Abstract
Alzheimer's disease (AD) is the most common cause of dementia in adulthood, followed by cognitive and behavioral deficits. Today, mesenchymal stem cell (MSC)-based therapy is a suitable therapeutic option to improve regenerative medicine approaches against neurodegenerative disorders, including AD. This study aimed to investigate the effects of human Wharton’s jelly-derived MSCs (WJ-MSCs) on AD-like rat models (rats treated with amyloid beta 1-42 (Aβ1-42)) by evaluating the expression of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), as well as the expression of apoptotic factors such as B-cell lymphoma 2 (BCL2, an anti-apoptotic factor to inhibit apoptosis) and BCL2-associated X protein (BAX, a pro-apoptotic factor to regulate apoptosis). After treatment of AD rat models with WJ-MSCs, behavioral tests (i.e., passive avoidance and Morris water maze) showed cognitive improvements, and amelioration of cells in the CA1 area of the hippocampus was detected by cresyl violet staining. Additionally, real-time polymerase chain reaction (RT-PCR) of the hippocampus indicated an increase in the expression level of the BDNF, NGF, and BCL2 genes and a decrease in the expression level of the BAX gene. Overall, the WJ-MSCs improved the cognitive function in AD rat models by increasing the neurotrophic and anti-apoptotic factors and decreasing the pro-apoptotic factor.
Graphical Abstract
This graphical abstract depicts the process of isolating mesenchymal stem cells (MSCs) from human Wharton’s jelly (WJ) and their subsequent use in an Alzheimer’s disease (AD) rat model. The left side of the image illustrates the steps involved in isolating MSCs from WJ. The right side of the image shows how AD rat models are generated under stereotaxic injection of Aβ. In the middle, intranasally administration of MSCs is shown and then how their effects are evaluated using behavioral tests, nissl staining, and RT-qPCR.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD), the most common cause of dementia in the elderly, is associated with memory loss and cognitive and behavioral dysfunctions (Madadi and Mehdizaded 2014). These impairments are mainly caused by the loss of cholinergic neurons, especially basal forebrain cholinergic neurons (BFCN), as a result of extracellular deposition of amyloid beta (Aβ) proteins (senile plaques) and intracellular formation of neurofibrillary tangles (NFTs) in the brain, especially in the hippocampus (Barati et al. 2016; Jahanshahi et al. 2014). It has been reported that nerve growth factor (NGF) controls amyloidogenesis by decreasing the phosphorylation of amyloid precursor protein (APP) (Sampaio et al. 2017). On the other hand, Aβ can cause the brain-derived neurotrophic factor (BDNF) to decrease by disrupting the conversion process from pro-BDNF to mature BDNF (Azman and Zakaria 2022; Sampaio et al. 2017). BDNF is significantly decreased during the end-stage of AD (Girotra et al. 2022; Mungmunpuntipantip and Wiwanitkit 2022), which may finally lead to apoptosis and cell death (Radi et al. 2014). Therefore, apoptosis, also known as programmed cell death, contributes to neuronal cell death in AD due to trophic factor deprivation (Dragunow et al. 1997), as neurotrophins such as NGF and BDNF suppress neuronal apoptosis (Sharma et al. 2021). The most important factors involved in apoptosis include the B-cell lymphoma 2 (BCL2) family (mainly anti-apoptotic BCL2 and pro-apoptotic BAX) and the Caspase family (mainly Caspase3) (Radi et al. 2014; Shaabani et al. 2011; Sharma et al. 2021).
Regarding treatment of AD, currently there is no definitive treatment, and available treatments such as administration of acetylcholinesterase inhibitors (AchEI) can only reduce the symptoms with possible side effects (Aisen et al. 2012; Joyce et al. 2010), which necessitate an effective treatment. Recent mesenchymal stem cell (MSC)-based breakthroughs in preclinical experiments have been shown to be more effective in the treatment of neurodegenerative diseases (Alipour et al. 2019). MSCs are considered the most appropriate option for the treatment of neurodegenerative diseases (Lo Furno et al. 2018) due to their wide range of activities, including self-renewal, replenishing lost cells via differentiation into other cell lineages (Kolf et al. 2007; Yang et al. 2020), triggering neurorestorative processes, providing neuroprotection by secretion of trophic factors and anti-inflammatory cytokines, decreasing oxidative stress and apoptosis, and stimulating in situ neurogenesis (Alipour et al. 2019).
Among different sources of MSCs, recently, the human umbilical cord Wharton’s jelly (WJ) has been considered in MSC-based therapies for neurodegenerative diseases (Lee et al. 2017), as they are non-invasively accessible and overexpress high levels of the neuroregulatory and neurotrophic factors (Donders et al. 2018; Ribeiro et al. 2012), such as BDNF and NGF (Hsieh et al. 2013; Ribeiro et al. 2012). They play an essential role in neurogenesis, synaptic plasticity, inhibition of apoptosis, immunomodulation, and cell survival (Hsieh et al. 2013; Mattson et al. 2004; Teixeira et al. 2013). Among various routes of cell delivery (Park et al. 2018; Qin et al. 2022a, b), intranasal (IN)-administration seems to be more effective and non-invasive because in IN-administration, the cells can easily bypass peripheral organs and the blood-brain barrier (BBB) and migrate along the olfactory nerve into the brain parenchyma and cerebrospinal fluid (CSF) (Danielyan et al. 2009; Tang et al. 2020).
This study aimed to investigate the effects of WJ-MSCs on the cognitive status of Aβ1-42-induced AD-like rat models by evaluating the levels of neurotrophins BDNF and NGF and apoptosis-related factors (BCL2 and BAX) after the IN-administration of these cells.
Materials and methods
Isolation of MSCs from WJ and confirmation of isolated cells
Stem cell isolation and characterization procedures were performed as described in our previous studies (Hour et al. 2020; Qiyami Hour et al. n.d.). Briefly, after getting mother’s consent, human umbilical cords were collected from full-term cesarean section births, WJ was separated, cut into small pieces of 5 mm3, and were transferred into a sterile centrifuge tube containing collagenase type I (300 U/mL) and hyaluronidase (1 mg/mL, Sigma-Aldrich, catalog number H1136) enzymes for the first enzymatic digestion in an incubator (37 °C in 5% CO2) for 1 h. Hyaluronidase specifically targets bonds within the extracellular matrix involving hyaluronic acid (also known as hyaluronan), a major component of the extracellular matrix. It hydrolyzes the β-1,3 bonds between N-acetylglucosamine and glucuronic acid residues in hyaluronic acid, leading to its degradation. This process disrupts the integrity of the extracellular matrix (Jung 2020). After filtering the lysed solution with a 70-μm cell strainer and centrifuging at 300 g for 5 minutes, the remaining tissue was transferred to another centrifuge tube, containing 0.1% trypsin enzyme (Sigma) for the second enzymatic digestion for 30 minutes. Next, it was filtered and centrifuged again. Both cell pellets derived from two enzymatic digestion steps were mixed, suspended, and cultured in DMEM (Gibco, Billings, USA), containing 10% fetal bovine serum (FBS; Sigma) and 1% penicillin-streptomycin solution (Invitrogen) for expansion. The viability of isolated cells was assessed by the trypan blue exclusion method, using 0.4% trypan blue dye (Sigma).
Flow cytometry
To quantitatively detect the mesenchymal CD markers in WJ-isolated cells, passage 3 cells were incubated with monoclonal mouse anti-human antibodies against mesenchymal markers (CD105 and CD73) as positive markers and hematopoietic markers (CD45 and CD34) as negative markers, followed by 10 mg/mL of fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG) antibodies (Abcam) for 1h at room temperature. Also, a fluorescence-activated cell sorting (FACS) analyzer (Becton, Dickinson) and FlowJo software were used for antibody binding analysis and data analysis, respectively.
Immunocytochemistry (ICC)
To qualitatively confirm the mesenchymal CD markers on WJ-isolated cells, passage 3 cells were incubated with primary anti-human nuclei antibodies against mesenchymal markers (CD105 and CD73), hematopoietic marker (CD31 as a negative marker), and neuronal marker (β-tubulin III as a negative marker). The coverslips were mounted and observed under a Nikon Eclipse TE300 inverted microscope (Spectra Services), and images were acquired by a charge-coupled device (CCD) camera connected to a microscope.
Osteogenic and adipogenic differentiation
The passage 3 cells were incubated in two differentiation media: i) an osteogenic induction medium (DMEM‐LG+10% FBS, 50 μg/mL of ascorbic acid 2-phosphate, 10−8 M dexamethasone, and 10 mM β‐glycerophosphate; Invitrogen) for 14 days, and ii) an adipogenic differentiation medium (DMEM+1 g/mL of glucose [DMEM‐LG], 10% FBS, 50 μg/mL of ascorbate 1 phosphate, 10−7 M dexamethasone, and 50 μg/mL of indomethacin; Invitrogen, Waltham, USA) for 21 days. The media in both cell types were changed every three days, and completion of cell differentiation was established by morphology and staining (i.e., Alizarin Red S for osteocytes and Oil Red O for adipocytes; Sigma).
AD modeling by Aβ1-42 treatment and IN-administration of WJ-MSCs
Adult 220–260 g male Wistar rats were obtained from the IUMS animal lab. All animal experiments were approved by IUMS Animal Care, and adequate measures were taken to minimize pain or discomfort. The rats were randomly divided into five groups (n = 8 in each group) as follows: i) control group: intact rats; ii) sham group: rats were subjected to anesthesia, surgery, intra-hippocampal PBS injection, and intranasal PBS and hyaluronidase administration at the appropriate time. This allows us to assert that manipulations such as anesthesia, surgery, and injection of PBS and hyaluronidase do not cause cognitive impairment or biochemical changes. Injecting hyaluronidase into the sham group is crucial because its potential entry via the same route as MSCs can lead to extracellular matrix degradation. This possible degradation has the potential to impact synaptic plasticity and serves as a method for inducing delayed status epilepticus (Balashova et al. 2019; Blondiaux et al. 2023); iii) MSCs group: IN-administration of WJ-MSCs to study the possible effects of the IN-MSCs administration to normal healthy rats; iv) AD model group: rats were subjected to intra-hippocampal Aβ1-42 injection to generate AD-like rat models; and v) IN-MSC-treated group: AD rat models were subjected to IN-administration of WJ-MSCs.
To generate AD rat models, Aβ1-42 treatment (Qin et al. 2022a, b) was performed as described in our previous study (Hour et al. 2020). Briefly, rats were anesthetized using ketamine/xylazine (50/4 mg/kg, i.p.), and fixed in the stereotaxic device. After exposing the skull, freshly prepared Aβ 1–42 (Sigma, 8 µg/kg of Aβ1-42 in 16 μl PBS – almost 2 µg of Aβ in 4 μl PBS for each rat) was administered using a Hamilton microsyringe during 3 min into the dorsal hippocampus bilaterally (1 µg Aβ in 2 μl PBS on each side) according to Paxinos rat brain atlas (coordinates: 3.6 mm posterior, ± 2 mm lateral to the bregma, and 3.2 mm ventral to the skull surface).
IN-administration of WJ-MSCs in the IN-MSC-treated group was performed on day 14 after Aβ1-42 treatment as previously described (Danielyan et al. 2009; Shahror et al. 2019). For IN-administration, briefly, animals were anesthetized and immobilized facing upward. First, 100U hyaluronidase was freshly dissolved in sterile PBS (4 U/μl) and 3 μl of the suspension administered in each nostril using a pipette, which was repeated 4 times up to almost 100U of hyaluronidase suspension. Next, after keeping treated rats facing upward for 30 min, 3×105 WJ-MSCs/rat was suspended in 36 μl PBS and administered 6 μl/nostril. After 30 seconds, the administration was repeated three times with a two-minute interval between each.
Behavioral assessments
Two months after MSCs administration (Hour et al. 2020; Simorgh et al. 2021b), to evaluate the effect of WJ-MSCs on spatial learning and memory, two tests, including passive avoidance (PA) response and Morris water maze (MWM), were conducted:
PA response
This test was performed using a shuttle box device with two connected equal-sized chambers, separated by a guillotine door, as previously described (Vafaee et al. 2018). First, a habituation trial was performed to familiarize all animals with the apparatus without any stimuli. Next, for the acquisition trial, the animals in all groups were guided individually into the illuminated chamber for ten seconds, and the guillotine door was opened to determine the latency to enter the dark chamber as the initial latency (IL). Once the animal entered the dark chamber, its feet were exposed to electrical stimulation (0.5 mA, 50 Hz, 2 sec) through a stainless-steel floor. Finally, after 24 hours, for the retention trial, the rats re-entered the light chamber without any foot shock to record latency to enter the dark chamber as retention time (step through latency [STL]). The total time spent in the dark chamber was also recorded as an indicator of contextual learning. The maximum cutoff time for STL and the time spent in the dark chamber were 300 and 600 seconds, respectively. If a rat avoided entering the dark chamber for up to 300 seconds, the acquisition of PA response was considered successful.
MWM test
spatial reference learning and memory were evaluated in the water maze task as previously described (Mehdizadeh et al. 2017; Morris et al. 1982). This test was performed over six days using a circular water tank (22 °C), which was divided into four imaginary quadrants with a platform inside and a camera above the tank, as previously described (Morris 1984; Tamtaji et al. 2017). The MWM test included three phases: (1) habituation phase on the first day with an apparent platform in the center of the tank; (2) acquisition phase in which the learning goals were achieved on days 2-5 with a hidden platform in one of the quadrants (each day for four trials, with each trial lasting 60 seconds); and (3) the probe trial phase on day six with no platform in which the time spent and the distance traveled in the target quadrant (in which the platform was situated in the acquisition phase) were measured as two criteria for spatial memory.
Histological assessments
RNA extraction and real-time quantitative reverse transcription-polymerase chain reaction (RT-qPCR)
Total RNA from the hippocampus was extracted and purified with a TRIzol™ reagent (Sigma, Pool, UK), according to the manufacturer's instructions, and its concentration was measured with a NanoDrop ND-100 spectrophotometer. The RT-qPCR assay was employed to quantify the gene expression levels of neurotrophic factors (BDNF and NGF) and apoptosis-related factors (BCL2 and BAX) in hippocampus. For this purpose, 500 ng of RNA was reverse-transcribed into complementary DNA (cDNA) with the Transcriptor High-Fidelity cDNA Synthesis Kit (Invitrogen, Paisley, UK) using oligo (dT) primers (Roche, Germany). Next, 1 µL of cDNA was amplified using Opticon II (Invitrogen, Paisley, UK) and SYBR Green PCR Master Mix (Invitrogen, Paisley, UK), based on the manufacturer's instructions.
The PCR assay was performed in 40 cycles at an annealing temperature of 60°C for all genes. The primers used in this study were specifically designed between two adjacent exons in the Gene Runner program, and the sequences are listed in Table 1. The mRNA levels for target genes were normalized to the reference gene (β-actin) by subtracting the cycle threshold (CT) of the reference gene (β-actin) from the CT value of the samples (ΔCT = CTsample-CTreference). The relative expression of the calibrator target gene was quantified using the 2-ΔΔCt method.
Cresyl violet (Nissl) staining
Two months after cell therapy, this test was performed to distinguish healthy neurons from damaged neurons in the cornu ammonis-1 (CA1) area of rats’ hippocampus (Faghani et al. 2016). Briefly, mice were transcardially perfused using PBS and 4% paraformaldehyde (PFA), and the samples were post-fixed in 4% PFA for overnight and cryopreserved in 30% sucrose. Then, 7μm coronal sections (5 sections) were taken from -3.84 to -5.8 from Bregma, the sections were gently transferred onto gelatinized slides and stained with cresyl violet stain. An optical microscope (Carl Zeiss) was used to take images from stained slides.
Statistical analysis
All results were considered significant at P<0.05 and expressed as mean±SEM. Using GraphPad Prism (V5; GraphPad), the normality of the data was checked by Kolmogorov-smirnov, and group differences in PA, MWM, and RT-qPCR were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. To analyze acquisition performance on MWM, repeated measures of two-way ANOVA for the swimming time among the groups were followed by Tukey’s test. Image analysis of cresyl violet (Nissl) staining was done using ImageJ software.
Results
WJ-MSCs
Adherent stem cells were isolated from the WJ of the human umbilical cord with a viability > 95%, with a spindle-shaped morphology (Figure 1a). The ability to differentiate into osteocytes by producing calcium nodules (Figure 1b) and into adipocytes by producing lipid droplets (Figure 1c), which is one of the important characteristics of MSCs, confirmed that the WJ-derived cells were mesenchymal cells. The flow cytometry indicated that these cells strongly expressed mesenchymal markers, CD105 and CD73, but did not noticeably express hematopoietic markers, CD34 and CD45 (Figure 1d). The ICC showed that the WJ-MSCs were significantly positive for mesenchymal markers, CD105 and CD73, and negative for hematopoietic and neuronal markers (CD31 and β-tubulin III, respectively) (Figure 1e).
Human Wharton’s jelly-derived cells (WJ-cells) and their in vitro differentiation towards other cell lineage types. a) The cells isolated from Wharton’s jelly are adherent with a spindle-shaped fibroblast-like phenotype. Differentiation into osteocytes (b) and adipocytes (c) caused a considerable amount of calcium nodules (arrows in b) and lipid droplets (arrows in c). d) Flow cytometry analysis showed that a large amount of WJ-SCs was positive for both CD105 and CD73 mesenchymal markers, while just a small amount of them expressed the hematopoietic markers of CD34 and CD45. e) Immunocytochemistry (ICC) analysis showed that the CD105 and CD73 mesenchymal markers are largely expressed in WJ-derived stem cells and came in green with FITC. Hematopoietic marker of CD31 and neuronal marker of β tubulin III are weakly expressed in this population of cells. Blue color: nuclei of cells stained by DAPI
IN-delivered WJ-MSCs alleviated memory deficits in AD rat models
PA response
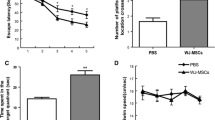
The step-through PA test indicated very short and almost similar IL in entering the dark chamber before applying electric shock in all animal groups (Figure 2a). After applying an electric shock, STL was significantly lower in the AD model group as compared to the control, sham, and MSCs groups; however, it was significantly higher in the IN-MSC-treated group as compared to the AD group (**** p < 0.0001; Figure 2b). The time spent in the dark chamber was significantly longer in the AD model group as compared to the other groups (**** p < 0.0001; Figure 2c), while it was significantly shorter in the IN-MSC-treated group as compared to the AD groups (**** p < 0.0001; Figure 2c). For both STL and time spent in the dark chamber, the difference between IN-MSC-treated group and the control, sham, and MSCs groups was less significant (* p < 0.05).
Passive avoidance (PA) response test. a) Initial latency (IL, before applying the electric shock), and b) step-through latency (STL, after applying the electric shock) in entering into the dark chamber. IL was very low and none of the groups differed in IL (p > 0.05). The IN-MSC-treated group showed an increased STL two months after treatment which was significantly higher than it was in the AD model group. STL was significantly lower in the AD model group than it was in the control, sham, and MSCs groups. c) Time spent in the dark chamber after applying electric shock was significantly lower in the IN-MSC-treated group than it was in the AD model group and significantly higher in the AD model group than it was in the other groups. **** p < 0.0001; * p < 0.05; ns: not-significant
MWM test
Acquisition performance was assessed two months after the treatments. During the training sessions in the MWM, all groups exhibited significant trial effects in the learning procedure. The IN-MSC-treated group learned to find the platform faster than the AD group (**** p < 0.0001; Figure 3a). None of the groups differed in swimming speed (Figure 3b). Both the time spent (Figure 3c) and the distance travelled (Figure 3d) in the target quadrant on the probe trial test were significantly lower in the AD model group as compared to the other groups. These parameters were significantly higher in the IN-MSC-treated group as compared to the AD model group (**** p < 0.0001). Also, regarding these parameters, the difference between the IN-MSC-treated group and the control, sham, and MSCs groups was less significant (* p < 0.05).
Morris Water Maze (MWM) test. a) Acquisition performance was assessed two months after the treatment. The results are the mean swimming time moved per trial toward the platform. The mean values of the 16 trials for 4 days for each group are shown (**** p < 0.0001 as compared to the corresponding data from the AD model group). b) None of the groups differed in swimming speed. c) Time spent and d) distance moved in the target quadrant. The IN-MSC-treated group showed an increased distance moved and also an increased time spent in the target quadrant, two months after of treatment which was significantly high as compared to AD model group. Also, both time spent and distance moved in the target quadrant were significantly low in AD model group as compared to the other groups. **** p < 0.0001; * p < 0.05; ns: not-significant
Gene expression levels in the hippocampus
The mean mRNA levels of neurotrophic factors BDNF, NGF, and anti-apoptotic factor BCL2 in the AD model group were significantly lower than they were in the control, sham, and MSCs groups (Figure 4; **** p < 0.0001). Two months after treatment with the WJ-MSCs in the IN-MSC-treated group, the mRNA level of these genes (i.e., BDNF, NGF, and BCL2) significantly increased as compared to the AD model group (Figure 4; *** p < 0.001). Considering the pro-apoptotic factor BAX, the mean mRNA level was significantly higher in the AD model group as compared to the other groups, while it significantly decreased in the IN-MSC-treated group as compared to the AD model group (Figure 4; **** p < 0.0001). The difference between the IN-MSC-treated group and the control, sham, and MSCs groups was not significant for BDNF and NGF, and it was less significant for BCL2 and BAX (** p < 0.001, * p < 0.01).
Evaluation of mRNA levels gene expression by RT-qPCR in the hippocampus. The mean mRNA level of neurotrophins BDNF and NGF and anti-apoptotic factor BCL2 in the AD model group was significantly low as compared to the control, sham, and MSCs groups (**** p < 0.0001) and IN-MSC-treated group (*** p < 0.001). Two months after the treatment with WJ-MSCs in the IN-MSC-treated group, the level of mRNA for BDNF, NGF, and BCL2 was significantly increased as compared to the AD model group (*** p < 0.001). Regarding apoptotic factor BAX, the mean mRNA level was significantly high in the AD rat models as compared to the control, sham, and MSCs groups (**** p < 0.0001), and it was significantly decreased in the IN-MSC-treated group as compared to the AD model group. Data are represented as mean ± SEM. β-actin was used as an internal housekeeping control. (** p < 0.01, * p < 0.05, ns: not-significant). BDNF: brain-derived neurotrophic factor, NGF: nerve growth factor, BCL2: B-cell lymphoma 2, BAX: BCL2-associated X protein.
Nissl-stained cells decreased following the IN delivery of WJ-MSCs
Figure 5a shows the Nissl-stained images of the CA1 area, where the number of dark cells (cells with a cell body shrinkage) was significantly higher in the AD rats as compared to the other groups. The quantitative analysis showed a significantly higher level of dark cells in the AD model group as compared to the other groups. However, significantly low level of dark cells was detected in the IN-MSC-treated group as compared to the AD model group (Figure 5b; **** p < 0.0001). The difference between IN-MSC-treated and control, sham, and MSCs groups was not significant.
a) Nissl staining of cornu ammonis-1 (CA1) region of the hippocampus in which arrows show the nissl-stained dead or dark cell. b) The quantitative analysis showed that the number of nissl-stained dark cells was significantly higher in the CA1 area of the hippocampus of the AD rat models as compared to other groups (**** p < 0.0001). Two months after treatment with WJ-MSCs, the number of dark cells was significantly decreased in IN-MSC-treated group as compared to the AD model group (* p < 0.05). Scale bars = 50 µm; ns: not-significant
Discussion
Neurodegeneration and apoptosis play a key role in memory and learning deficits (Mattson 2000; Wozniak et al. 2004). In this study, IN-administered human WJ-MSCs in AD rat models effectively improved behavioral and cognitive performance by improving memory and learning capabilities. This finding was assessed by measuring the levels of trophic and apoptosis-related factors in the hippocampus. Aligned with previous studies (Beigi Boroujeni et al. 2020; Salehi et al. 2022; Shahror et al. 2019; Simorgh et al. 2021a), the IN route of MSC delivery to the brain was more effective in the present study. In this regard, Danielyan et al. examined the possible routes of IN-administered cells and hypothesized that these cells could bypass the BBB by migrating from the nasal mucosa through the cribriform plate along the olfactory neural pathway into the brain and CSF (Danielyan et al. 2009). In addition, owing to MSCs’ preferential tendency to migrate to degenerating brain lesions (Yu-Taeger et al. 2019), the IN route could be closer and less invasive.
Throughout all the experiments, no differences were found between the control and MSCs groups. This suggests that administering WJ-MSCs in healthy animals does not impact the behavior and expression levels of neurotrophic and apoptosis-related factors. However, the WJ-MSCs effectively enhanced the trophic support in AD rat models by increasing the mRNA levels of BDNF and NGF in the hippocampus, leading to improved cognitive performance on MWM and PA response tests. Moreover, it has been reported that application of fetal human neural stem cells (hNSCs) in the brain of transgenic mice could excrete high levels of neurotrophic factors, including BDNF and NGF, and activate the Akt/GSK3β (protein kinase B/glycogen synthase kinase-3beta) signaling pathway, resulting in the prevention of tau phosphorylation, attenuation of Aβ synaptotoxicity, promotion of synaptic plasticity, and therefore, enhanced spatial memory (Lee et al. 2015; Pang et al. 2023). In a previous study, although NSC transplantation in a transgenic model of AD had no significant effects on either Aβ or tau, it led to cognitive improvements by elevating the BDNF level and increasing the hippocampal synaptic density (Blurton-Jones et al. 2009).Similarly, other studies demonstrated that these neurotrophins are essential to neural survival. For instance, overexpression of BDNF or BDNF gene delivery have demonstrated the ability to diminish behavioral impairments, decrease neuronal abnormality, mitigate synaptic degeneration, and hinder neuron depletion (Jiao et al. 2016). Furthermore, BDNF can potentially have positive feedback in stem cell-based therapy. Elevated BDNF levels enhance the effectiveness of transplanted neural stem cells (NSCs) in treating AD by promoting neuronal replacement and neurogenic impacts, thereby enhancing the viability of transplanted cells (or IN-administered WJ-MSCs in our study), synaptic maturation, and synaptic density (Wu et al. 2016). Recently, it has been reported that IN-delivered bone marrow-derived stem cells (BMSCs) in Parkinson’s disease (PD) mice could successfully migrate into the hippocampus, olfactory bulb, substantia nigra, striatum, and lateral ventricle and enhance the BDNF level in these areas, resulting in the survival of existing dopaminergic neurons and, finally, functional and motor improvements (Tang et al. 2020). Also, in the aforementioned study, after IN-administration of BMSCs, the expression of Nestin, a neuronal precursor cell marker, was increased in the subventricular zone (SVZ), indicating the stimulation of endogenous neurogenesis (Tang et al. 2020). Regarding NGF, a clinical trial of NGF gene therapy in AD patients found these factors to be useful in preventing cognitive decline (Tuszynski et al. 2005). In addition, we anticipate positive feedback or reinforcement from overexpression of NGF in our research. Implantation of neural stem cells (NSCs) transduced with human NGF via an adeno-associated virus (AAV) vector into the cerebral cortex of rats with cognitive impairments has been demonstrated to effectively merge with the host brain and enhance cognitive function post-transplantation (Wu et al. 2008).
The current study demonstrated that WJ-MSCs suppressed apoptosis in Aβ1-42-induced AD-like rat models by enhancing the expression of anti-apoptotic factor (BCL2) and suppressing the expression of apoptotic factor (BAX). WJ-MSCs may help the rat overcome the internal triggers (starvation, hypoxia, and cellular dysfunction) for initiator Caspase 9 which triggers the executioner Caspase 3 (Sharma et al. 2021). Besides its neurotrophic activity, BDNF can induce multiple neuroprotective mechanisms, including anti-apoptosis (by expression of anti-apoptotic BCL2 proteins), anti-oxidation (by expression of antioxidative thioredoxins), and suppression of autophagy and neuronal death in neurodegenerative diseases (Chen et al. 2017). The transplantation of bone marrow-derived mesenchymal stem cells (BMMSCs) in mice with AD-like symptoms have the potential to reduce apoptotic cell death by suppressing caspase-3 activity via the enhancement of anti-apoptotic protein BCL2 (Qin et al. 2022a, b). The development of AD involves a dynamic interplay between autophagy and apoptosis, in which reduced autophagy coincides with neuronal apoptosis (Qin et al. 2022a, b). The interaction between autophagy and apoptosis involves shared regulators where the BCL2 protein can influence signaling molecules such as Beclin1 (Qin et al. 2022a, b), in which overexpression of Beclin-1 decreases amyloid pathology in AD transgenic mouse models (Pickford et al. 2008). Furthermore, treatment with membrane-free stem cell extract (MFSCE), derived from adipose-tissue stem cells, improved learning and memory in Aβ25-35-induced AD mice by inhibiting BAX and cleaved caspase-3 protein expression (Choi et al. 2022). Another in vitro study demonstrated that dental pulp stem cells (DPSC) secretome increases the BCL2 and decreases the apoptotic regulator BAX (Ahmed et al. 2016). Therefore, secretome and/or exosome of IN-administered WJ-MSCs, in our work, have the potential to regulate apoptosis and autophagy. However, one of the limitations of this study is the suboptimal selection of primers for BCL2 and BDNF, potentially leading to off-target products affecting the measured expression levels. According to the Primer Blast database for Wistar rats (taxid: 10116) it seems that the used primers for BCL2 in this study lack specific annealing, while predicted off-target products have similar lengths, a concern shared with the BDNF primers. Furthermore, Aβ1-42 may influence actin dynamics in neurons at the protein level (Mendoza-Naranjo et al. 2012), suggesting the consideration of alternative housekeeping genes instead of β-actin in future studies since its expression level does not change in experimental groups after amyloid administration compared to the control group.
Since there are many controversies about whether MSCs differentiate into neurons, or into mesenchymal tissue supporting neurons or directly excrete neurotrophic factors or even stimulate the neural stem cells (NSCs) in the brain regions such as SVZ and subgranular zone (SGZ), more research regarding the mechanisms behind the neuronal differentiation would be necessary. In addition, further investigation is required here to define optimal amount of MSCs needed for IN-administration before the conducting of this approach in clinical trials. The research faces limitations due to the inability to track mesenchymal stem cells (MSCs) effectively in the brain using staining methods like immunohistochemistry (IHC). This hinders the verification of their penetration into the brain and survival without succumbing to factors such as induced immune responses. Future studies will focus on overcoming this constraint by implementing improved tracking methods. Additionally, WJ-MSCs typically express CD44 (Hour et al. 2020; Qiyami Hour et al. n.d.; Ranjbaran et al. 2018), which is an established receptor for hyaluronic acid. The interaction between MSCs and hyaluronic acid, particularly through the CD44 receptor, is disrupted by hyaluronidase treatment, potentially altering their behavior within the tissue environment. This could affect crucial cellular processes and consequently impact the efficacy of cell-based therapy. Furthermore, hyaluronidase treatment may impair the olfactory epithelium, potentially leading to anosmia and modifying behavioral test results (van Rijzingen et al. 1995). To address these issues, future research should explore alternative methods to hyaluronidase and assess the sensitivity of animals to odors to better understand its effects on behavioral outcomes.
Conclusion
Neurotrophic factors play an essential role in neuron survival, and a decline in their expression levels seems to lead to the progression of apoptosis and neurodegeneration. Among new drug delivery and gene therapy approaches, MSC-based cell therapy can be considered the main therapeutic tool since MSCs can play a supporting role in the secretion of necessary trophic factors, according to the needs of the target organ. The results of this study showed that IN delivery of human WJ-MSCs in AD rats could significantly improve learning and memory by enhancing trophic support and suppressing apoptosis. Neurotrophic support of cholinergic neurons could result from the increased levels of BDNF and NGF in the hippocampus. Also, the increased level of anti-apoptotic factor (BCL2), besides the decreased levels of apoptotic factor (BAX) in the hippocampus, indicated suppressed apoptosis in WJ-MSC-treated AD rat models.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed, N. E.-M. B., Murakami, M., Hirose, Y., & Nakashima, M. (2016). Therapeutic potential of dental pulp stem cell secretome for Alzheimer’s disease treatment: an in vitro study. Stem Cells International, 2016.
Aisen, P. S., Cummings, J., & Schneider, L. S. (2012). Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harbor perspectives in medicine, 2(3), a006395.
Alipour, M., Nabavi, S. M., Arab, L., Vosough, M., Pakdaman, H., Ehsani, E., & Shahpasand, K. (2019). Stem cell therapy in Alzheimer’s disease: possible benefits and limiting drawbacks. Molecular biology reports, 46(1), 1425-1446.
Azman, K. F., & Zakaria, R. (2022). Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int J Mol Sci, 23(12). https://doi.org/10.3390/ijms23126827
Balashova, A., Pershin, V., Zaborskaya, O., Tkachenko, N., Mironov, A., Guryev, E., … Mukhina, I. (2019). Enzymatic digestion of hyaluronan-based brain extracellular matrix in vivo can induce seizures in neonatal mice. Frontiers in neuroscience, 13, 1033.
Barati, E., Asl, S. S., Jamshidian, M., & Shahidi, S. (2016). Effect of Borage on hipocampal TNF-α protein and gene in the Amyloid β-Peptide (25–35)-Induced of Alzheimer model in rat. Biosciences Biotechnology Research Asia, 13(1), 37-42.
Beigi Boroujeni, F., Pasbakhsh, P., Mortezaee, K., Pirhajati, V., Alizadeh, R., Aryanpour, R., … Ragerdi Kashani, I. (2020). Intranasal delivery of SDF‐1α‐preconditioned bone marrow mesenchymal cells improves remyelination in the cuprizone‐induced mouse model of multiple sclerosis. Cell biology international, 44(2), 499-511.
Blondiaux, A., Jia, S., Annamneedi, A., Çalışkan, G., Nebel, J., Montenegro-Venegas, C., … Stork, O. (2023). Linking epileptic phenotypes and neural extracellular matrix remodeling signatures in mouse models of epilepsy. Neurobiology of disease, 188, 106324.
Blurton-Jones, M., Kitazawa, M., Martinez-Coria, H., Castello, N. A., Müller, F.-J., Loring, J. F., … LaFerla, F. M. (2009). Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proceedings of the National Academy of Sciences, 106(32), 13594-13599.
Chen, S.-D., Wu, C.-L., Hwang, W.-C., & Yang, D.-I. (2017). More insight into BDNF against neurodegeneration: anti-apoptosis, anti-oxidation, and suppression of autophagy. International journal of molecular sciences, 18(3), 545.
Choi, J. M., Park, H. S., He, M. T., Kim, Y. S., Kim, H. Y., Lee, A. Y., & Cho, E. J. (2022). Membrane-Free Stem Cells and Pyridoxal 5′-Phosphate Synergistically Enhance Cognitive Function in Alzheimer’s Disease Mouse Model. Antioxidants, 11(3), 601.
Danielyan, L., Schäfer, R., von Ameln-Mayerhofer, A., Buadze, M., Geisler, J., Klopfer, T., … Ayturan, M. (2009). Intranasal delivery of cells to the brain. European journal of cell biology, 88(6), 315-324.
Donders, R., Bogie, J. F., Ravanidis, S., Gervois, P., Vanheusden, M., Marée, R., … Gijbels, K. (2018). Human Wharton's Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem cells and development, 27(2), 65-84.
Dragunow, Μ., MacGibbon, G., Lawlor, P., Butterworth, N., Connor, B., Henderson, C., … Faull, R. (1997). Apoptosis, neurotrophic factors and neurodegeneration. Reviews in the Neurosciences, 8(3-4), 223-265.
Faghani, M., Ejlali, F., Sharifi, Z. N., Molladoost, H., & Movassaghi, S. (2016). The Neuroprotective Effect of Atorvastatin on Apoptosis of Hippocampus Following Transient Global Ischemia/Reperfusion. Galen Medical Journal, 5(2), 82-89.
Girotra, P., Behl, T., Sehgal, A., Singh, S., & Bungau, S. (2022). Investigation of the molecular Role of brain-derived neurotrophic factor in Alzheimer’s disease. Journal of Molecular Neuroscience, 72(2), 173-186.
Hour, F. Q., Moghadam, A. J., Shakeri-Zadeh, A., Bakhtiyari, M., Shabani, R., & Mehdizadeh, M. (2020). Magnetic targeted delivery of the SPIONs-labeled mesenchymal stem cells derived from human Wharton's jelly in Alzheimer's rat models. Journal of Controlled Release.
Hsieh, J.-Y., Wang, H.-W., Chang, S.-J., Liao, K.-H., Lee, I.-H., Lin, W.-S., … Cheng, S.-M. (2013). Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PloS one, 8(8).
Jahanshahi, M., Shabani, R., Nikmahzar, E., & Babakordi, F. (2014). Female rat hippocampal cell density after conditioned place preference. Folia Biologica (Czech Republic), 60(1), 47-51.
Jiao, S., Shen, L., Zhu, C., Bu, X., Liu, Y., Liu, C., … Walker, D. (2016). Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Translational psychiatry, 6(10), e907-e907.
Joyce, N., Annett, G., Wirthlin, L., Olson, S., Bauer, G., & Nolta, J. A. (2010). Mesenchymal stem cells for the treatment of neurodegenerative disease. Regenerative medicine, 5(6), 933-946.
Jung, H. (2020). Hyaluronidase: An overview of its properties, applications, and side effects. Archives of plastic surgery, 47(04), 297-300.
Kolf, C. M., Cho, E., & Tuan, R. S. (2007). Mesenchymal stromal cells: biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis research & therapy, 9(1), 204.
Lee, I.-S., Jung, K., Kim, I.-S., Lee, H., Kim, M., Yun, S., … Park, K. I. (2015). Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Molecular neurodegeneration, 10(1), 38.
Lee, N. K., Park, S. E., Kwon, S. J., Shim, S., Byeon, Y., Kim, J.-H., … Chang, J. W. (2017). Agouti related peptide secreted via human mesenchymal stem cells upregulates proteasome activity in an Alzheimer’s disease model. Scientific reports, 7(1), 1-9.
Lo Furno, D., Mannino, G., & Giuffrida, R. (2018). Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. Journal of cellular physiology, 233(5), 3982-3999.
Madadi, S., & Mehdizaded, M. (2014). Alzheimer diseases. Avicenna Journal of Neuro Psych Physiology, 1(2).
Mattson, M. P. (2000). Apoptosis in neurodegenerative disorders. Nature reviews Molecular cell biology, 1(2), 120-130.
Mattson, M. P., Maudsley, S., & Martin, B. (2004). BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends in neurosciences, 27(10), 589-594.
Mehdizadeh, M., Dabaghian, F. H., Shojaee, A., Molavi, N., Taslimi, Z., Shabani, R., & Asl, S. S. (2017). Protective effects of cyperus rotundus extract on amyloid β-peptide (1-40)-induced memory impairment in male rats: a behavioral study. Basic and clinical neuroscience, 8(3), 249.
Mendoza-Naranjo, A., Contreras-Vallejos, E., Henriquez, D. R., Otth, C., Bamburg, J. R., Maccioni, R. B., & Gonzalez-Billault, C. (2012). Fibrillar amyloid-β 1-42 modifies actin organization affecting the cofilin phosphorylation state: a role for Rac1/cdc42 effector proteins and the slingshot phosphatase. Journal of Alzheimer's disease, 29(1), 63-77.
Morris, R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods, 11(1), 47-60.
Morris, R. G., Garrud, P., Rawlins, J. a., & O'Keefe, J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297(5868), 681-683.
Mungmunpuntipantip, R., & Wiwanitkit, V. (2022). Brain-derived neurotrophic factor and its clinical applications. Medical Journal of Dr. DY Patil University, 15(5), 619-628.
Pang, S., Li, S., Cheng, H., Luo, Z., Qi, X., Guan, F., … Gao, X. (2023). Discovery of an evodiamine derivative for PI3K/AKT/GSK3β pathway activation and AD pathology improvement in mouse models. Frontiers in Molecular Neuroscience, 15, 1025066.
Park, S. E., Lee, N. K., Na, D. L., Chang, J. W., & Chang, J. W. (2018). Optimal mesenchymal stem cell delivery routes to enhance neurogenesis for the treatment of Alzheimer’s disease. Histology and histopathology: cellular and molecular biology, 33(6), 533-541.
Pickford, F., Masliah, E., Britschgi, M., Lucin, K., Narasimhan, R., Jaeger, P. A., … Levine, B. (2008). The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. The Journal of clinical investigation, 118(6), 2190-2199.
Qin, C., Bai, L., Li, Y., & Wang, K. (2022a). The functional mechanism of bone marrow-derived mesenchymal stem cells in the treatment of animal models with Alzheimer’s disease: crosstalk between autophagy and apoptosis. Stem cell research & therapy, 13(1), 1-13.
Qin, C., Wang, K., Zhang, L., & Bai, L. (2022b). Stem cell therapy for Alzheimer’s disease: An overview of experimental models and reality. Animal models and experimental medicine, 5(1), 15-26.
Qiyami Hour, F., Shabani, R., Ashtrai, B., Moinzadeh, A., & Mehdizadeh, M. Labelling of human Wharton's jelly‐derived mesenchymal stem cells with gold nanorods by biomimicry method. Cell Biochemistry and Function.
Radi, E., Formichi, P., Battisti, C., & Federico, A. (2014). Apoptosis and oxidative stress in neurodegenerative diseases. Journal of Alzheimer's disease, 42(s3), S125-S152.
Ranjbaran, H., Abediankenari, S., Mohammadi, M., Jafari, N., Khalilian, A., Rahmani, Z., … Ebrahimi, P. (2018). Wharton's jelly derived-mesenchymal stem cells: Isolation and characterization. Acta Medica Iranica, 28-33.
Ribeiro, C. A., Fraga, J. S., Grãos, M., Neves, N. M., Reis, R. L., Gimble, J. M., … Salgado, A. J. (2012). The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem cell research & therapy, 3(3), 18.
Salehi, M. S., Jurek, B., Karimi-Haghighi, S., Nezhad, N. J., Mousavi, S. M., Hooshmandi, E., … Miyan, J. A. (2022). Intranasal application of stem cells and their derivatives as a new hope in the treatment of cerebral hypoxia/ischemia: a review. Reviews in the Neurosciences.
Sampaio, T. B., Savall, A. S., Gutierrez, M. E. Z., & Pinton, S. (2017). Neurotrophic factors in Alzheimer's and Parkinson's diseases: implications for pathogenesis and therapy. Neural regeneration research, 12(4), 549.
Shaabani, R., Jahanshahi, M., Nowrouzian, M., Sadeghi, Y., & Azami, N. (2011). Effect of morphine based CPP on the hippocampal astrocytes of male Wistar rats. Asian Journal of Cell Biology, 6(3), 89-96.
Shahror, R. A., Wu, C.-C., Chiang, Y.-H., & Chen, K.-Y. (2019). Tracking Superparamagnetic Iron Oxide-labeled Mesenchymal Stem Cells using MRI after Intranasal Delivery in a Traumatic Brain Injury Murine Model. JoVE (Journal of Visualized Experiments)(153), e60450.
Sharma, V. K., Singh, T. G., Singh, S., Garg, N., & Dhiman, S. (2021). Apoptotic pathways and Alzheimer’s disease: probing therapeutic potential. Neurochemical Research, 46(12), 3103-3122.
Simorgh, S., Alizadeh, R., Shabani, R., Karimzadeh, F., Seidkhani, E., Majidpoor, J., … Kasbiyan, H. (2021a). Olfactory mucosa stem cells delivery via nasal route: a simple way for the treatment of Parkinson disease. Neurotoxicity Research, 39(3), 598-608.
Simorgh, S., Bagher, Z., Farhadi, M., Kamrava, S. K., Boroujeni, M. E., Namjoo, Z., … Alizadeh, R. (2021b). Magnetic targeting of human olfactory mucosa stem cells following intranasal administration: a novel approach to Parkinson’s disease treatment. Molecular Neurobiology, 58, 3835-3847.
Tamtaji, O. R., Hosseinzadeh, H., Talaei, S. A., Behnam, M., Firoozeh, S. M. T., Taghizadeh, M., & Alipoor, R. (2017). Protective Effects of Red Onion (Allium cepa) Ethanolic Extract on Learning and Memory Impairments in Animal Models of Diabetes. Galen Medical Journal, 6(3), 249-257.
Tang, Y., Han, L., Bai, X., Liang, X., Zhao, J., Huang, F., & Wang, J. (2020). Intranasal Delivery of Bone Marrow Stromal Cells Preconditioned with Fasudil to Treat a Mouse Model of Parkinson’s Disease. Neuropsychiatric Disease and Treatment, 16, 249.
Teixeira, F. G., Carvalho, M. M., Sousa, N., & Salgado, A. J. (2013). Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cellular and Molecular Life Sciences, 70(20), 3871-3882.
Tuszynski, M. H., Thal, L., Pay, M., Salmon, D. P., Bakay, R., Patel, P., … . Tong, G. (2005). A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature medicine, 11(5), 551-555.
Vafaee, F., Zarifkar, A., Emamghoreishi, M., Namavar, M. R., Shahpari, M., & Zarifkar, A. H. (2018). Effect of Recombinant Insulin-like Growth Factor-2 Injected into the Hippocampus on Memory Impairment Following Hippocampal Intracerebral Hemorrhage in Rats. Galen Medical Journal, 7, 1353.
van Rijzingen, I. M., Gispen, W. H., & Spruijt, B. M. (1995). Olfactory bulbectomy temporarily impairs Morris maze performance: an ACTH (4–9) analog accellerates return of function. Physiology & behavior, 58(1), 147-152.
Wozniak, D. F., Hartman, R. E., Boyle, M. P., Vogt, S. K., Brooks, A. R., Tenkova, T., … Muglia, L. J. (2004). Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiology of disease, 17(3), 403-414.
Wu, C.-C., Lien, C.-C., Hou, W.-H., Chiang, P.-M., & Tsai, K.-J. (2016). Gain of BDNF function in engrafted neural stem cells promotes the therapeutic potential for Alzheimer’s disease. Scientific reports, 6(1), 27358.
Wu, S., Sasaki, A., Yoshimoto, R., Kawahara, Y., Manabe, T., Kataoka, K., … Yuge, L. (2008). Neural stem cells improve learning and memory in rats with Alzheimer’s disease. Pathobiology, 75(3), 186-194.
Yang, H., Liu, Y., Liu, X., Gu, H., Zhang, J., & Sun, C. (2020). Concise Review: The Regulatory Mechanism of Lysine Acetylation in Mesenchymal Stem Cell Differentiation. Stem Cells International, 2020.
Yu-Taeger, L., Stricker-Shaver, J., Arnold, K., Bambynek-Dziuk, P., Novati, A., Singer, E., … Riess, O. (2019). Intranasal administration of mesenchymal stem cells ameliorates the abnormal dopamine transmission system and inflammatory reaction in the R6/2 mouse model of Huntington disease. Cells, 8(6), 595.
Acknowledgments
The authors gratefully acknowledge the financial support of the Cellular and Molecular Research Center (CMRC) of IUMS (94-05-117-27524) and the Iran National Science Foundation (INSF) for funding this work through a grant (95849005). Experiments were performed at the Cellular and Molecular Research Center (CMRC) and IUMS Core Laboratory (ICL), Tehran, Iran.
Funding
This work was supported by grants from Iran University of Medical Sciences (IUMS) (97-4-75-13504) and Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, and Investigation [Ebrahim Eslami, Farshid Ghiyamihoor, Marjan Sadr, Marziyeh Ajdary, Sahar Hakimpour, Rana Mehdizadeh, Ronak Shabani, Mehdi Mehdizadeh]; Writing – original draft preparation [Farshid Ghiyamihoor]; Writing – Review and Editing [Ebrahim Eslami, Farshid Ghiyamihoor, Marjan Sadr, Marziyeh Ajdary, Sahar Hakimpour, Rana Mehdizadeh, Ronak Shabani, Mehdi Mehdizadeh]; Funding Acquisition and Resources [Mehdi Mehdizadeh]; Supervision, [Mehdi Mehdizadeh and Ronak Shabani]
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Ethics Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional research ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures performed on animals were in accordance with the ethical standards of the IUMS (IR.iums.rec.1397.1299).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of data in this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eslami, E., Ghiyamihoor, F., Sadr, M. et al. Intranasal delivery of human Wharton’s jelly-derived mesenchymal stem cells alleviates Aβ-induced Alzheimer’s symptoms in rat models by regulating neurotrophic and apoptotic factors. Neurosci Behav Physi 54, 374–387 (2024). https://doi.org/10.1007/s11055-024-01582-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-024-01582-1