Abstract
Interdisciplinary sciences have paved the way for the creation of new technologies that could potentially alter how things were conventionally done. Biotechnology and nanotechnology have resulted in the creation of various techniques, one of which is upconversion nanoparticles (UCNPs), which can be conjugated with biomaterials for added functionality. UCNPs work based on optical phenomena referred to as anti-Stoke shift, wherein the nanoparticle absorbs light of a high wavelength (lower energy) and emits light having a lower wavelength (higher energy). This ability to provide luminescent signals when irradiated with near-infrared (NIR) light sources is particularly useful as a consequence of its minimal toxicity and high depth of penetration. When coupled with the appropriate biomaterials, the UCNPs can conjugate with the target under study and via quenching/luminescence recovery and quantitative and qualitative tests can be performed relating to the target molecule, in vivo. These UCNP-nanomaterial structures have proven to be very useful in the in vivo image detection, diagnostic, therapeutic as well as combined approaches for disease treatment, drug delivery, disease diagnosis etc. This review aims at providing an in-depth explanation about the origins of UCNPs, how they may be synthesized, their mechanisms of upconversion (UC), their adaptability and flexibility under in vivo conditions, and the future of the field.
Graphical Abstract
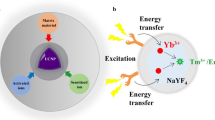
Overall representation of Upconverting Nanoparticle (UCNP) synthesis followed by the currently researched/applied modes of utilization for the same. The drug delivery aspect involves loading the porous layer over the UCNP with the required drug while the bio-imaging and therapeutic application (via photothermal therapy and/or photodynamic therapy) involves irradiation of the nanoparticles by tissue-penetrating near-infrared radiation (NIR).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is a leading science in the twenty-first century. It goes without saying that the capability to invent and utilize small particles that are not visible to the naked eye is not a recent tradition. In 1959, Richard Feynman brought attention to the concept of nanotechnology to the world in his famous talk titled “There’s Plenty of Room at the Bottom” [1]. Since then, concepts and ideas based on his talk have played a major role in almost every field of science.
Free nanoparticles are created either by breaking down bigger particles (top-down approach) or by carefully controlling assembly processes (bottom-up approach) or by natural occurrences when released into the atmosphere. Nanoparticles (NPs) are a wide classification of materials that comprise particulate substances with a maximum diameter of 100 nm [2]. Upconversion nanoparticles are a revolutionary type of nanomaterials that are capable of exhibiting anti-stokes phenomenon. Anti-Stokes emission is accomplished via successive immersion and “piling up” in the energy of infrared excitation photons via uniformly placed enduring excited states of lanthanide dopants [3]. UCNPs are a type of nanoparticle capable of producing higher-energy photons in the UV–visible emission region from a low-power NIR laser [4], achieved by employing an anti-Stokes shift. The lanthanide ions within the nanoparticles’ lattice enable this. It is a lanthanide system with a main sensitizer, bridge sensitizer, and emitter, which is usually Y3+ or Nd3+ [5]. The easiest way to explain the upconversion process is to compare it to the downconversion, also known as the Stokes shift. Quantum dots, silica/gold nanoparticles, and various dyes are examples of nanomaterials that emit via a Stokes shift. The electrical energy level from the ground state (S0) to the excited state (S1) increases when a photon of light is absorbed by a molecule. The energy is lost during internal conversion, which is discharged as heat. The fluorophore is de-excited, and the energy is subsequently expelled as spectra of light to return to S0. This is referred to as a down-conversion since the light produced has a lower energy state than the photon involved. The UC process, on the other hand, results in the release of a photon with a greater state of energy [6].
When excited by NIR, UCNPs coated with rare earth components release a photon. They are commonly doped with lanthanum (Ln3+), ytterbium (Yb3+), thulium (Tm3+), erbium (Er3+), neodymium (Nd3+), europium (Eu3+), terbium (Tb3+), holmium (Ho3+), cerium (Ce3+), gadolinium (Gd3+), dysprosium (Dy3+), samarium (Sm3+), and samarium (Sm2+) ions in various configurations [7,8,9]. Those illuminated particulates have exceptional tissue perforation and photochemical constancy, making them suitable for use as a sensitive nanodevice. UCNPs have emerged as a potential fluorescent material for applications such as biodetection, bioimaging, and photodynamic treatment [10]. To make fluorescence resonance energy transfer (FRET) sensors, UCNPs are mixed with certain nanomaterials (gold nanoparticles, graphene, cadmium telluride quantum dots, a mixture of MoS2 and WS2) [11]. Furthermore, UCNPs are laminated to create a core–shell figure and are in a hexagonal crystal juncture with an average diameter of 22 ± 3 nm, and their fluorescence power can be greatly increased, which improves sensitivity in biological applications [12]. Blocking the decay of energy transfer induced by the interaction between the interface of core UCNPs with errors and their surroundings is a strategy for ameliorating the luminescence productivity of coating shells on UCNPs. Several synthetic substances, such as ethylene glycol (EG), polyethylene glycol (PEG), polyethyleneimine (PEI), oleic acid (OA), polyacrylic acid (PAA), polyvinylpyrrolidone (PVP), methylmethacrylate, and others, have illustrated significant dominance in interface functionalities and biomedical applications [13]. As can be seen in Fig. 1, the final UCNP structure resembles a multi-layered sphere with the polymer making up the outermost coating. Polymers possessing amphiphilic segments in their spines are used in coating with hydrophobic and hydrophilic polymer shells [14]. Hydrophobic interactions between the oleate ligand and the polymer’s hydrocarbon chain adsorb amphiphilic polymers onto the UCNP exterior. The hydrophilic polymers’ polar organic functional groups are absorbed externally (into H2O), making oleate-capped UCNPs H2O-dispersible [15].
UCNPs have outstanding optical properties and grew into a helpful devices in the biomedical industry, including biosensing, cancer diagnosis and therapy, and biomarker identification [16]. UCNP has a number of advantages as luminescent reporters, including bright fluorescence patterns at adjustable wavelengths, the ability to perform multicolor detection, and fluorescence existence in tens to hundreds of seconds, which allows the UCNP to be identified without background interference. UCNPs also have minimal toxicity, no photoblinking [17], chemical reactivity, large anti-Stokes shifts, and excellent photostability, permitting them to be recognized with extremely little ocular background interference even in intricate biomolecules like bodily secretions. While UCNPs’ unique properties make them ideal for some applications, their inherent constraints may preclude them from being widely used as luminous probes in preclinical and clinical contexts. UCNPs have a lower brightness than other NIR fluorescent probes. Because of their restricted emission bands as well as interfacial quenching phenomena, they have a low absorption cross-section. Several methods for improving UCNP luminescence efficiency have been put forth to address this issue. These entail either altering the host lattice (by adding more dopant ions) or manipulating the interface of nanoparticles (through stabilization, integrating plasmonic materials or compounds with broad-ranging absorption, such as dyes). Using high-energy excitation light has various drawbacks, such as genetic mutation, apoptosis from durable luminosity, and auto-luminescence from cytological materials, which results in a modest transmission quality and shallow density depth for biomolecules. The only stumbling block of UCNPs in bioimaging techniques is their poor upconversion quantum yield, which is addressed in a variety of ways (such as coating the UCNPs with a polymeric layer).
UCNPs have proven to be of great importance in the field of medical diagnosis and hold great promise as a therapeutic agent as well. Their ability to both diagnose a condition as well as treat it makes them a potential theranostic agent. UCNPs conjugated with the appropriate biomaterials could be used for the in vivo detection of biomolecules like DNA, RNA, micro-RNA, proteins, specific antibodies/antigens, etc. Their therapeutic application lies in their ability to deliver drugs directly to the target site to improve efficiency or via photothermal therapy or photodynamic therapy. All of this will be discussed in-depth in the subsequent sections [18].
Mechanisms of upconversion
The many energy states of lanthanide-based ions provide huge opportunities for up-energy transfer processes in upconversion nanoparticles. Energy transfer upconversion (ETU), excited-state absorption (ESA), and photon avalanche (PA) are some of the processes that make up the complicated energy transfer system. Their mechanism of action has been detailed diagrammatically in Fig. 2.
In recent years, the UC process has been intensively researched and has shown to be effective for converting NIR to radiation within the visible spectrum. UC is an anti-Stokes phenomenon as it involves nonlinear optical processes during which low-energy radiation (NIR light) excites lower electronic levels, resulting in greater energy emission (visible or UV light) at higher electronic levels. This process is facilitated by the sequential uptake of two (or more) photons in order for the UC emission to take place. The abovementioned processes can lead to a successful absorption of two or more photons [19, 20].
Excited state absorption
Bloembergen presented ESA, sometimes referred to as the successive absorption of two photons, as the most widely recognized UC model in 1959. Bloem et al. claimed that the excited-state absorption (ESA) process is based on the idea that the same ion can make a transition from ground state (G) to higher-energy level, which is in an excited state by continuous multiphoton absorption, which is the most fundamental UC mechanism [21]. ESA is a UC process that happens in those materials, whose dopant concentration will be very low, and it includes photon absorption by a single ion. It comprises consecutive absorption of two photons. The ground state absorption (GSA) occurs when an ion transitions from G (state 1) to an intermediate excited state (state 2), which is very long-lived by the action of a photon. In an optical transition, the consecutive photon drives the ion from state 2 to a further higher energy state 3 and causes the emission of UC [22].
Energy transfer upconversion
The ETU mechanism usually requires the interaction of two adjacent lanthanide ions. A sensitizer ion undergoes transformation to the metastable E1 state from the ground state by the absorption of a pump photon. This sensitizer ion is referred to as ion 1. It then relays the energy it has accumulated to the ground state G and the excited state E1 of a second ion referred to as the activator, which stimulates ion 2 to the E2 upper emission state. This is accompanied by the sensitizer ion 1 relaxing back to the ground state in an ETU process. The upconversion effectiveness of an ETU process depends on the average distance between close sensitizer-activators, which is controlled by dopant concentrations. Contrary to ETU, the efficiency of an ESA process is independent of the dopant concentration due to its single ion feature [23, 24].
For example: Red, blue, and green light, for instance, can be produced in the system of NaYF4:Yb3+ Er3+ UCNPs through this method. It can operate as a sensitizer for Er3+ and transmit its energy to an unexcited Er3+ ion by the energy transfer process: 2F5/2 (Yb3+) + 4I15/2 2F7/2 (Yb3+) + 4I11/2 (Er3+). The Yb3+ ion, with its excited state 2F5/2, has an energy comparable to 4I11/2 (Er3+). Through further cross-relaxation and a phonon-assisted process, 4F9/2 [2F5/2 (Yb3+) + 4I13/2 (Er3+) + 2F7/2 (Yb3+) + 4F9/2 (Er3+)] can generate red emission (654 nm). In a similar manner, 2H9/2 − 4I15/2 and 2H11/2 − 4I15/2 (also 4S3/2 − 4I15/2) produce blue (408 nm) and green luminescence emissions, respectively, at 526 nm and 533 nm [25,26,27].
Photon avalanche
Photon avalanche which is also sometimes called as absorption avalanche is often considered to be one of the most efficient and widely used upconversion processes. Its discovery took place in the year 1979 by Chivian. The PA process is the least seen of all the UC mechanisms [28]. Ground state absorption (GSA) initially promotes the sensitizing ion (ion 1) to the second state from its first. Then, ESA advances it to the third state using an incident photo. In order to produce two ions (ion 1 and 2) in state 2 as a result of cross-relaxation, one sensitizing ion (ion 1) in state 3 can interact with a nearby ion (ion 2) in the ground state. The 2 resulting ions function as sensitizing ions and can produce up to another eight ions in a chain reaction. Eventually, an avalanche of the ion population in state 2 can be formed because the intermediate excited state (state 2) functions as an energy storage reservoir [29]. The energy scheme for PA is given in Fig. 2.
Versatility of UCNPs
The versatility of UCNPs makes it all the more attractive for use. By controlling and optimizing specific parameters, we can drastically change the output of emission as well as its interactivity with biological samples. Some of the ways by which UCNPs can be customized and fine-tuned are mentioned below.
Host material and dopants
An upconverting nanoparticle system has two major components, the host material, which serves as the lattice for the entire system and the dopant. Dopants, which are usually lanthanide ions or rare-earth ions, have been used, are the luminescent centers, and are responsible for the actual process of upconversion. Many factors have to be taken into account to optimize the UCNP when dealing with the dopants and host material. An appropriate choice of host material, dopant ion, as well as their concentrations are important factors for consideration with respect to UCNP synthesis.
The distance between individual dopant ions, their surrounding environments and their spatial arrangement are all determined by the host material. Quenching of the dopant ions by the host material is a major concern and must be avoided at all costs. Fluoride materials are generally used as the host. Due to the low phonon energies of the lattice, long lifetimes of the excited states are frequently found in fluoride materials. In general, calcium and sodium fluorides are suitable host materials for upconversion systems because their lattices are based upon cations like sodium, calcium, and yttrium ions having an ionic radius that is similar to that of the dopant material, i.e., lanthanide ions. This is because these cations suppress crystal defect and lattice stress formation [30].
As already mentioned, lanthanide ions are usually used as the dopants for UCNPs. The electron shell configuration of these elements lends them unique optical properties, namely upconversion and downconversion. Most rare-earth nanomaterials fall into one of two main classes: downconversion nanoparticles (DCNPs) or upconversion nanoparticles (UCNPs). DCNPs are capable of splitting a single high-energy photon into multiple lower-energy photons. On the other hand, UCNPs convert one or more low-energy photons into a single high energy photon. Their ability to perform upconversion is why rare-earth elements are used as the dopant for UCNP systems [31].
Surface modifications
Various synthesis routes exist for creating UCNPs. Solvothermal synthesis, thermal decomposition, and coprecipitation synthesis routes are just a few of the more commonly used approaches. All these methods yield nanoparticles with an upconverting luminescent core protected by a hydrophobic layer of surface ligand (most commonly oleic acid). This hydrophobic layer stabilizes the nanoparticle and prevents the aggregation of individual UCNPs. However, for the vast majority of biological applications, we would prefer using systems which are hydrophilic, thus necessitating surface modification steps. If the hydrophobic ligands are swapped out for hydrophilic ligands as the growth-controlling agent, the surface modification step can be avoided [32]. Ligand engineering is the method of choice when it comes to surface-coated modification. It entails either a direct oxidation of the terminal molecule or a ligand exchange with hydrophilic molecules. A range of hydrophilic substances, such as 6-aminohexanoic acid, citrate, hexanedioic acid, polyacrylic acid, and compounds generated from phosphate, can transform hydrophobic ligand-coated nanoparticles into hydrophilic by ligand exchange processes [33].
It is crucial to confirm not only that the surface functionalization was successful but also that the modification would have no detrimental effects on the upconversion efficiency or the colloidal stability. This is because each surface functionalization process can change a number of characteristics of the UCNPs. Additionally, post-surface modification to replace the hydrophobic layer with a hydrophilic ligand layer may be necessary to functionalize a biomolecule to allow the nanoparticle system to interact with biomolecules.
For example, we may need to attach ssDNA molecules to the UCNPs to facilitate biodetection for disease prognosis. Electrostatic adsorption, coordination and covalent cross-linking are the three main methods for the same.
If the charges of the two materials are opposite, electrostatic adsorption is an easy approach to join them. DNA molecules have phosphate group backbones that are negatively charged. This characteristic enables UCNPs to bind nucleic acids through a modification of the surface charge. Due to the relatively small force of positive and negative electrostatic adsorption, DNA might not completely attach to UCNPs, despite the adsorption process being convenient and easy to characterize. Therefore, this surface-modification process is mostly useful for the temporary attachment of DNA to UCNPs for delivery into cells where they dissociate from it.
Coordination of the DNA molecules onto the UCNP structure is facilitated by the strong interaction that occurs between the phosphate group present in the strand and the Ln3+ ion on the surface of the nanoparticle. Nucleic acids attached via the coordination method are stably conjugated with the nanoparticle and can be used for in vivo studies. However, as the nucleic acid strand lies on the surface, it may not exhibit proper recognition. The final mode of attachment is via covalent cross-linking. For this approach, the DNA strand must be conjugated with functional groups like NH2 for attachment. Additionally, the UCNPs also need to be functionalized with complementary function groups to facilitate ligand exchange. DNA strands attached via this method are very stably hybridized onto the UCNP and show very little desorption or leakage [34].
Applications of UCNPs
Bioimaging
Bioimaging techniques provide a thorough view into the state of functioning of specific regions or tissues within the body. The greatest advantage attributed to bioimaging techniques, in general, is their non-invasive nature, making them much more patient-friendly. While a variety of methods are available for these bioimaging studies, there are certain points that make UCNPs better suited for it. NIR light sources allow deeper penetration of the radiation into the tissues and eliminate tissue autofluorescence, providing us with a better and more sensitive detection route. Additionally, the ability to use both NIR excitation and emission (NIR-to-NIR upconversion) is especially useful in vivo imaging. It allows for deeper penetration into the sample and lowers the absorption and scattering by the biological samples (Fig. 3) [35].
Polyethyleneimine (PEI)-coated NaYF4:Yb,Er nanoparticles were used in a study for the bioimaging of bone marrow-derived stem cells. The study was able to conclude that the uptake of the UCNPs did not induce any substantial cytotoxic effects on the cells when used in specific concentration ranges with the appropriate incubation periods. When injected into female Wistar rats, the nanoparticles accumulated the fastest within the lungs, and in the next 7 days, the nanoparticles had fallen to below-detectable levels. The study was also able to conclude that while cell-surface binding is quite quick and can be detected fluorescently within an hour, uptake of the UCNPs is much slower [36].
Diabetes detection
Unlike other fluorescent nanoparticles used in sensors that rely on hydrogen peroxide quenching as the primary detection technique for testing for the presence of glucose, UCNPs wholly rely on turn-on approaches in the sensors they are used in. We can utilize the UCNPs to detect overall blood and serum glucose levels to identify the state of diabetes progression in the patient [37].
A study performed by Liu et al. utilized a UCNP-polydopamine detection system to test for glucose levels. The free UCNPs were incubated along with the test sample, dopamine and glucose oxidase. The dopamine molecules attached themselves onto the free UCNPs to create a PDA shell that would hinder the upconversion process, resulting in almost complete quenching. The quenching could be reversed by the H2O2 released when glucose oxidase enzymatically acts on glucose. H2O2 in an alkaline solution produces free radical species which interact with the polymerization of dopamine on the UCNP in a concentration-dependent manner. This allows the systemic detection of glucose levels in the sample based on the quenching/fluorescence levels. The study was able to test glucose in samples with an LOD of 1.2 µM with a high degree of accuracy and efficiency [38].
Another study utilized a UCNP bio-conjugate system to test for the presence of vaspin, a serpin associated with T2DM. A lateral flow strip assay (LFSA) which works in an aptamer conjugated sandwich mode was created for the detection of vaspin levels. A primary aptamer (capture aptamer) was conjugated onto the assay strip to which the target molecule would attach as the sample was passed over it. A secondary aptamer acting as the detection probe was hybridized with UCNPs for the fluorescent detection of the target. The quantity of vaspin directly correlates with the amount of fluorescence generated by the UCNPs, and the study was able to detect it within a concentration range of 0.1–55 ng/ml with a LOD of 39 pg/ml [39].
HIV detection
A similar detection strategy as developed in the previous work was demonstrated by Martiskainen et al. Their work focused on the detection of anti-HIV 1 or 2 antibodies. The UCNPs were conjugated with recombinant HIV 1 and 2 glycoproteins which would serve as the epitope for attachment of anti-HIV antibodies from the sample [40].
Cancer detection
UCNPs are already being studied widely with respect to cancer diagnosis and added therapeutic application. The standard diagnostic approach to detecting cancer is biopsy. The technique is however time-consuming and invasive. UCNPs serve as potential alternatives because of their already listed properties.
A recent study demonstrated the use of UCNPs coated with cancer cell membranes to create cancer cell membrane-coated upconversion nanoparticles, which have proved to be successful in differentially diagnosing the type of tumor. The effectiveness of the modified probe was attributed to its low immunogenicity and its ability to diagnose via a three-way UCL/MRI/PET imaging system in vivo. The UCNP was synthesized with Gd3+ and was modified to be compatible with and provide information via magnetic resonance imaging (MRI) and positron emission tomography (PET) in addition to the upconversion luminescence (UCL) properties of the nanoparticle itself. Combining three different imaging techniques vastly improves the data we obtain regarding the tumor with a much better accuracy. The main complication that the probe faced was the immune response it generated, which considerably shortened its blood residence time. The surface modification of the UCNP with MDA-MB-231 cell membrane helped circumvent the immune detection issue. Additionally, coating with the membrane helps in the homologous targeting of MDA-MB-231 cancer cells. The cell membranes of cancerous cells have membrane antigens and unique membrane structures which impart properties such as long blood circulation, immune escaping, and specific recognition [41]. UCNPs coated with MDA-MB-231 membranes showed targeted-tumor accumulation only when cp-incubated with MDA-MB-231 cells and not for other human breast cancer cells [42].
Drug delivery
A variety of papers have already established the benefit of using nanoparticle carriers and nanoencapsulated drug delivery. Nagalakshmi et al. (2017) have stated in their work that nanoencapsulated drug DDA (14-deoxy-11, 12-didehydroandrographolide) was found to have increased the oral availability of the drug aiding in maintaining blood glucose levels in rats [43]. Another study developed a chitosan-based nanoparticle platform for the efficient delivery and uptake of two varieties of lipopeptide vaccines. They concluded that the nanoparticles were found to be safe, showed minimal cytotoxicity, and produced good quantities of antigen-specific antibodies as well [44].
Another study demonstrated the benefits of using nanocarriers of Poly(2-oxazoline) (POx) for the loading and site-specific delivery of the drug paclitaxel for breast cancer therapy. They concluded that the anti-tumor effects of the drug system were considerably improved by the nano-drug loading and delivery [45].
The diversity in the nature of UCNPs makes them extremely suited for the delivery of a wide variety of therapeutic substances. Their small size combined with their large surface area increases the effectiveness and retention of drugs for cell/tissue-specific therapy. Far and away superior, UCNP-based composites utilized as medication delivery frameworks will empower following and productivity assessment of medication discharge progressively. Analysts have arranged UCNPs for drug stacking and designated a change of the delivery surface to deliver their load into specific target cells. Additionally, having the option to surface modify the UCNPs further increases the options available [46].
The UCNP drug delivery strategy is based on the conjugation of an additional module that utilizes the upconverted light by transferring it to their immediate surroundings and facilitating the release of the drug/load (Fig. 4). These conjugated photo-labile compounds are the key to releasing the drug. When they are excited by NIR, which has a deeper penetration range than other parts of the EM spectrum for biological samples while maintaining low cytotoxicity, the conjugated compounds induce changes in the delivery system which allows for the specific release of the drug at the desired tissue. Yet again, surface polymerization is a crucial component which aids in this kind of UCNP-based drug delivery [47].
UCNPs are also being parallelly used as chemotherapeutic drug delivery vehicles. However, they are generally combined with other PDT or PTT approaches to enhance therapeutic effect. This particular approach will be discussed in the next section [48].
Therapy
In addition to bioimaging, biodetection, and drug delivery, UCNP systems can be used directly for therapeutic applications. In a recent study, researchers were able to control glucose metabolism rates for the treatment of Type-2 Diabetes Mellitus (T2DM). Their technique combined upconversion fluorescence with optogenetic techniques that helped control T2DM levels by targeting and selectively activating the PI3K/AKT signaling pathway. Various advantages of the therapeutic system include rapid response (within seconds) to NIR, tissue penetration (several centimeters in depth), and alterable dosages of irradiation. Studies have successfully shown the ability to control glucose metabolism via this approach (both in vitro and in vivo) [49].
Another mode of action for UCNPs is via photodynamic therapy (PDT) (Fig. 5). UCNPs configured to exhibit PDT have garnered attention recently. Upon irradiation with NIR, UCNPs liberate light that is capable of prompting the photosensitizer molecules in the vicinity to produce singlet oxygen that eliminates target/tumor cells. PDT is gaining acceptance as an alternative to harsher modes of treatment like chemo and radiotherapy for the treatment of a wide array of diseases in addition to cancer. Due to its minimally invasive approach when compared to radio and chemotherapy, PDT is starting to be considered a viable and much more effective mode of treatment than the others [50].
Therapeutic application—UCNP loaded with phothermal coupling agent (PTCA) for photothermal therapy (PTT) or photosensitizer for photodynamic therapy (PDT) is introduced to the target cell. Excitation with NIR generates UCL that activates the effects of the PTCA/photosensitizer, ultimately causing cell death
A recent study by Chen et al. demonstrated the use of UCNPs functionalized with a photosensitizer drug for PDT. The photosensitizer in use was the Ce6 or Chlorin e6 molecule. It is a widely used photosensitizer with low dark toxicity and high efficacy [51]. The NaYF4:Yb3+, Tm3+ / NaYF4: Yb3+, Er3+ core–shell nanoparticles were initially surface coated with a PEG-phospholipid layer for good dispersion stability, and then it was surface loaded with the Ce6 molecules. When the UCNP mix was incubated with QGY-7703, a human hepatocellular carcinoma cell line, it was observed that the NIR-induced activation of the photosensitizer resulted in significant cell death of the tumorous cells. Irradiation was provided at a wavelength of 980 nm, and emission was observed at approximately 660 nm, which also happens to be the absorbance maxima for Ce6 photoactivation. Ultimately, the NIR irradiation causes the UCNP to emit light of a particular wavelength which activates the Ce6 molecules to liberate singlet oxygen species and promote tumor death. The cytotoxicity tests for the UCNP-Ce6 system showed that they were highly biocompatible (more than 70% cells survived incubation with the nanoparticle) and that they were also taken up by the tumor cells in sufficient quantities for tumor detection [52].
Photothermal therapy (PTT) is another mode by which UCNPs can contribute to therapeutic applications. PTT involves conjugating the nanoparticle with an appropriate photothermal converting agent (PTCA) that absorbs incoming energy and converts it to thermal or heat energy to kill the targeted cells. Ideally, the PTCA should be non-toxic, should not exhibit any photothermal conversion without input from the UCNPs, and should have the appropriate size and morphology depending on the use.
Dibaba et al. demonstrated the use of such a setup to create a dual-modal imaging UCNP structure that also exhibited PTT via the antimony nanoshell coating of the as-prepared UCNP. In vivo imaging could be achieved via UCL and MRI imaging and was tested for using HeLa cell lines. The cells showed good uptake of the UCNPs, and strong MRI, as well as UCL signals were obtained. Viability tests showed that the cells were negligibly affected by UCNP uptake, and they concluded that the nanoparticles were biocompatible. Photothermal conversion was also observed with a high degree of success in in vitro studies, in a concentration-dependent manner. The higher the concentration of UCNP accumulation within the HeLa cells, the higher the temperature could be achieved. But this aspect must be taken into account along with the biocompatibility concentration range of the UCNPs [53].
Future applications
Considering the range of sizes in which UCNPs are available alongside their biocompatible nature, they possess the potential to bind to various biological components, which plays a crucial role in recently developed biological platforms including bio-detection assays as well as therapeutic modalities. The UCNPs have certain distinctive optical properties which makes them very unique. Some of the properties which brings UCNPs to the limelight in the biomedical field are luminescence; their ability to penetrate deep into the biological tissue without actually damaging the cells acts as a good contrast agent in not only in vitro but also in vivo by maintaining a low background as well as a high resistance to photo-bleaching. Now let us look into the various ways in which UCNPs can contribute to biomedical science in the near future.
UCNPs in (bio)assays
Although traditional fluorescence has long been an important tool in biodetection, there are certain downsides to this approach. Fluorescent dyes which were used traditionally, which also includes quantum dots, were excited by ultraviolet or near-ultraviolet lights. These high-energy radiations not only have poor tissue penetration but also severely harm biological tissues when exposed over an extended period of time. More significantly, the high-intensity light source is the primary contributor to a substantial amount of auto-fluorescence, which gives rise to a low signal-to-noise ratio and negatively impacts the detection’s sensitivity. Here, UCNP plays a major role by compensating for all the deficiencies. FRET-based and non-FRET-based models have played an important role in revealing the potential of UCNP in the detection of biomolecules.
We can use UCNPs to detect oligonucleotides like miRNA. Certain miRNA in the serum gets upregulated during various metabolic disorders like T2DM, whereas there is a global downregulation of miRNA during cancer. A sandwich-type of hybridization format is commonly used in a FRET-based nucleic acid detection. To capture the longer target oligonucleotides, two shorter oligonucleotides (probes) were created. One of the probes is covalently attached to UCNP, while the other is fluorescently marked and has an absorption spectrum that coincides with the emission spectrum of UCNP. The fluorophore-labeled probe would come closer to the UCNP surface in the presence of the target oligonucleotides. The photon generated by UCNP would be transferred to the fluorophore and result in the fluorophore’s emission upon illumination by an NIR light source. One can assess how much of the targeted oligonucleotide is present by measuring the fluorophore’s fluorescence intensity after NIR excitation. Correspondingly, we can detect the associated disease at an early onset by measuring the intensity of the fluorescence. Just like the detection of nucleic acids, even certain proteins can be detected as well. Research is going on to be able to fabricate microarrays, which can be used to detect proteins like cancer proteins; as a result, it can help in the detection and diagnosis at an early stage [54].
Gong et al. (2019) have designed and tested the use of a UCNP-based lateral flow assay (LFA) in the identification of a variety of targets. The designed UCNP-LFA point-of-care testing device was successfully used in the detection of targets like bacteria, nucleic acids, and proteins. The device used a smartphone-bound analysis application for reading the output of the LFA. The device showed a 0.992 coefficient of correlation to the accepted gold standards for the same, making the device accurate and sensitive given the extremely low LOD [55].
Upconversion-based gene therapy
Gene therapy plays a crucial part in the treatment of cancer as well as diseases which are inherited by making the use of targeted cells along with nucleic acids which are therapeutic in nature [56]. An attempt is made to try and repair the genes which show abnormal gene expression. In recent times, UCNP has come to the limelight in the biomedical field. To increase the biological effectiveness of UCNP-based gene carriers, surface modifications can be implemented. There are two benefits to the surface alteration. The first is the enhanced efficiency of gene loading, and the second is the enhanced physical and chemical properties imparted to the nanoparticles allowing for improved in vitro execution [57]. In recent times, Zhang’s team carried out ground-breaking research on UCNPs for nucleic acid delivery. In their investigation, siRNAs were electrostatically linked to the base of UCNP-antiHer2 by conjugation of an anti-Her2 antibody to amino-fitted UCNPs for objective distribution. The satisfactory confirmation of material absorption by Her2-overexpressing SK-BR-3 cells was indicated by the negatively regulated expression of the luciferase reporter gene. The aforementioned delivery technique was then further used with surface enhancements to boost the targeted dispersion and raise gene loading efficiency [58]. In order to silence the MDR1 gene and enhance the treatment reactivity of ovarian cancer cells, Lin et al. created stratified UCNPs for scrutinizing and delivering MDR1 gene-silencing siRNA (MDR1-siRNA). UCNPs were coated with 146 PAA and PEI, which were subsequently electrostatically adsorbed with MDR1-siRNA [59]. MDR1-siRNA was more effectively taken up by cells due to the UCNP-based carrier, which also helped them avoid nuclease-mediated degeneration and allow endosomal release for efficient MDR1 gene knockdown [60].
Upconversion-based immunotherapy
Cancers that are resistant to conventional therapy may benefit from using nanoparticles as a treatment. Numerous research focused on immunotherapy in combination with various cancer therapies in recent years. Research has illuminated a number of prospective remedies that demonstrate cooperative advantages, in addition to being utilized as discrete remedies. Several scholars have looked into combining PDT with immunotherapy to promote antitumor immunity [61,62,63]. The escape of tumor-associated antigens was induced by PDT as anticipated, whereas the R837-activated TLR7 pathway might cause potent systemic immune reactions that can be additionally amplified by CTLA-4 inhibition [64]. The system’s main advantage is the nanodevice’s ability to use the immunological memory effect to stop tumor recurrence. The system’s main advantage is the nanodevice’s ability to use the immunological memory effect to stop tumor recurrence [65]. PDT, chemotherapy, and immunotherapy together should have a significant impact on distant metastases. Chemo-PDT exhibits potent synergistic anticancer activity and induces immunogenic cell death (ICD), which results in immunity specific to the tumor. When used in conjunction with ICD-elicited tumor therapy, the anti-CD73 antibody avoids immunosuppression by tumors that serve as an adequate immune checkpoint blockade [66].
Combining various therapeutic modalities—such as cancer vaccines, immune checkpoint blockade (ICB) therapy [67,68,69,70], chimeric antigen receptor (CAR)-T cell therapy [71,72,73], cytokine therapy [74,75,76], and immunological adjuvant therapy [77, 78]—has proven to be an effective way to treat cancer. All of the new technologies exhibit clinical promise for eliminating orthotopic tumors, but their abscopal benefits against metastases remain insufficient. For instance, immunotherapy medicines’ non-specific side effects on healthy tissues, including increased cytokine production, can have fatal consequences. After a medicine is administered to a patient, the immunostimulatory activity is not accurately controlled, which increases the risk of severe toxicity. Current methods are ineffective at preventing systemic toxicity. A major focus of cancer immunotherapy continues to be methods for finely tuning the arrangement and duration of immune reactions exogenously. Construction of administrative scaffolding to track anti-tumor immunity with high configurational accuracy is still difficult. UCNPs offer a method to manage the transport, release, and activation of pharmaceuticals because of their distinctive effects. As a result, UCNPs have been the subject of substantial research as a cancer immunotherapy in recent years.
Toxicity of UCNPs
Comprehensive studies annually explore the biomedical applications of various nanoparticles. Earlier research suggests upconversion nanoparticles (UCNPs) pose no apparent toxicity in vitro and in vivo. However, their long-term impact on small animals, interaction with the immune system, and influence on blood proteins are unclear. Lanthanide-doped nanoparticles, including UCNPs, lack sufficient toxicity data crucial for understanding their impact on biological cells. Systematic research is essential to address these gaps and draw accurate conclusions about their safety and biomedical potential [79, 80].
Recent research has explored diverse properties of nanoparticles, such as chemical composition, size, morphology, structure, lanthanide ion concentration, and surface ligands. However, comparing findings across studies on the potential toxicity of upconversion nanoparticles (UCNPs) is challenging due to these variations. In vitro cytotoxicity studies using various cell cultures suggest the need for in vivo animal studies to assess long-term safety.
In a thorough in vivo investigation conducted by Xiong et al., the prolonged toxicity of NaYF4:Yb3 + , Tm3 + UCNPs coated with PAA was explored in mice. Post intravenous administration, UCNPs were initially present in the liver, spleen, and lungs with minor traces in the kidney and heart. Within the initial 24 h, accumulation took place in the spleen, gradually diminishing in the liver. Even after 2 weeks, UCNPs were still observable in the liver and spleen, highlighting their crucial role in clearance. Three months after injection, UCNPs were still noticeable in the intestinal tract, decreasing by the fourth month. Despite the persistent luminescence, mice exhibited no signs of health decline, with normal blood parameters, organ function markers, and behavior. The spleen’s white pulp displayed minimal hyperplasia, suggesting negligible nanotoxic effects [81].
Chatterjee et al. investigated the effects of PEI-coated UCNPs on rats and cell cultures, finding no harmful impact on bone marrow-derived stem cells [36]. Similarly, Xing et al. demonstrated the low in vitro toxicity of lanthanide-doped UCNPs. In their study, HL-7702 and RAW264.7 cells treated with a high concentration of NaYbF4 (1.6 mg Yb3 + mL − 1) for 24 h exhibited robust survival, with more than 82.7% and 88.9% viability, respectively [82]. Another examination of NaYF4 UCNPs interaction with living cells (HeLa, LO2, KB) revealed minimal toxicity, as over 80% of cells survived following 24h incubation with 800 µg mL − 1 UCNPs [83]
Research on the toxicity of upconversion nanoparticles (UCNPs) in biological cells primarily focuses on their chemical impact and stabilizing ligands. In vitro studies examine effects on protein regulation, internalization, targeting, and organelle deposition. However, limitations exist, and broader considerations, like exposure to external factors or interactions with other nanoparticles, are gaining attention.
While in vitro cell models provide insights, in vitro tissue models are underexplored but offer a more comprehensive understanding of UCNP behavior in different tissues and vessels. Combining these models with conventional assays could enhance the assessment of nano-toxicity, providing a fuller picture of UCNP interactions with biological components over time.
Conclusion
UCNPs provide us with the opportunity to exponentially improve diagnostic and treatment approaches for a wide range of disease, disorders, and medical conditions. From their use as either single-mode diagnostic tools or targeted therapeutic/drug delivery vehicles to multi-mode uses, UCNPs can be used for a plethora of functions. Even now, significant work is being done on these nanoparticles. Current research models and studies that are testing the use of UCNPs are understandably limited to cancer detection and treatment given the widespread nature of the disease and the amount of academic/research interest it draws. However, this cannot remain the case forever. There are diseases and medical conditions that are slowly gaining prevalence, either due to how deadly or how widespread they are. Detection of chronic disorders like Alzheimer’s [84], cardiovascular disease (CVD) [85], and diabetes [86] can allow the affected to pursue early treatment options and adopt lifestyle changes that can significantly lower or even completely prevent the progression of the condition. Additionally, UCNPs are capable of treating the condition (if treatment necessitates cellular destruction or via the delivery of appropriate drugs) and as such can be designed in such a way that the system is capable of both detection and treatment if necessary. The scope of this theranostic system is practically endless. We could even hypothesize the fabrication of a system that exists within our bodies that periodically pumps these UCNPs tailored for specific diseases. However, before any of that can be visualized, we need to understand the limits of UCNPs with respect to individual conditions and diseases.
New discoveries and approaches regarding their applicability are under study, but there is still massive scope left for improvement. We have yet to realize the full effects of the nanoparticles within biological systems. Studies relating to their cytotoxicity and biological viability are few and far in between. To fully harness the power of UCNPs, we need to divert more focus onto the effects these particles would have on biological systems in the long run. Once this and a few other aspects, such as luminescence intensity, choice of dopants, and host materials have been resolved, UCNPs will be set to become the preferred choice of approach for diagnostic as well as combined diagnostic and treatment avenues.
Data availability
No datasets were generated or analyzed during the current study.
References
Feynman RP (2011) There’s plenty of room at the bottom. Reson 16:890–905. https://doi.org/10.1007/S12045-011-0109-X
Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F (2020) The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules 25:112. https://doi.org/10.3390/MOLECULES25010112
Sirkka N, Lyytikäinen A, Savukoski T, Soukka T (2016) Upconverting nanophosphors as reporters in a highly sensitive heterogeneous immunoassay for cardiac troponin I. Anal Chim Acta 925:82–87. https://doi.org/10.1016/J.ACA.2016.04.027
Li Z, Zhang Y, La H, Zhu R, El-Banna G, Wei Y, Han G (2015) Upconverting NIR photons for bioimaging. Nanomater 5:2148–2168. https://doi.org/10.3390/NANO5042148
Shen J, Chen G, Vu AM, Fan W, Bilsel OS, Chang CC, Han G (2013) Engineering the upconversion nanoparticle excitation wavelength: cascade sensitization of tri-doped upconversion colloidal nanoparticles at 800 nm. Adv Opt Mater 1:644–650. https://doi.org/10.1002/ADOM.201300160
Gulzar A, Xu J, Yang D, Xu L, He F, Gai S, Yang P (2018) Nano-graphene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalt Trans 47:3931–3939. https://doi.org/10.1039/C7DT04141A
Wen S et al (2018) Advances in highly doped upconversion nanoparticles. Nat Commun 2018 9:1 9:1–12
Du K, Feng J, Gao X, Zhang H (2022) Nanocomposites based on lanthanide-doped upconversion nanoparticles: diverse designs and applications. Light Sci Appl 2022 11:1 11:1–23
Jethva P, Momin M, Khan T, Omri A (2022) Lanthanide-doped upconversion luminescent nanoparticles-evolving role in bioimaging, biosensing, and drug delivery. Materials (Basel) 15
Smith AM, Mancini MC, Nie S (2009) Bioimaging: second window for in vivo imaging. Nat Nanotechnol 4:710–711. https://doi.org/10.1038/NNANO.2009.326
Xu S, Xu S, Zhu Y, Xu W, Zhou P, Zhou C, Dong B, Song H (2014) A novel upconversion, fluorescence resonance energy transfer biosensor (FRET) for sensitive detection of lead ions in human serum. Nanoscale 6:12573–12579. https://doi.org/10.1039/C4NR03092C
Thakur MK, Gupta A, Fakhri MY, Chen RS, Wu CT, Lin KH, Chattopadhyay S (2019) Optically coupled engineered upconversion nanoparticles and graphene for a high responsivity broadband photodetector. Nanoscale 11:9716–9725. https://doi.org/10.1039/C8NR10280E
Johnson NJJ, Sangeetha NM, Boyer JC, Van Veggel FCJM (2010) Facile ligand-exchange with polyvinylpyrrolidone and subsequent silica coating of hydrophobic upconverting β-NaYF4: Yb 3+/Er3+ nanoparticles. Nanoscale 2:771–777. https://doi.org/10.1039/B9NR00379G
Xiong LQ, Chen ZG, Yu MX, Li FY, Liu C, Huang CH (2009) Synthesis, characterization, and in vivo targeted imaging of amine-functionalized rare-earth up-converting nanophosphors. Biomaterials 30:5592–5600. https://doi.org/10.1016/J.BIOMATERIALS.2009.06.015
Ansari AA, Parchur AK, Thorat ND, Chen G (2021) New advances in pre-clinical diagnostic imaging perspectives of functionalized upconversion nanoparticle-based nanomedicine. Coord Chem Rev 440:213971. https://doi.org/10.1016/J.CCR.2021.213971
DaCosta MV, Doughan S, Han Y, Krull UJ (2014) Lanthanide upconversion nanoparticles and applications in bioassays and bioimaging: a review. Anal Chim Acta 832:1–33. https://doi.org/10.1016/J.ACA.2014.04.030
Lee J, Lee H, Kang M, Baday M, Lee SH (2022) High spatial and temporal resolution using upconversion nanoparticles and femtosecond pulsed laser in single particle tracking. Curr Appl Phys 44:40–45. https://doi.org/10.1016/J.CAP.2022.09.002
Del Rosal B, Jaque D (2019) Upconversion nanoparticles for in vivo applications: limitations and future perspectives. Methods Appl Fluoresc 7:022001. https://doi.org/10.1088/2050-6120/AB029F
Joubert MF (1999) Photon avalanche upconversion in rare earth laser materials. Opt Mater (Amst) 11:181–203. https://doi.org/10.1016/S0925-3467(98)00043-3
Wang M, Abbineni G, Clevenger A, Mao C, Xu S (2011) Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomed Nanotechnol Biol Med 7:710–729. https://doi.org/10.1016/J.NANO.2011.02.013
Bloembergen N (1959) Solid state infrared quantum counters. Phys Rev Lett 2:84–85. https://doi.org/10.1103/PHYSREVLETT.2.84
Wilhelm S, Kaiser M, Würth C, Heiland J, Carrillo-Carrion C, Muhr V, Wolfbeis OS, Parak WJ, Resch-Genger U, Hirsch T (2015) Water dispersible upconverting nanoparticles: effects of surface modification on their luminescence and colloidal stability. Nanoscale 7:1403–1410. https://doi.org/10.1039/C4NR05954A
Chen J, Zhao JX (2012) Upconversion nanomaterials: synthesis, mechanism, and applications in sensing. Sensors 12:2414–2435. https://doi.org/10.3390/S120302414
Hong E, Liu L, Bai L, Xia C, Gao L, Zhang L, Wang B (2019) Control synthesis, subtle surface modification of rare-earth-doped upconversion nanoparticles and their applications in cancer diagnosis and treatment. Mater Sci Eng C 105:110097. https://doi.org/10.1016/J.MSEC.2019.110097
Gee A, Xu X (2018) Surface functionalisation of upconversion nanoparticles with different moieties for biomedical applications. Surfaces 1:96–121. https://doi.org/10.3390/SURFACES1010009
Lin C, Berry MT, Anderson R, Smith S, May PS (2009) Highly luminescent NIR-to-visible upconversion thin films and monoliths requiring no high-temperature treatment. Chem Mater 21:3406–3413. https://doi.org/10.1021/CM901094M
Auzel F (2004) Upconversion and anti-stokes processes with f and d ions in solids. Chem Rev 104:139–173. https://doi.org/10.1021/CR020357G
Chivian JS, Case WE, Eden DD (1979) The photon avalanche: a new phenomenon in Pr3+-based infrared quantum counters. Appl Phys Lett 35:124–125. https://doi.org/10.1063/1.91044
Arai MS, de Camargo ASS (2021) Exploring the use of upconversion nanoparticles in chemical and biological sensors: from surface modifications to point-of-care devices. Nanoscale Adv 3:5135–5165. https://doi.org/10.1039/D1NA00327E
Haase M, Schäfer H (2011) Upconverting nanoparticles. Angew Chemie - Int Ed 50:5808–5829. https://doi.org/10.1002/ANIE.201005159
Yu Z, Eich C, Cruz LJ (2020) Recent advances in rare-earth-doped nanoparticles for NIR-II imaging and cancer theranostics. Front Chem 8:496. https://doi.org/10.3389/FCHEM.2020.00496
Sedlmeier A, Gorris HH (2015) Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem Soc Rev 44:1526–1560. https://doi.org/10.1039/C4CS00186A
Tan H, Yu L, Gao F, Liao W, Wang W, Zeng W (2013) Surface modification: how nanoparticles assemble to molecular imaging probes. J Nanoparticle Res 15:2100. https://doi.org/10.1007/S11051-013-2100-9
Zhang D, Peng R, Liu W, Donovan MJ, Wang L, Ismail I, Li J, Li J, Qu F, Tan W (2021) Engineering DNA on the surface of upconversion nanoparticles for bioanalysis and therapeutics. ACS Nano 15:17257–17274. https://doi.org/10.1021/ACSNANO.1C08036
González-Béjar M, Francés-Soriano L, Pérez-Prieto J (2016) Upconversion nanoparticles for bioimaging and regenerative medicine. Front Bioeng Biotechnol 4:47. https://doi.org/10.3389/FBIOE.2016.00047
Chatterjee DK, Rufaihah AJ, Zhang Y (2008) Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials 29:937–943. https://doi.org/10.1016/J.BIOMATERIALS.2007.10.051
Mello GPC, Simões EFC, Crista DMA, Leitão JMM, Pinto da Silva L, Esteves da Silva JCG (2019) Glucose sensing by fluorescent nanomaterials. Crit Rev Anal Chem 49:542–552. https://doi.org/10.1080/10408347.2019.1565984
Liu Y, Tu D, Zheng W, Lu L, You W, Zhou S, Huang P, Li R, Chen X (2018) A strategy for accurate detection of glucose in human serum and whole blood based on an upconversion nanoparticles-polydopamine nanosystem. Nano Res 11:3164–3174. https://doi.org/10.1007/S12274-017-1721-1
Ali M, Sajid M, Khalid MAU, Kim SW, Lim JH, Huh D, Choi KH (2020) A fluorescent lateral flow biosensor for the quantitative detection of vaspin using upconverting nanoparticles. Spectrochim Acta - Part A Mol Biomol Spectrosc 226:117610. https://doi.org/10.1016/J.SAA.2019.117610
Martiskainen I, Juntunen E, Salminen T, Vuorenpää K, Bayoumy S, Vuorinen T, Khanna N, Pettersson K, Batra G, Talha SM (2021) Double-antigen lateral flow immunoassay for the detection of anti-hiv-1 and-2 antibodies using upconverting nanoparticle reporters. Sensors (Switzerland) 21:1–17. https://doi.org/10.3390/S21020330
Fang RH, Hu CMJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L (2014) Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 14:2181–2188. https://doi.org/10.1021/NL500618U
Fang H, Li M, Liu Q, Gai Y, Yuan L, Wang S, Zhang X, Ye M, Zhang Y, Gao M, Lan X, Hou Y (2020) Ultra-sensitive nanoprobe modified with tumor cell membrane for UCL/MRI/PET multimodality precise imaging of triple-negative breast cancer. Nano-Micro Lett 12:62. https://doi.org/10.1007/S40820-020-0396-4
Kamaraj N, Sundaresan S, Devaraj A (2017) DDA loaded PCL nanoparticles enhances the oral bioavailability of DDA in diabetes induced experimental rats. Int J Pharm Pharm Sci 9:198. https://doi.org/10.22159/IJPPS.2017V9I4.16860
Norpi ASM, Nordin ML, Ahmad N, Katas H, Fuaad AAHA, Sukri A, Marasini N, Azmi F (2022) New modular platform based on multi-adjuvanted amphiphilic chitosan nanoparticles for efficient lipopeptide vaccine delivery against group A streptococcus. Asian J Pharm Sci 17:435–446. https://doi.org/10.1016/J.AJPS.2022.04.002
Dong S, Ma S, Chen H, Tang Z, Song W, Deng M (2022) Nucleobase-crosslinked poly(2-oxazoline) nanoparticles as paclitaxel carriers with enhanced stability and ultra-high drug loading capacity for breast cancer therapy. Asian J Pharm Sci 17:571–582. https://doi.org/10.1016/J.AJPS.2022.04.006
Liang G, Wang H, Shi H, Wang H, Zhu M, Jing A, Li J, Li G (2020) Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J Nanobiotechnology 18:154. https://doi.org/10.1186/S12951-020-00713-3
Jalani G, Tam V, Vetrone F, Cerruti M (2018) Seeing, targeting and delivering with upconverting nanoparticles. J Am Chem Soc 140:10923–10931. https://doi.org/10.1021/JACS.8B03977
Chu H, Cao T, Dai G, Liu B, Duan H, Kong C, Tian N, Hou D, Sun Z (2021) Recent advances in functionalized upconversion nanoparticles for light-activated tumor therapy. RSC Adv 11:35472–35488. https://doi.org/10.1039/D1RA05638G
Yan J, Li C, Liu J (2021) Remotely ameliorating blood glucose levels in type 2 diabetes via a near-infrared laser. Adv Funct Mater 31:2007215. https://doi.org/10.1002/ADFM.202007215
Wang C, Cheng L, Liu Z (2013) Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 3:317–330. https://doi.org/10.7150/THNO.5284
Feng C, Zhu D, Chen L, Lu Y, Liu J, Kim NY, Liang S, Zhang X, Lin Y, Ma Y, Dong C (2019) Targeted delivery of chlorin E6 via redox sensitive diselenide-containing micelles for improved photodynamic therapy in cluster of differentiation 44-overexpressing breast cancer. Front Pharmacol 10:369. https://doi.org/10.3389/FPHAR.2019.00369
Chen X, Zhao Z, Jiang M, Que D, Shi S, Zheng N (2013) Preparation and photodynamic therapy application of NaYF4:Yb, Tm-NaYF4:Yb, Er multifunctional upconverting nanoparticles. New J Chem 37:1782–1788. https://doi.org/10.1039/C3NJ00065F
Dibaba ST, Xie Y, Xi W, Bednarkiewicz A, Ren W, Sun L (2022) Nd3+-sensitized upconversion nanoparticle coated with antimony shell for bioimaging and photothermal therapy in vitro using single laser irradiation. J Rare Earths 40:862–869. https://doi.org/10.1016/J.JRE.2021.05.015
Nguyen PD, Son SJ, Min J (2014) Upconversion nanoparticles in bioassays, optical imaging and therapy. J Nanosci Nanotechnol 14:157–174. https://doi.org/10.1166/JNN.2014.8894
Gong Y, Zheng Y, Jin B, You M, Wang J, Li XJ, Lin M, Xu F, Li F (2019) A portable and universal upconversion nanoparticle-based lateral flow assay platform for point-of-care testing. Talanta 201:126–133. https://doi.org/10.1016/J.TALANTA.2019.03.105
Lin M, Gao Y, Hornicek F, Xu F, Lu TJ, Amiji M, Duan Z (2015) Near-infrared light activated delivery platform for cancer therapy. Adv Colloid Interface Sci 226:123–137. https://doi.org/10.1016/J.CIS.2015.10.003
Lai WF, Rogach AL, Wong WT (2017) Molecular design of upconversion nanoparticles for gene delivery. Chem Sci 8:7339–7358. https://doi.org/10.1039/C7SC02956J
Jiang S, Zhang Y, Lim KM, Sim EKW, Ye L (2009) NIR-to-visible upconversion nanoparticles for fluorescent labeling and targeted delivery of siRNA. Nanotechnology 20:155101. https://doi.org/10.1088/0957-4484/20/15/155101
Lin M, Gao Y, Diefenbach TJ, Shen JK, Hornicek FJ, Il PY, Xu F, Lu TJ, Amiji M, Duan Z (2017) Facial layer-by-layer engineering of upconversion nanoparticles for gene delivery: near-infrared-initiated fluorescence resonance energy transfer tracking and overcoming drug resistance in ovarian cancer. ACS Appl Mater Interfaces 9:7941–7949. https://doi.org/10.1021/ACSAMI.6B15321
Chan MH, Chen SP, Chen CW, Chan YC, Lin RJ, Tsai DP, Hsiao M, Chung RJ, Chen X, Liu RS (2018) Single 808 nm laser treatment comprising photothermal and photodynamic therapies by using gold nanorods hybrid upconversion particles. J Phys Chem C 122:2402–2412. https://doi.org/10.1021/ACS.JPCC.7B10976
Castano AP, Mroz P, Hamblin MR (2006) Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer 6:535–545. https://doi.org/10.1038/NRC1894
Ding B, Shao S, Yu C, Teng B, Wang M, Cheng Z, Wong KL, Ma P, Lin J (2018) Large-pore mesoporous-silica-coated upconversion nanoparticles as multifunctional immunoadjuvants with ultrahigh photosensitizer and antigen loading efficiency for improved cancer photodynamic immunotherapy. Adv Mater 30:e1802479. https://doi.org/10.1002/ADMA.201802479
Song W, Kuang J, Li CX, Zhang M, Zheng D, Zeng X, Liu C, Zhang XZ (2018) Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano 12:1978–1989. https://doi.org/10.1021/ACSNANO.7B09112
Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, Dong Z, Zhu W, Peng R, Liu Z (2017) Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano 11:4463–4474. https://doi.org/10.1021/ACSNANO.7B00715
Wang J, Qi J, Jin F, You Y, Du Y, Liu D, Xu X, Chen M, Shu G, Zhu L, Ying X, Ji J, Li W, Du Y (2022) Spatiotemporally light controlled “drug-free” macromolecules via upconversion-nanoparticle for precise tumor therapy. Nano Today 42:. https://doi.org/10.1016/J.NANTOD.2021.101360
Aghajanzadeh M, Zamani M, Kouchi FR, Eixenberger J, Shirini D, Estrada D, Shirini F (2022) Synergic antitumor effect of photodynamic therapy and chemotherapy mediated by nano drug delivery systems. Pharmaceutics 14:322. https://doi.org/10.3390/PHARMACEUTICS14020322
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348:56–61. https://doi.org/10.1126/SCIENCE.AAA8172
Topalian SL, Taube JM, Anders RA, Pardoll DM (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275–287. https://doi.org/10.1038/NRC.2016.36
Auslander N, Zhang G, Lee JS, Frederick DT, Miao B, Moll T, Tian T, Wei Z, Madan S, Sullivan RJ, Boland G, Flaherty K, Herlyn M, Ruppin E (2018) Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med 24:1545–1549. https://doi.org/10.1038/S41591-018-0157-9
Morrison AH, Diamond MS, Hay CA, Byrne KT, Vonderheide RH (2020) Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc Natl Acad Sci U S A 117:8022–8031. https://doi.org/10.1073/PNAS.1918971117
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D’Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B (2016) Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 375:2561–2569. https://doi.org/10.1056/NEJMOA1610497
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, Westin J, Gulbis AM, Loghin ME, De Groot JF, Adkins S, Davis SE, Rezvani K, Hwu P, Shpall EJ (2018) Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat Rev Clin Oncol 15:47–62. https://doi.org/10.1038/NRCLINONC.2017.148
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O’connor RS, Hwang WT, Pequignot E, Ambrose DE, Zhang C, Wilcox N, Bedoya F, Dorfmeier C, Chen F, Tian L, Parakandi H, Gupta M, Young RM, Johnson FB, Kulikovskaya I, Liu L, Xu J, Kassim SH, Davis MM, Levine BL, Frey NV, Siegel DL, Huang AC, Wherry EJ, Bitter H, Brogdon JL, Porter DL, June CH, Melenhorst JJ (2018) Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 24:563–571. https://doi.org/10.1038/S41591-018-0010-1
Dranoff G (2004) Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 4:11–22. https://doi.org/10.1038/NRC1252
Van Gorp H, Lamkanfi M (2019) The emerging roles of inflammasome-dependent cytokines in cancer development. EMBO Rep 20:e47575. https://doi.org/10.15252/EMBR.201847575
Qiao J, Fu YX (2020) Cytokines that target immune killer cells against tumors. Cell Mol Immunol 17:722–727. https://doi.org/10.1038/S41423-020-0481-0
Napolitano S, Brancaccio G, Argenziano G, Martinelli E, Morgillo F, Ciardiello F, Troiani T (2018) It is finally time for adjuvant therapy in melanoma. Cancer Treat Rev 69:101–111. https://doi.org/10.1016/J.CTRV.2018.06.003
Tsujimura K, Ikehara Y, Nagata T, Koide Y, Kojima N (2009) Induction of anti-tumor immune responses with oligomannose-coated liposomes targeting to peritoneal macrophages. Procedia Vaccinol 1:127–134. https://doi.org/10.1016/J.PROVAC.2009.07.024
Mettenbrink EM, Yang W, Wilhelm S (2022) Bioimaging with upconversion nanoparticles. Adv Photonics Res 3:2200098
Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA (2015) Upconverting nanoparticles: assessing the toxicity. Chem Soc Rev 44:1561–1584
Xiong L, Yang T, Yang Y, Xu C, Li F (2010) Long-term in vivo biodistribution imaging and toxicity of polyacrylic acid-coated upconversion nanophosphors. Biomaterials 31:7078–7085
Xing H et al (2012) A NaYbF4: Tm3+ nanoprobe for CT and NIR-to-NIR fluorescent bimodal imaging. Biomaterials 33:5384–5393
Cao T et al (2011) High-quality water-soluble and surface-functionalized upconversion nanocrystals as luminescent probes for bioimaging. Biomaterials 32:2959–2968
Agraharam G, Saravanan N, Girigoswami A, Girigoswami K (2022) Future of Alzheimer’s disease: nanotechnology-based diagnostics and therapeutic approach. Bionanoscience 12:1002–1017. https://doi.org/10.1007/S12668-022-00998-8
Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS (2012) Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol 60:1207–1216. https://doi.org/10.1016/J.JACC.2012.03.074
Menezes G, Menez P, Meneze C (2011) Nanoscience in diagnostics: a short review. Internet J Med Updat - EJournal 6. https://doi.org/10.4314/IJMU.V6I1.63971
Author information
Authors and Affiliations
Contributions
R.S. drafted the applications of UCNP; P.S. contributed in drafting the upconversion process; S.D. drafted the introduction and the surface modification of UCNP; S.S. conceived the idea and formatted and corrected the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ajee, R.S., Roy, P.S., Dey, S. et al. Upconversion nanoparticles and their potential in the realm of biomedical sciences and theranostics. J Nanopart Res 26, 50 (2024). https://doi.org/10.1007/s11051-024-05960-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-05960-1