazonia during the first 2 years was 13 times higher in the M-B-M-M than in the C-S treatment (HR = 13.7, 95 % CI 6.7–28.2), and nearly twice as high as that for the FP treatment (HR = 1.8, 95 % CI 1.29–2.41) (Table 1). Apart from the M-B-M-M treatment, trees of all species in the agroforestry treatments tended to have a lower probability of mortality than those in the FP treatment. The hazard for mortality of T. grandis trees was, for example, six times higher in pure plantation than in the agroforestry treatment with ginger and pigeon pea (HRFP/GP = 6.37, 95 % CI 3.9–10.3).
Controlling for treatment effects, the likelihood of death for individuals of all tree species was significantly higher when planted in a relative depression (p < 0.006). The magnitude of the HR between relative planting positions was highest for T. grandis and A. graveolens (Table 1). The HR for trees of these species, planted in a relative depression vs. all other planting positions was 3.3 (95 % CI 2.7–4.1) and 3.2 (95 % CI 2.4–4.5), respectively.
Tree growth
Figure 3 reveals the nonlinear growth trajectories of the trees previously mentioned in the “Materials and methods” section. While growth increment was low in the first 9 months after planting, a period which included an exceptionally severe dry season, it subsequently accelerated with the onset of the rainy season. The graph also demonstrates the overall superior growth performance of the exotic T. grandis which resulted in a mean height after 2 years that was twice as high as that of any of the native species (Fig. 3, OR 1 Table S3).
Concerning differences in growth performance among the intercropping treatments, Fig. 3 reveals two major effects—first, the height-growth-inducing effect of the maize intercropping (treatment M-B-M-M) during the first 3 months (Figs. 3, 4) and second, the overall superior growth performance of nearly all tree species in the G-P treatment (see circle symbol in Fig. 3) from month 9 onwards. In the G-P treatment, this point in time corresponds to the start of the pigeon pea rotation (Fig. 1).
Mean monthly height increment of the target trees of each tree species during the periods from a 0–3 months, b 0–9 months, c 9–18 months and d 18–24 months after tree planting. Different letters denote significant differences between treatments within a tree species. Different letters denote significant differences between treatments within tree species according to the LSD test (p < 0.05) applied in cases where the fixed effect “treatment” was found to be significant (Table 2). Bars denote 95 % confidence interval
The data on mean monthly height increment displayed in Fig. 4 reveals that height growth during the first 3 months was particularly high for C. odorata, D. retusa, and T. amazonia in the maize fields as compared to that for these species in all other treatments (Fig. 4a). This increase in growth, however, did not continue after the maize harvest (at 3 months after tree planting), as demonstrated by the increment for the 0–9 month period, which did not significantly differ among treatments except in the case of T. amazonia and A. graveolens (Fig. 4b; Table 2). The latter species showed superior growth in the pure forest plantation plots (FP) as compared to that in any of the agroforestry treatments during the first 9 months. Still, a negative impact of the intercropping treatments on this tree species appears unlikely, as the growth increment in the G-P treatment—in which management was identical to that in the FP plots during this phase—did not differ from the other intercropping treatments. Thus, this difference might rather be explained by other factors that remain unknown.
Figure 4 further demonstrates that in the period from nine to 18 months after tree planting, the height increment was higher in the G-P plots than in all other treatments for both T. amazonia (pair-wise comparisons p < 0.045) and C. odorata (p < 0.008). A. graveolens also performed significantly better in association with G-P than in all other treatments (p < 0.008) with the exception of the B-R-R treatment (Fig. 4c). These increases in height growth can most likely be attributed to a facilitative effect from pigeon pea, considering that all species demonstrated average to low height increments in the G-P treatment during the first 9 months. This facilitative effect was particularly obvious after month 12, when the shrub had reached its final height of up to 4 m (Figs. 1, 3).
A further notable effect that occurred during this interval was the clear decrease in the height growth of T. grandis in the C-S-treatment (p < 0.011 compared to all other treatments) that followed the cassava harvest, which might be attributed to less intensive weeding after this time.
During the 6-month period after crop cultivation had stopped (months 18–24), no mid-term effects on height growth were observed (Fig. 4d). Only T. grandis showed a significantly greater height increment in the individuals of this species in the former G-P plots compared to those in the former M-B-M-M (p = 0.011), B-R-R (p = 0.003) and FP treatments (p = 0.024). This reveals that even though pigeon pea did not appear to have a growth-inducing effect on T. grandis during its cultivation period, it might have had lasting impacts on soil conditions that resulted in better growth performance in this species after the pigeon pea was harvested.
Despite the small differences in the growth increments between months 18 and 24, tree heights still differed significantly among treatments at the end of the observation period (Table 2, see also OR 1 Table S3): Accordingly, even 6 months after the harvest of the pigeon pea shrubs, A. graveolens, C. odorata and T. amazonia trees were significantly taller in the former G-P plots than those in all other treatments. C. odorata reached significantly greater heights in all of the agroforestry systems (p < 0.021) (with the exception of the B-R-R treatment (p = 0.162) than it did in pure forest plantation. This can be attributed to the significantly lower infestation rates of this tree species with Hypsipyla grandella which were observed in the agroforestry treatments that contained tall crops or shrubs.Footnote 2
The differences in diameter growth found among the various treatments were generally smaller than those found for height growth (Fig. 5). Diameter growth did not increase during intercropping with either maize or pigeon pea (Fig. 5). This finding suggests that trees in the agroforestry treatments invested in height growth at the cost of diameter growth in order to compete with crops for light. Accordingly, diameter growth during the first 18 months was generally higher in the pure forest plantation plots than in the agroforestry plots (Fig. 5). However, between months 18 and 24, T. amazonia showed a higher diameter growth increment in the GP treatment than that which occurred in the former M-B-M-M (p = 0.002), B-R-R (p = 0.001) and FP treatments (p = 0.007), (Fig. 5d; Table 2).
Mean monthly root collar diameter (rcd) increment of target trees during the periods from a 0–3 months, b 0–9 months, c 9–18 months and d 18–24 months after tree planting. Different letters denote significant differences between treatments within tree species according to the LSD test (p < 0.05) applied in cases where the fixed effect “treatment” was found to be significant (Table 2). Bars denote 95 % confidence intervals
Diameter measurements of C. odorata should generally be interpreted with caution here, as stem infestation with H. grandella usually led to the development of a thick buttress. Hence, larger diameters tend to reflect relatively poor stem quality, rather than greater vitality.
The relative position of trees within the microrelief was proven to have a significant effect on both height and diameter growth performance. This effect was particularly evident for D. retusa and T. grandis (Table 2). The estimated regression coefficients for the effect of microrelief (b2 in Eq. 1) were negative for all tree species, which—given the coding scheme used here—means that trees growing in a relative depression showed smaller growth increments, for both height and diameter, than trees growing on the flank or top of a mound. This finding underlines the sensitivity of all species to the planting position in high clay content soils, both in terms of survival and growth performances.
Discussion
Based on the findings presented above, the hypothesis that survival of tree seedlings is not negatively affected by intercropping can be accepted for all of the crop rotations investigated here except the maize intercropping system. The high rate of mortality of tree seedlings of all species after 3 months in the M-B-M-M treatment is most likely related to the sudden modification of the environmental conditions through the maize harvest and the simultaneous onset of the dry season. This drastic change in microclimatic conditions might have led to a solar shock and a higher level of transpiration of the tree seedlings. Accordingly, tree species known to be sensitive to direct sunlight, such as D. retusa (Marin and Flores 2002), showed the highest rates of mortality after the maize harvest. However, when associated with crops with a longer cultivation cycle, such as cassava or pigeon peas, particularly the native tree species tended to have a higher survival rate than those planted in the open field (FP treatment). This means that these crops might actually create a “nurse effect” (Vandermeer 1989) for tree seedlings. By moderating the intensity of sunlight and wind, they can reduce the level of evapotranspiration, and thus maintain a more humid microclimate (Vieira et al. 2009). This effect has mainly been studied in the reverse, where trees have been planted in order to create this same effect in favor of crop species (Rao et al. 1997).
Concerning growth performance, the hypothesis that planting crops in young forest plantations does not negatively influence the growth of tree seedlings was accepted for the tree species C. odorata, D. retusa, T. grandis and T. amazonia. Only two tree species—A. graveolens and H. alchorneoides—showed significantly negative effects of intercropping on tree growth. In the case of A. graveolens this was, however, only true during the first 9 months, while a significantly better height increment was found for this tree species during the entire period in which it was intercropped with pigeon pea. The measurements of growth increment for H. alchorneoides should be interpreted carefully due to the high mortality rate that occurred in this species in the trial and, therefore, the reduced sample size. It is likely that this species had difficulty coping with the heavy clay soil conditions in our trial, as high survival rates have been found for this species on other sites in Panama (Piotto et al. 2003, 2004; van Breugel et al. 2011).
For the other tree species studied here, the effects of the associated crops were not merely supplementary, with crops using excess available space, light and soil resources without impeding tree growth (Jordan 1992), but even complementary, and thus, facilitative (Vandermeer 1989): Among such facilitative effects that were consistent across the majority of tree species studied were the positive effects of both the first maize rotation and the pigeon pea rotation on height growth. It could be argued that the apparent facilitative effect of maize during the first 3 months was actually due to the fertilizer applied to the maize plants during this period. This is, however, unlikely in this experiment, as the same fertilizer was applied to the tree seedlings themselves only 2 weeks prior to the maize application. Thus, tree seedlings were unlikely to benefit from further fertilization. Due to the small rooting systems of tree seedlings during the first 3 weeks after planting, the distance of 50 cm which was kept free of crops (and hence, additional fertilizer) around each tree should have been sufficient to avoid supplemental fertilization of trees in the M-B-M-M treatment. It appears more likely that the competition for light led to the superior height growth of the tree seedlings in both the maize and pigeon pea fields. Increased height growth has been shown to occur in shaded tree seedlings as a natural response intended to secure access to light (Ammer 2003; Coomes and Allen 2007; Imo 2009). Such an allocation of resources to height growth is usually realized at the expense of diameter growth, as was observed in this study. Reduced diameter growth can be viewed as unfavorable in terms of wood production. However, Evans and Turnbull (2004) and Potvin and Dutilleul (2009) among others, argue that such induced height growth in tree seedlings will eventually lead to a total accumulation of a larger amount of wood due to the concomitant development of larger crowns, and the resulting higher photosynthesis rate. Accordingly, in this trial, diameter growth increased for both T. amazonia and A. graveolens after the pigeon pea shrubs were cut.
Not only trees were shaded by the dense crop canopies, but also competing vegetation. Kreuzer (2013) found for instance, that the transmission of photosynthetically active radiation at the ground level was less than 10 % in the pigeon pea treatments, which resulted in the shading-out of abundant fodder grasses, such as Brachiara spp. This is an important finding, as these invasive grasses often impede the successful restoration of abandoned pastures (Zimmermann 2002; Palomeque 2012) or require extremely intensive management (Griscom and Ashton 2011). Accordingly, tree growth was rather negatively affected by intercropping with cassava, beans and rice, which did not succeed in suppressing these competitive grasses. Even though cassava also reached final heights of up to three meters, the large amount of light transmitted through the open crown of cassava allowed grasses to grow in the understory. This effect was exacerbated by the low planting success for cassava as a high percentage of stem cuttings deteriorated in the waterlogged soil before developing a rooting system. A negative effect of cassava on hardwood seedling growth has also been observed by Coster and Hardjowasono (1935) and Schlönvoigt and Beer (2001), while a positive effect of pigeon peas was also found by Beltrame and Rodrigues (2007). The latter effect might partly be attributed to the ability of pigeon pea to fix nitrogen. However, as none of the tree species showed a positive reaction to the intercropping with beans, it can be assumed that the ability to suppress grasses was more relevant in this case than the nitrogen effect, as was also reported by Imo (2009). Pigeon pea has also been found to reduce soil compaction (Young 1997) and provide improved nutrient input due to the nutrient-rich foliage (Loss et al. 2009; Nair et al. 1999). In addition, pigeon pea helped drain the waterlogged site of this trial due to its fast root development and high water needs.
Due to the design of this study, inferences can only be made from an intercropping period of 18 months and a total observation period of 24 months. Although this is a rather short time in relation to the life span of most tree species, this interval corresponds well to the intercropping periods most often used in traditional Taungya systems (Schlönvoigt and Beer 2001). Finally, this study provides empirical proof for the suggestion made by Vieira et al. (2009) that tropical forest recovery can be promoted through the use of agro-successional restoration.
Conclusions and recommendations
We conclude that introducing an agricultural component into tropical timber plantations can be an effective management tool to facilitate the productive restoration of abandoned pastures. This is particularly true for native tree species that are often not adapted to the harsh conditions found on degraded pasture sites. Crops that create a dense “canopy” and thus, quickly shade out grasses have the highest potential to aid reforestation under conditions similar to those in the trial. Crops with longer rotation periods might be a better choice than short rotation crops, as the sudden changes in microclimate in the latter case can have negative effects on tree survival. Shrubs such as C. cajan can help shade out invasive herbaceous species, thus reducing the need for herbicide use, while at the same time improving soil conditions and creating a “nurse effect” for tree seedlings.
The results of this trial demonstrate that in order to facilitate reforestation on clay soils, to improve growth performance and to reduce costs for replacing dead trees, careful consideration of the local environmental variation—particularly the microrelief—should be given. This might not appear to be a new finding. However, in fact, the majority of tree planting activities in the region are still carried out using marked ropes and a strict rectangular planting design.
In addition to the silvicultural advantages of the system outlined above, we believe that foresters might be forced to consider such timber-based agrisilvicultural systems in the future, due to the pressure that is emerging from the alarming increase in the food and energy demands of the rising world population. Increasing the spatial extent of forest through conservation and reforestation is often seen as inherently in conflict with fulfilling the basic right to food. We, therefore, encourage further research and, in particular, long-term studies to investigate the potential of different tree-crop combinations along an ecosystem gradient. We are convinced that such interdisciplinary research projects will generate results which are highly relevant for modern forest plantation management, thus supporting the productive restoration of degraded lands.
Notes
In the case of H. alchorneoides, mortality was so high that in some plots less than five of the original seedlings planted were still alive at the end of the observation period (Fig. 2 and OR 1, Table S3 and S4). Though the statistical evidence for treatment effects is, therefore, weak for this species, we have provided the observed values here, as very little empirical data are available on the growth performance of this valuable native tree species in Panama.
More information on infestation rates of C. odorata with H. grandella in this trial can be found in Paul and Weber (2013).
References
Aguirre NM (2007) Silvicultural contributions to the reforestation with native species in the tropical mountain rainforest region of South Ecuador. Dissertation at the Institute of Silviculture, Technische Universität München, Freising, Weihenstephan
Aide TM, Cavelier J (1994) Barriers to lowland tropical forest restoration in the Sierra Nevada de Santa Marta, Colombia. Rest Ecol 2(4):219–229. doi:10.1111/j.1526-100X.1994.tb00054.x
Aide TM, Zimmerman JK, Pascarella JB, Rivera L, Marcano-Vega H (2000) Forest regeneration in a chronosequence of tropical abandoned pastures: implications for restoration ecology. Rest Ecol 8(4):328–338. doi:10.1046/j.1526-100x.2000.80048.x
Ammer C (2003) Growth and biomass partitioning of Fagus sylvatica L. and Quercus robur L. seedlings in response to shading and small changes in the R/FR-ratio of radiation. Ann For Sci 60(2):163–171
ANAM (2010) Guía Técnica de la Reforestación en Panamá. (Manual for the reforestation in Panama). Autoridad Nacional del Ambiente, Panama
ANAM (2011) Atlas Ambiental de la Republica de Panamá. Autoridad Nacional del Ambiente; Gobierno Nacional de la Rebública de Panamá, Panama
Ashton MS, Gunatilleke CVS, Singhakumara BMP, Gunatilleke IAUN (2001) Restoration pathways for rain forest in southwest Sri Lanka: a review of concepts and models. New Dir Trop For Res 154(3):409–430. doi:10.1016/S0378-1127(01)00512-6
Beltrame T, Rodrigues E (2007) Guandu bean (Cajanus cajan) on tropical forest restoration. Feijao (Cajanus cajan) na restraucao de florestas tropicais. Ciencias Agrárias Londrina 28(1):19–28
Bertomeu M (2012) Growth and yield of maize and timber trees in smallholder agroforestry systems in Claveria, northern Mindanao, Philippines. Agrofor Syst 84(1):73–87. doi:10.1007/s10457-011-9444-x
Bradburn MJ, Clark TG, Love SB, Altman DG (2003) Survival analysis part II: multivariate data analysis—an introduction to concepts and methods. Br J Cancer 89(3):431–436. doi:10.1038/sj.bjc.6601119
Ceccon E (2008) Production of bioenergy on small farms: a two-year agroforestry experiment using Eucalyptus urophylla intercropped with rice and beans in Minas Gerais, Brazil. New For 35(3):285–298
Chamshama SAO, Monela GC, Sekiete KEA, Persson A (1992) Suitability of the taungya system at North Kilimanjaro forest plantation, Tanzania. Agrofor Syst 17(1):1–11. doi:10.1007/BF00122924
Clark TG, Bradburn MJ, Love SB, Altman DG (2003) Survival analysis part I: basic concepts and first analyses. Br J Cancer 89(2):232–238. doi:10.1038/sj.bjc.6601118
Collett D (2003) Modelling survival data in medical research, 2nd edn. Chapman & Hall/CRC, Boca Raton
Coomes DA, Allen RB (2007) Effects of size, competition and altitude on tree growth. J Ecol 95(5):1084–1097. doi:10.1111/j.1365-2745.2007.01280.x
Coster C, Hardjowasono M (1935) Influence of agricultural crops in taungya plantations on the growth of teak. Tectona 28:464–487
Current D (1995) Economic and institutional analysis of projects promoting on-farm tree planting in Costa Rica. Costs, benefits, and farmer adoption of agroforestry. Project experience in Central America and the Caribbean. The World Bank, Washington, pp 45–80
Ehiagbonare JE (2006) Effect of Taungya on regeneration of endemic forest tree species in Nigeria: Edo State Nigeria as a case study. Afr J Biotechnol 5(18):1608–1611
ETESA (2011a) El Fenómeno de El Nino en Panamá. http://www.hidromet.com.pa/nino_nina.php. Accessed on 13 Feb 2013
ETESA (2011b) Historical data on mean annual temperature and rainfall in Tortí (1977-2011). http://www.hidromet.com.pa/clima_historicos.php. Accessed on 13 Feb 2013
Evans J, Turnbull JW (2004) Plantation forestry in the tropics. The role, siviculture, and use of planted forests for industrial, social, environmental and agroforestry purposes, 3rd edn. Oxford University Press, Oxford
FAO (2010) Global forest resources assessment 2010. Main report. Food and Agriculture Organization of the United Nations, Rome
Fischer A, Vasseur L (2000) The crisis in shifting cultivation practices and the promise of agroforestry: a review of the Panamanian experience. Biodiv Cons 9(6):739–756. doi:10.1023/A:1008939425511h
Fischer J, Batary P, Bawa KS, Brussaard L, Chappell MJ, Clough Y, Daily GC, Dorrough J, Hartel T, Jackson LE, Klein AM, Kremen C, Kuemmerle T, Lindenmayer DB, Mooney HA, Perfecto I, Philpott SM, Tscharntke T, Vandermeer J, Wanger TC, von Wehrden H (2011) Conservation: limits of land sparing. Science 334(6056):593. doi:10.1126/science.334.6056.593-a
Garen E, Saltonstall K, Slusser J, Mathias S, Ashton M, Hall J (2009) An evaluation of farmers’ experiences planting native trees in rural Panama: implications for reforestation with native species in agricultural landscapes. Agrofor Syst 76(1):219–236
Garen EJ, Saltonstall K, Ashton MS, Slusser JL, Mathias S, Hall JS (2011) The tree planting and protecting culture of cattle ranchers and small-scale agriculturalists in rural Panama: opportunities for reforestation and land restoration. The ecology and ecosystem services of native trees: implications for reforestation and land restoration in Mesoamerica. For Ecol Manag 261(10):1684–1695. doi:10.1016/j.foreco.2010.10.011
Goers L, Lawson J, Garen E (2012) Economic drivers of tropical deforestation for agriculture. In: Ashton MS, Tyrrell ML, Spalding D, Gentry B (eds) Managing forest carbon in a changing climate. Springer, Netherlands, pp 305–320
Griscom HP, Ashton MS (2011) Restoration of dry tropical forests in Central America: a review of pattern and process. The ecology and ecosystem services of native trees: implications for reforestation and land restoration in Mesoamerica. For Ecol Manag 261(10):1564–1579. doi:10.1016/j.foreco.2010.08.027
Griscom HP, Griscom BW, Ashton MS (2009) Forest regeneration from pasture in the dry tropics of Panama: effects of cattle, exotic grass, and forested Riparia. Rest Ecol 17(1):117–126. doi:10.1111/j.1526-100X.2007.00342.x
Günter S, Weber M, Erreis R, Aguirre N (2007) Influence of distance to forest edges on natural regeneration of abandoned pastures: a case study in the tropical mountain rain forest of Southern Ecuador. Eur J For Res 126(1):67–75. doi:10.1007/s10342-006-0156-0
Günter S, Cabrera O, Weber M, Stimm B, Zimmermann M, Fiedler K, Knuth J, Boy J, Wilcke W, Iost S, Makeschin F, Werner F, Gradstein SR, Mosandl R (2008) Natural forest management in neotropical mountain rain forests—an ecological experiment. In: Beck E, Bendix J, Kottke I, Makeschin F, Mosandl R, Caldwell MM, Heldmaier G, Jackson RB, Lange OL, Mooney HA, Schulze E, Sommer U (eds) Gradients in a tropical mountain ecosystem of ecuador, vol 198. Springer, Berlin, pp 347–359
Haggar J, Rheingans R, Arroyo P, Alvarado B (2003) Benefits and costs of intercropping reforestation in the Atlantic lowlands of Costa Rica. New For 25(1):41–48
Holdridge LR (1967) Life zone ecology, Revised edn. Tropical Science Center, San José
Holl KD (2007) Old field vegetation succession in the neotropics. In: Cramer V, Hobbs RJ (eds) Old fields: dynamics and restoration of abandoned farmland. Island Press, Washington
Imo M (2009) Interactions amongst trees and crops in taungya systems of western Kenya. Agrof Syst 76(2):265–273. doi:10.1007/s10457-008-9164-z
INEC (2011) VII Censo Nacional Agropecuario 2011. Resultados Finales Básicos. Instituto Nacional de Estadística y Censo, Contraloría General de la República de Panamá
Jordan CF (ed) (1992) Taungya. Forest plantations with agriculture in Southeast Asia. CAB International, Wallingford
Kapp G, Beer J (1995) A comparison of agrisilvicultural systems with plantation forestry in the Atlantic Lowlands of Costa Rica. Agrof Syst 32:207–223
Khybri M, Gupta R, Ram S, Tomar H (1992) Crop yields of rice and wheat grown in rotation as intercrops with three tree species in the outer hills of Western Himalaya. Agrof Syst 17(3):193–204. doi:10.1007/BF00054147
Kikvidze Z, Armas C (2010) Plant interaction indices based on experimental plant performance data. In: Pugnaire FI (ed) Positive plant interactions and community dynamics. CRC Press; Fundación BBVA, Boca Raton, pp 17–38
Kiriinya C (1994) Comparative growth of seven years old Grevillea robusta A. cunn. grown under Taungya system and pure plantation. J Trop For 10(1)
Knoke T, Huth A (2011) Modelling forest growth and finance: often disregarded tools in tropical land management. In: Günter S, Weber M, Stimm B, Mosandl R (eds) Silviculture in the tropics, vol 8. Springer, Berlin, pp 129–142
Knoke T, Calvas B, Moreno SO, Onyekwelu JC, Griess VC (2013) Food production and climate protection—What abandoned lands can do to preserve natural forests. Global Env Change 23(5):1064–1072. doi:10.1016/j.gloenvcha.2013.07.004
Kreuzer S (2013) Untersuchungen zur Konkurrenz sowie zur Interaktion zwischen Cajanus Cajan und sechs verschiedneen tropischen Wertholzarten in Panama. Master thesis, Institute of Silviculture, Technische Universität München, Freising
Kuusipalo J, Ådjers G, Jafarsidik Y, Otsamo A, Tuomela K, Vuokko R (1995) Restoration of natural vegetation in degraded Imperata cylindrica grassland: understorey development in forest plantations. J Veg Sc 6(2):205–210. doi:10.2307/3236215
Lamb D, Erskine PD, Parrotta JA (2005) Restoration of degraded tropical forest landscapes. Science 310(5754):1628–1632
Langton S (1990) Avoiding edge effects in agroforestry experiments; the use of neighbour-balanced designs and guard areas. Agrofor Syst 12(2):173–185. doi:10.1007/BF00123472
Loss A, Pereira MG, Ferreira EP, Santos LLd, Beutler SJ, Ferraz Júnior ASdL (2009) Frações oxidáveis do carbono orgânico em argissolo vermelho-amarelo sob sistema de aleias. Revista Brasileira de Ciência do Solo 33:867–874
Marin W, Flores E (2002) Dalbergia retusa—species description. In: Vozzo J (ed) Tropical tree seed manual. USDA Forest Service, Washington, DC
Martin F, van Noordwijk M (2009) Trade-offs analysis for possible timber-based agroforestry scenarios using native trees in the Philippines. Agrofor Syst 76:555–567. doi:10.1007/s10457-009-9208-z
Mermut A, Dasog G, Dowuona G (1996) Soil morphology. In: Ahmad N, Mermut A (eds) Vertisols and technologies for their management. Elsevier, Amsterdam
Moran EF, Brondizio ES, Tucker JM, da Silva-Forsberg MC, McCracken S, Falesi I (2000) Effects of soil fertility and land-use on forest succession in Amazônia. For Ecol Manag 139(1–3):93–108. doi:10.1016/S0378-1127(99)00337-0
Nair PK, Buresh RJ, Mugendi D, Latt C (1999) Nutrient cycling in tropical agroforestry systems: myths and science. In: Buck L, Lassoie JP, Fernandes ECM (eds) Agroforestry in sustainable agricultural systems. CRC Press, Boca Raton
Norgrove L, Hauser S (2002) Measured growth and tree biomass estimates of Terminalia ivorensis in the 3 years after thinning to different stand densities in an agrisilvicultural system in southern Cameroon. For Ecol Manag 166(1–3):261–270. doi:10.1016/S0378-1127(01)00614-4
Oelmann Y, Potvin C, Mark T, Werther L, Tapernon S, Wilcke W (2010) Tree mixture effects on aboveground nutrient pools of trees in an experimental plantation in Panama. Plant Soil 326(1):199–212. doi:10.1007/s11104-009-9997-x
Onyekwelu J, Stimm B, Evans J (2011) Review Plantation Forestry. In: Günter S, Weber M, Stimm B, Mosandl R (eds) Silviculture in the Tropics, vol 8. Springer, Berlin, pp 399–454
Palomeque FP (2012) Natural succession and tree plantation as alternatives for restoring abandoned lands in the Andes of Southern Ecuador: aspects of facilitation and competition. Dissertation, Technische Universität München
Paul C (2014) Timber-based agrisilvicultural systems to facilitate reforestation in Panama—a silvicultural and economic evaluation. Dissertation, Technische Universität München
Paul C, Weber M (2013) Intercropping Cedrela odorata with shrubby crop species to reduce infestation with Hypsipyla grandella and improve the quality of timber. ISRN Forestry 2013:10. doi:10.1155/2013/637410
Paul C, Griess V, Havardi-Burger N, Weber M (2014) Timber-based agrisilviculture improves financial viability of hardwood plantations: a case study from Panama. Agrofor Syst. doi:10.1007/s10457-014-9755-9
Peterson St-Laurent G, Gélinas N, Potvin C (2013) Diversity of perceptions on REDD + implementation at the agriculture frontier in Panama. Int J For Res 2013:16. doi:10.1155/2013/657846
Piepho HP, Büchse A, Emrich K (2003) A Hitchhiker’s guide to mixed models for randomized experiments. J Agr Crop Sc 189(5):310–322. doi:10.1046/j.1439-037X.2003.00049.x
Piotto D, Montagnini F, Ugalde L, Kanninen M (2003) Performance of forest plantations in small and medium-sized farms in the Atlantic lowlands of Costa Rica. For Ecol Manag 175(1–3):195–204
Piotto D, Viquez E, Montagnini F, Kanninen M (2004) Pure and mixed forest plantations with native species of the dry tropics of Costa Rica: a comparison of growth and productivity. For Ecol Manag 190(2–3):359–372. doi:10.1016/j.foreco.2003.11.005
Potvin C, Dutilleul P (2009) Neighborhood effects and size-asymmetric competition in a tree plantation varying in diversity. Ecology 90(2):321–327. doi:10.1890/08-0353.1
Prasad J, Korwar G, Rao K, Mandal U, Rao C, Rao G, Ramakrishna Y, Venkateswarlu B, Rao S, Kulkarni H, Rao M (2010) Tree row spacing affected agronomic and economic performance of Eucalyptus-based agroforestry in Andhra Pradesh, Southern India. Agrofor Syst 78(3):253–267. doi:10.1007/s10457-009-9275-1
Pretzsch H (2009) Forest dynamics, growth and yield. From measurement to model. Springer, Berlin
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rao M, Nair P, Ong C (1997) Biophysical interactions in tropical agroforestry systems. Agrofor Syst 38(1):3–50. doi:10.1023/A:1005971525590
Schlönvoigt A, Beer J (2001) Initial growth of pioneer timber tree species in a Taungya system in the humid lowlands of Costa Rica. Agrofor Syst 51(2):97–108. doi:10.1023/A:1010674402907
Schuchmann J (2011) A participatory survey on current integration of trees on farms and pastures within land use systems in the township of Tortí in Panama: Bachelor thesis, Institute of Silviculture, Technische Universität München, Freising, Weihenstephan
Sloan S (2008) Reforestation amidst deforestation: simultaneity and succession. Globalisation and environmental governance: Is another world possible? Global Env Change 18(3):425–441
Somarriba E, Valdivieso R, Vásquez W, Galloway G (2001) Survival, growth, timber productivity and site index of Cordia alliodora in forestry and agroforestry systems. Agrofor Syst 51(2):111–118
van Breugel M, Hall JS, Craven DJ, Gregoire TG, Park A, Dent DH, Wishnie MH, Mariscal E, Deago J, Ibarra D, Cedeño N, Ashton MS (2011) Early growth and survival of 49 tropical tree species across sites differing in soil fertility and rainfall in Panama. The ecology and ecosystem services of native trees: implications for reforestation and land restoration in Mesoamerica. For Ecol Manag 261(10):1580–1589. doi:10.1016/j.foreco.2010.08.019
Vandermeer JH (1989) The ecology of intercropping. Cambridge University Press, Cambridge
Vieira DLM, Holl KD, Peneireiro FM (2009) Agro-successional restoration as a strategy to facilitate tropical forest recovery. Rest Ecol 17(4):451–459. doi:10.1111/j.1526-100X.2009.00570.x
Young A (1997) Agroforestry for soil management, 2nd edn. CAB International and ICRAF, New York
Zimmermann JK (2002) Barriers to forest regeneration in an abandoned pasture in Puerto Rico. Rest Ecol 8(4):350–360
Acknowledgments
The authors are grateful to the German Research Foundation (DFG) (Project WE 2069/6-1), the Elite Network of Bavaria and the program “Equal Opportunity for Women in Research and Teaching” by the Technische Universität München for funding this work. This project was furthermore supported by the Forest Finance Group that provided land and labor as well as BARCA SA who provided logistic help. The authors furthermore wish to thank Donna Ankerst for support with statistical analysis and Jörg Prietzel, Peter Schad, Carlos Him and Manuela Theobald for help with analysis of soil samples and all students who helped with measurements. We are grateful to Thomas Knoke for continuous support and collaboration in this project. The authors also thank Laura Carlson for language editing. Finally we would like to express our gratitude to the Editors and two anonymous referees for their valuable suggestions on an earlier version of this manuscript.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paul, C., Weber, M. Effects of planting food crops on survival and early growth of timber trees in eastern Panama. New Forests 47, 53–72 (2016). https://doi.org/10.1007/s11056-015-9477-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-015-9477-5