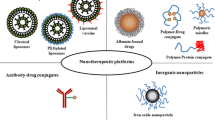
Abstract
Cancer remains a serious health problem in terms of incidence and mortality worldwide. As a result, researchers are working to identify new chemotherapeutic therapies or, potentially, to use innovative drug delivery methods in existing therapies. Recently, there has been a lot of interest in using nanocarriers as drug delivery systems, particularly for the treatment of cancer. Several novel nanocarrier-mediated drug delivery systems are currently being used to deliver chemotherapeutic agents to specific sites. Polymeric nanoparticles, liposomes, polymeric micelles, carbon nanotubes, dendrimers, solid lipid nanoparticles, magnetic nanoparticles and quantum dots are all examples of important nanocarriers. One of the most often prescribed chemotherapeutics for first-line therapy is gemcitabine hydrochloride, which has a broad spectrum of effects. Gemcitabine hydrochloride is an intriguing example of a drug for which various nanostructured targeted delivery methods are being explored over history. Even though some of these systems already exist on the market, there is continued research on this topic and new solutions are continually sought. In this context, the present review examines gemcitabine not as a specific drug, but as a proof of concept study that has drawn upon a wide range of innovative nanotechnology approaches.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, cancer is the leading cause of death, accounting for nearly 10 million deaths in 2020. Among the most common cancers are breast, lung, colon, rectum and prostate cancers. In many cases, cancer can be cured if it is detected early and treated effectively [1, 2]. The heterogeneity of tumours, drug resistance and systemic toxicities are major obstacles in cancer treatment [3]. The advent of nanoscale delivery systems as vehicles for anticancer drugs is gaining importance because of their multifunctionality and ability to target cancer cells. In cancer, cells differentiate rapidly and uncontrollably [4]. Due to fast cell differentiation, the tumour grows fast; however, the angiogenesis is slower, so these tissues have nonmature or formative vasculature. Due to leaky blood vessels, nanoparticles can penetrate cancer tissue, whereas tight junctions between endothelial cells prevent penetration in healthy tissue [5]. Furthermore, cancer tissues lack a well-formed lymphatic circulation that is responsible for maintaining tissue homeostasis. As a result, particles are retained in cancer tissue for a longer period [6, 7]. In cancer, this phenomenon is called enhanced permeation and retention (EPR). The drug carrier system’s size has a significant impact on the retention process [8, 9]. Therefore, the application of nanoparticles may present a fantastic opportunity for the treatment of cancer.
Nanotherapeutics, a fast-evolving field of science, have the potential to completely transform cancer diagnosis and therapy. Because of their small size (diameter within 1-100 nm) and high surface area to volume ratio, they have special biological properties that enable them to bind, absorb and transport chemotherapeutic agents, such as drugs, DNA, RNA and proteins, as well as imaging agents, with high effectiveness [10]. The two main categories of nanocarriers utilized in chemotherapy for targeted or non-targeted administering drugs are those that use organic molecules as a primary building block and those that use inorganic elements (usually metals) as a core. Lipids, carbon nanotubes, dendrimers, emulsions and synthetic polymers are examples of organic nanocarriers [11]. In contrast to polymer-based nanocarriers, inorganic nanocarrier platforms have undergone extensive research in recent years for therapeutic and imaging treatments due to their many benefits, including a large surface area, improved drug loading capacity, improved bioavailability, fewer toxic side effects, controlled drug release and tolerance to most organic solvents [12]. Quantum dots, carbon nanotubes, layered double hydroxides, mesoporous silica and magnetic nanoparticles are all frequently employed in the treatment of cancer. Quantum dots have previously been shown to be superior imaging probes, particularly for long-term, integrated and accurate imaging and detection [13].
Current therapies and their drawbacks
There has been a notable advancement in our awareness of the putative hallmarks of tumour growth and therapy during the last 10 years. As time passes, both the aggregate and individual cancer burdens are shifting. But with cancer’s rising occurrence, clinical care for the disease remains a dire problem in the twenty-first century [14]. Concerning the core biological functions that are disturbed in cancer, such as disruptions in growth-factor binding, cell signalling, transcriptional regulation control, cell-cycle checkpoints, apoptosis and angiogenic, a tremendous amount of detailed data has been gathered over the past couple of decades. These have in turn prompted the search for logical anticancer medications and led to the production of an unprecedented number of unique chemicals, which are currently being tested in cancer therapy trials [15, 16].
The current methods of treating cancer are centred on repairing the genetic mechanisms that cause damage, cutting off the blood supply to the cancer cells, or eliminating the cancer cells themselves. Radiation therapy, chemotherapy and surgical excision to remove malignant tissue are examples of traditional therapeutic techniques, but they all have drawbacks. Surgery cannot be used to treat all forms of cancer and has a risk of organ loss in addition to the possibility of developing cancer. Cancerous cells are damaged by radiation treatment, but nearby healthy cells are also harmed [17]. Chemotherapy, the most commonly used therapeutic strategy, is used either alone or in combination with other therapeutic strategies that either kill cancer cells through drug toxicity or prevent cell division by preventing nutrient uptake or inhibiting the mechanism responsible for cell division [18]. This strategy is crude and ineffective for advanced stages of cancer because pharmacologically active cancer medications have low tumour site selectivity and dose-limiting toxicity. Chemotherapeutic drugs on the market today have a proven track record, but they only provide good disease-free survival benefits for a limited time [19]. However, drug resistance and nontarget tissue harm limit the effectiveness of these medications. There is room for newer agents or site-specific delivery systems to overcome the major challenges of toxicity and drug resistance to deliver these chemotherapeutic agents [20]. Current requirements include delivering high doses of drug molecules to tumour areas for maximum treatment efficacy while minimizing damage to healthy tissues and cancer cells [21].
Gemcitabine: a potential anticancer agent
Gemcitabine hydrochloride (GEM), 20-deoxy-20,20-difluorocytidine; dFdC is a nucleoside analogue and a chemotherapeutic agent. It kills cancer cells and other rapidly growing cells by preventing them from making DNA and RNA [22]. It was originally investigated for its antiviral effects, but it is now used as an anticancer therapy for various cancers. GEM is a cytidine analogue with two fluorine atoms replacing the hydroxyl on the ribose. As a prodrug, GEM is transformed into its active metabolites that work by replacing the building blocks of nucleic acids during DNA elongation, arresting tumour growth and promoting apoptosis of malignant cells. The structure, metabolism and mechanism of action of GEM are similar to cytarabine , but GEM has a wider spectrum of antitumour activity [23, 24]. GEM activity is dependent on its entry into cells, where it is immediately phosphorylated by deoxycytidine kinase (DCK), producing monophosphate and diphosphate (dFdCDP). The anticancer activity of diphosphate is due to the inhibition of ribonucleotide reductase. Another active metabolite of GEM that can be incorporated into DNA is triphosphate metabolite (dFdCTP). It binds to DNA polymerase, causing chain termination, which is required for DNA synthesis [25].
Chemoresistance is one of the leading problems associated with this drug. To overcome the side effects caused by GEM, it has been formulated in other forms for effective administration and therapeutic outcome [24]. Nanotechnology approaches for GEM delivery began several decades ago in an attempt to reduce the severe side effects frequently observed after its use [26]. Nanocarriers have unique physicochemical and biological properties that endow them with multifunctional abilities that allow for the simultaneous delivery of multiple drugs with improved retention, controlled release and effective delivery of payloads specifically to target cells, thereby reducing the overall dose and minimizing side effects. The purpose of this review is to demonstrate the potential of nanomaterials in cancer treatment, primarily as drug delivery vehicles, using GEM-based nanosystems as a shining example. Since the effectiveness of a nanocarrier depends on its ability to deliver the drug in the therapeutic target, the biological barriers that may interfere in this process must be considered in its design. The biological and physicochemical properties of the action site should be considered when developing targeted and/or smart nanocarriers (sensitive to environmental conditions) [24,25,26]. Due to the importance of these aspects in the design of a nanocarrier, we begin by providing a brief overview of these elements. Following that, a summary of the research on GEM-based nanotherapeutics is provided, highlighting the fundamental traits of the various systems under investigation and providing illustrative examples in the form of tables.
Role of nanocarrier in cancer therapy
Nanocarriers are colloidal nanosystems loaded with therapeutic agents (anticancer agents or any macromolecule, such as proteins or genes), enabling drugs to accumulate selectively at the site of cancerous tumours. They are used in cancer treatment due to their unique nanometre range of 1-1000 nm (drug administration is preferable in the 5-200 nm range) [27]. They allow these anticancer agents to avoid normal tissues and accumulate in tumours, achieving a cytotoxic concentration several-fold higher in these tumours while causing less toxicity in the rest of the body than free drugs. Nanocarriers protect the drug from degradation, reduce renal clearance and increase its half-life in the bloodstream, augment the payload of cytotoxic drugs, allow control of anticancer drug release kinetics and improve the solubility of those insoluble drugs [28, 29]. Because of the drug’s improved stability and targeting when it is encapsulated or integrated into a nanocarrier, a smaller dosage of the drug is required to produce a given effect. A timely, targeted release increases the medicines’ potency, expands the spectrum of their applications and ensures the right dosage, increasing the product’s cost-effectiveness. By encasing or trapping reactive or delicate substances inside of nanocarrier systems, reactive or delicate substances like polynucleotides and polypeptides can be transformed into stable components. Chemotherapeutic medicines can now be delivered directly to tumours, minimizing systemic adverse effects [30]. This is made feasible by nanocarrier-mediated medication targeting. In actuality, the majority of nanotechnological cancer therapies are based on nanocarrier science. Since nanotechnology is a relatively young scientific topic, its potential contributions to the realm of human health care have not yet been extensively examined. However, current developments indicate that nanoscale will significantly affect illness prevention, diagnosis and therapy [31]. Nanotechnology applications in medical are highly promising, and many technologies are currently undergoing clinical trials in fields including molecular imaging, illness detection, medication encapsulation and targeted delivery at particular places in the body [32]. Targeting and targeted drug distributions are important factors in cancer therapy. Additionally, intracellular delivery techniques are necessary for newer generations of molecular therapies, including gene therapy and siRNA, to achieve the best outcomes.
Studies to date have shown that pharmacokinetic parameters can be changed by using nanocarrier-based formulations in a range that would otherwise be challenging to obtain [33]. In particular, it has been demonstrated that utilizing formulations based on nanocarriers enhanced circulation time by up to tens of hours, significantly decreased the severity of side effects and found and utilized the processes of passive and active targeting [34].
Advantages of nanocarriers in chemotherapeutics
-
i.
Site-specific delivery: The main objective of targeted therapy is to direct anticancer drugs onto cancerous cells, which eventually minimizes adverse effects. Several NDDSs that actively or passively target particular sites have been created recently to reduce toxicity and improve the specificity of existing drugs [27, 29].
-
ii.
Resolve multidrug resistance: Main resistance occurs when a disease does not initially react to chemotherapeutic drugs, as in the instance of non-small-cell lung cancer and rectal cancer, whereas obtained resistance occurs when certain sensitive tumours initially respond favourably to chemotherapy drugs but later develop acquired resistance. MDR is primarily caused by more efflux systems, including P-glycoprotein, in the cell membrane. The use of NP-based drug delivery devices to combat MDR was described in several research [35].
-
iii.
Improve aqueous solubility: Most anticancer drugs have poor solubility, which reduces absorption, raises the risk of food effects, frequently results in inadequate release in dosage form and increases interpatient variability [36].
Various approaches towards cancer treatment with different nanomaterials
Targeted drug delivery via nanocarriers
A fascinating method of treating cancer has been developed, and that method is called targeted therapy. Different targeting techniques hint at the potential impact of nanocarrier systems and could transform the way cancer is now treated. The goals of nanocarrier systems include various important angiogenesis, unregulated cell proliferation and tumour mass events in cancer mechanisms. The capacity of nanocarrier systems to lessen tumours or associated events without causing harm to healthy tissues is a key factor in their efficacy. Additional significant advancements offered by nanocarrier technologies include increased efficacy, fewer side effects, site specificity, efficient distribution and the ability to combat multidrug resistance (MDR) [27, 32, 37].
Targeting tumour cells
The most common targeting method uses targeted interactions between nanocarriers and the surface of cancer cells through the use of ligands. When choosing target molecules for the efficient distribution of nanocarriers, longer circulation durations and simpler endocytosis are important considerations [31]. These ligand-targeted nanocarriers are anticipated to enhance intracellular drug accumulation by selectively and specifically delivering lethal drugs to tumour cells through receptor-mediated endocytosis. A variety of tumour-targeting ligands, such as antibodies, folate or growth factors and cytokines, have been used to facilitate carriers’ entry into target cells [33, 39]. Monoclonal antibodies and antibody fragments have also been demonstrated to improve pharmacokinetics and reduce immunogenicity [37]. Additionally, synthetically produced antibodies have been discussed as a conjugate to thermosensitive liposomes (affisomes) and poly-(d,l-lactic acid)-polyethylene glycol (PLA-PEG) maleimide copolymer for the delivery of paclitaxel [35, 38]. As a compensating strategy for diffusion, passive targeted strategies for NP delivery in angiogenesis have also been described. It is based on nanocarrier characteristics such as size, surface make-up and circulation half-life [40]. Other intrinsic properties of nanocarriers (viz. charge) that can induce tumour targeting are also used in passive targeting. According to reports, negatively charged lipids that are selectively produced on tumour endothelial cells can connect to cationic liposomes via electrostatic interactions. Folate-conjugated nanocarriers cannot be taken up by human cervical cancer cells that lack the folate receptor [36, 41]. The possibility of tailored therapeutic nanocarriers as efficient anticancer drug delivery platforms was raised by a number of such studies.
Targeting the tumour microenvironment
It has been argued that the increased penetration and durability (EPR) effect of the tumour microenvironment may be a major justification for the creation of nanoscale carriers for solid tumours. Nanotherapeutics are anticipated to increase medication and diagnostic probe delivery as a result of EPR, have fewer side effects and lead to better tumour detection and therapy [42]. Another method of cancer treatment involves taking use of the aberrant tumour microenvironment to deliver nanomedicines to tumour locations in a targeted and homogeneous manner [43, 44].
Nanocarriers and EPR effect
Theoretically, nano-sized agents have several advantages over conventional low molecular weight agents, including a high loading capacity, the capacity to shield the payload from deterioration, precise targeting and controlled or sustained release. By altering attributes like size, shape, payload and surface features, their features can be improved. As a result, the field of nanomedicine has been rapidly developing, especially for the detection and treatment of cancer [27, 41].
Drugs that are nano-sized, however, are larger than most drugs and as a result, leak from capillary beds more slowly. Fortunately, the vasculature of solid tumours is characterized by leaky vessels with inadequate lymphatic drainage [2, 32]. If nano-sized agents are not small enough to be excreted by the kidney or large enough to be quickly detected and trapped by the reticuloendothelial system when administered intravenously, they tend to circulate for a long time (RES) [45]. Long-circulating nano-sized agents are retained in the tumour bed by reduced lymphatic drainage after preferentially penetrating tumour tissue through the permeable tumour vasculature (Fig. 1). The enhanced permeability and retention (EPR) effect is the name given to this phenomenon [37].
The EPR effect causes the drug to accumulate inside tumours before releasing its therapeutic payload, which is the basis for nano-sized drug delivery. EPR effects, in contrast to important normal organs, only offer a delivery increase of less than twofold [43]. The EPR effect makes it more likely for a drug to extravasate into a tumour the longer it is in circulation, though it can also extravasate into normal tissues, albeit more slowly [40]. To enhance the specific uptake of the drug within the tumour and thereby enhance its therapeutic effect, techniques that even momentarily increase the local EPR effect within the tumour are required [46]. They will then be able to take advantage of the special qualities of the tumour vasculature. Nanocarrier size should not be greater than 400 nm for this purpose in order to achieve extravasation into tumours via the EPR effect, which is noticeably more successful with diameters below 200 nm [47]. These nanoscale carriers also require hydrophilic, neutral, or mildly anionic surfaces in order to avoid the plasma proteins (opsonins) and delay the macrophage attack. This is done by coating the surfaces of the carriers with either amphiphilic or hydrophilic polymers, such as poly (ethylene glycol) (PEG) or synthetic copolymers of polyethylene oxide (hydrophilic block) and propylene oxide (hydrophobic block) [48]. It should be noted that positively or slightly negatively charged surfaces are preferable because negatively charged components on the surface of blood vessels and cells may resist nanocarriers with positively charged surfaces [49].
Proof-of-concept studies on the delivery of GEM-based nanocarriers in cancer therapeutics
The significance of GEM in the context of anticancer drugs justifies the large number of scientific studies conducted in this area, as well as the variety of nanoscale systems investigated for its delivery in cancer cells (Fig. 2). The following section discusses the various studies that has explored GEM-based nano-delivery in cancer therapeutics.
GEM-based polymeric nanocarriers
Polymer-based systems are among the most successful nanocarriers in nanomedicine due to their versatility. Their properties can be easily modified by adjusting their chemical composition, size and structure/architecture. Polymers have demonstrated the ability to maintain sustained drug release of encapsulated drugs, protect them from the environment and target cancer tissues in both passive (via the EPR effect) and active forms [50]. Furthermore, by varying the chemical composition, polymer toxicity and biodegradability can be modified, both of which are important considerations for nanomaterials used in medicine. One of the most well-known biodegradable and biocompatible polymers is poly(lactic-coglycolic acid) (PLGA). When exposed to normal physiological conditions, PLGA hydrolyzes, releasing the original monomers (lactic acid and glycolic acid), which are then metabolized via normal metabolic pathways. PLGA is considered safe and has been approved for human use by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA, in Europe) [51]. Table 1 lists the numerous polymeric nanocarriers for GEM delivery that have been discovered in the literature.
GEM-based polymeric micelles
Polymeric micelles have recently been widely used in pre-clinical studies to deliver poorly soluble chemotherapeutic agents in cancer. Polymeric micelles are formed by self-assembly of amphiphilic polymers. The wide availability of hydrophobic and, to a lesser extent, hydrophilic polymeric blocks allows researchers to explore various polymeric combinations for optimum loading, stability, systemic circulation and delivery to target cancer tissues. Furthermore, polymeric micelles could be easily tailored by varying the number of monomers in each polymeric chain. Poly(lactide) (PLA), poly(caprolactone) (PCL), poly(lactide-co-glycolide) (PLGA), polyesters, poly(amino acids) and lipids are some of the most widely used hydrophobic polymers. Poly(ethylene glycol), poly(oxazolines), chitosan, dextran and hyaluronic acids are the hydrophilic polymers used to wrap the hydrophobic core. Drugs could be conjugated to polymers at the distal ends to create pharmacologically active polymeric systems that improve the conjugates’ solubility and stability and allow for combination drug delivery. Because of their nanosize, they can accumulate in the tumour microenvironment through the enhanced permeability and retention (EPR) effect. Additionally, the stimuli-sensitive breakdown allows the micelles to effectively deliver the therapeutic cargo [62].
In a study carried out by Di et al., they investigated an effective method for the co-delivery of GEM and paclitaxel (PTX) into tumour cells. GEM and PTX were modified with functional (+)-α-tocopherol (VE) to obtain similar water solubility. Folic acid-poly(ethylene glycol)–(+)-α-tocopherol (FA–PEG–VE) was designed to co-encapsulate the modified GEM and PTX. Methoxy poly(ethylene glycol)–poly(lactide-co-glycolide) (MPEG–PLGA) was used as a control. It was found that two drug-loaded FA–PEG–VE micelles, GPF and MPEG–PLGA micelles (GPM), had a spherical morphology with an average diameter of 127 nm and 118.9 nm, respectively. In vitro releases of GPF, 2.73% of GEM–VE and 2.88% of PTX–VE, were accumulatively released in 72 h (4.04% of GEM–VE and 3.88% of PTX–VE from GPM). The comparisons of cytotoxicity were made with different formulations. The IC50 of GPF after 72-h incubation was lowest. FA–PEG–VE micelle showed higher uptake efficiency than that of MPEG–PLGA micelle. These results demonstrated that GEM–VE and PTX–VE-loaded FA–PEG–VE micelles would be a potentially useful prodrug-based nano-drug delivery system for cancer treatment [63].
Dual functional polymeric micelles (PMs) have been emerged as potent nanocarriers for combinational cancer therapy. In another study, Norouzi et al. investigated the potential of tri-layer PMs loaded with anti-nuclear factor-κB (NF-κB) siRNA and 4-(N)-stearoyl GEM-C18 for cancer treatment. PMs with different core hydrophobicity were prepared by using poly(ε-caprolactone), polyethyleneimine and polyethylene glycol (PCL-PEI-PEG) copolymers and evaluated. The results revealed that GEM-C18-loaded PMs were significantly more cytotoxic than free drug on breast and pancreatic cancer cells. However, the cytotoxicity of drug-loaded micelles was decreased by increasing the micellar core hydrophobicity because of decreasing drug release rate. Moreover, siRNA-loaded PMs could considerably inhibit NF-κB expression. PMs loaded with both GEM-C18 and siRNA exhibited higher capability to induce apoptosis and inhibit migration of both cells. PMs with the most hydrophobic core indicated higher tumour accumulation efficiency via in vivo imaging study [64].
GEM-based dendrimers
Dendrimers are a distinct type of polymer because of their regular and well-defined architecture, narrow polydispersity (especially when compared to classical polymers) and a large number of terminal groups (multivalency) that allow for further modification [65]. The basic dendrimer structure is made up of a core, branched shells (the number of which determines dendrimer generation) and outer functional groups [66]. Drugs can be transported by dendrimers through electrostatic interaction, chemical conjugation to their surface functional groups, or encapsulation within their inner voids [67]. Because of their intrinsic chemical nature, dendrimers can be controlled in terms of drug release rate regardless of whether the drug has been encapsulated or conjugated [65, 66].
The bio permeability of cationic PAMAM-NH2 (G0-G4) dendrimers for oral drug delivery was investigated, and it was discovered that they passed through the membrane via endocytosis and paracellular pathways [67]. Functionalization increased dendrimer size and water solubility, which aided in biodistribution and retention. Numerous studies have discovered a strong link between dendrimer termination and cell toxicity. Surface functional groups studied on dendrimers include proton-decorated amines, ethylenediamine ligands with benzyloxycarbonyl- or tert-butoxycarbonyl-protecting groups and dansyl fluorescent labels [68,69,70].
Hanurry et al. investigated GEM-containing biotin-coupled poly (amido) amine (PAMAM) (PG4.5) dendrimer nanoparticles that transported and absorbed inside of cells via receptor-mediated endocytosis. According to cytotoxicity studies, GEM-loaded PG4.5-DETA-biotin reduced HeLa cell viability and induced apoptosis. In order to assess the biocompatibility, cellular internalization effectiveness and antiproliferative efficacy of PG4.5-DETA-biotin/GEM, cell viability and uptake were measured using the MTT assay and flow cytometry. The biotin-coupled PG4.5-DETA nanocarrier can deliver GEM to tumour cells in an efficient and targeted manner. The best way to administer NPs and treat cancer cells may therefore involve techniques for administering biotin-coupled poly (amido) amine (PAMAM) (PG4.5) dendrimer-based NPs into the precise location of cancer cells [71].
Table 2 presents some examples of research studies on dendrimer-based systems for the release of GEM.
GEM-based gold nanoparticles
Gold nanoparticles (AuNPs) are one of the most extensively researched metal nanomaterials in biomedicine. They are popular because of their distinct optical, chemical and biological properties, which provide advantages over other nanoparticles. AuNPs have shown great promise in cancer therapy as nanocarriers for the targeted release of chemotherapeutic agents into tumoural cells, as well as intrinsic inhibitory activity against various tumour cell lines.
According to recent studies, nanogold has many advantages over other nanomaterials. This is largely because of highly optimized production processes that result in gold nanoparticles of all different sizes and shapes, each with their own special properties. The ability to modify the surface of nanogold particles with different targeting and functional compounds significantly expands the range of their potential biomedical applications, with a focus on cancer treatment. Functionalized gold nanoparticles have good biocompatibility and controllable biodistribution patterns, making them excellent candidates for the foundation of novel therapies [80, 81].
Conventional chemotherapy of pancreatic cancer (PaCa) suffers the problems of low-drug permeability and inherent or acquired drug resistance. Development of new strategies for enhanced therapy still remains a great challenge. Lin et al. reported a new ultrasound-targeted microbubble destruction (UTMD)-promoted delivery system based on dendrimer-entrapped gold nanoparticles (Au DENPs) for co-delivery of GEM and miR-21 inhibitor (miR-21i). In the study, Gem-Au DENPs/miR-21i was designed and synthesized. The designed polyplexes were characterized via transmission electron microscopy (TEM), gel retardation assay and dynamic light scattering (DLS). Then, the optimum exposure parameters were examined by an ultrasound exposure platform. The cellular uptake, cytotoxicity and anticancer effects in vitro were analysed by confocal laser microscopy, spectra microplate reader, flow cytometry and a chemiluminescence imaging system. Lastly, the anticancer effects in vivo were evaluated by contrast-enhanced ultrasound (CEUS), haematoxylin and eosin (H&E) staining, TUNEL staining and comparison of tumour volume. The results showed that the Gem-Au DENPs/miR-21i can be uptake by cancer cells and the cellular uptake was further facilitated by UTMD with an ultrasound power of 0.4 W/cm2 to enhance the cell permeability. Furthermore, the co-delivery of Gem and miR-21i with or without UTMD treatment displayed 82-fold and 13-fold lower IC50 values than the free Gem, respectively. The UTMD-promoted co-delivery of Gem and miR-21i was further validated by in vivo treatment and showed a significant tumour volume reduction and an increase in blood perfusion of xenografted pancreatic tumours [82].
In a recent study, Huai et al. investigated whether gold nanoparticles (AuNPs) could sensitize pancreatic cancer cells to the chemotherapeutic agent GEM. The study demonstrated that treatment with AuNPs of 20 nm diameter inhibited migration and colony-forming ability of pancreatic cancer cells. Pre-treatment with AuNPs sensitized pancreatic cancer cells to GEM in both viability and colony-forming assays. Mechanistically, pre-treatment of pancreatic cancer cells with AuNPs decreased GEM-induced EMT, stemness and MAPK activation. Taken together, these findings suggest that AuNPs could be considered a potential agent to sensitize pancreatic cancer cells to GEM [83].
Furthermore, Devi L. and colleagues claim that targeted drug administration is crucial for treating breast cancer since it produces therapeutic effects over a long period of time with few side effects. The improved water solubility and drug release from GEM-GA-colloidal AuNPs could be attributed to a gold cargo supply in the GEM. GEM’s targeting effectiveness enhanced as a result of a considerable particle sizing increase, better water solubility and improved drug release profile [84].
Some examples of utilization of GEM-based gold nanoparticles in cancer therapy are shown in Table 3.
GEM-based carbon nanotubes
Carbon nanotubes (CNTs) have become a popular tool in cancer diagnosis and treatment due to their unique physicochemical properties. CNTs have cylindrical hollow structures with walls that are the thickness of a carbon atom, whereas C60 is a spherical molecule with 60 carbon atoms that has the shape of a soccer ball [95]. With the ability to both detect cancerous cells and deliver medications or other small therapeutic molecules to these cells, they are regarded as one of the most promising nanomaterials. Nearly all cancer treatment modalities, including drug delivery, lymphatic targeted chemotherapy, thermal therapy, photodynamic therapy and gene therapy, have been investigated with CNTs over the past few years [96].
In a study reported by Das et al., they formulated GEM-loaded functionalized carbon nanotubes to achieve tumour targeted drug release and thereby reducing GEM toxicity. Multiwalled carbon nanotubes were functionalized using 1,2-distearoylphosphatidyl ethanolamine-methyl polyethylene glycol conjugate 2000. Optimized ratio 1:2 of carbon nanotubes:1,2-distearoylphosphatidyl ethanolamine-methyl polyethylene glycol conjugate 2000 was taken for loading of GEM. The formulation was evaluated for different parameters. The results showed that maximum drug loading efficiency achieved was 41.59% with an average particle size of 188.7 nm and zeta potential of −10 to 1 mV. Scanning electron microscopy and transmission electron microscopy images confirmed the tubular structure of the formulation. The carbon nanotubes were able to release GEM faster in acidic pH than at neutral pH indicating its potential for tumour targeting. GEM release from carbon nanotubes was found to follow Korsmeyer-Peppas kinetic model with non-Fickian diffusion pattern. Cytotoxic activity of formulation on A549 cells was found to be higher in comparison to free GEM [97].
In another study, Razzazan et al. designed a drug delivery system based on single-walled carbon nanotubes (SWCNTs) for the anticancer drug GEM, which has limitations under biological conditions, by using polyethylene glycol (PEG) to obtain nanoconjugates with high loading capacity, controlled drug release and effective cytotoxicity. Raw SWCNTs were functionalized through carboxylation, acylation, PEGylation and finally GEM conjugation via a cleavable ester bond. Different characterization techniques such as Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance spectrometer (NMR) and differential scanning calorimetry analysis (DSC) were performed to confirm the successful functionalization. Next, the influence of molecular weight (MW) of PEG on the drug loading capacity, drug release and cytotoxicity were studied. Experimental results showed that the drug loading capacity was dependent on the MW of PEG, but the drug release was independent. Also, the results revealed that the nanoconjugates with lower PEG MW caused higher cytotoxicity in A549 and MIA PaCa-2 cancer cells [98].
GEM’s clinical application is limited due to its short plasma half-life and poor uptake by cells. To address this problem, a drug delivery three-component composite, multiwalled carbon nanotubes (MWNTs)/GEM/lentinan (Le); (MWNTs- GEM -Le), was fabricated by Zhang et al. To enhance antitumour efficacy, the combination of chemotherapy and photothermal therapy was also employed in the study. They conjugated GEM and lentinan with MWNTs via a covalent and noncovalent way to functionalize with MWNTs. Using the composite and an 808-nm laser, tumours were treated and investigated for the photothermal responses and anticancer efficacy. The study showed MWNT-GEM-Le composite could efficiently cross cell membrane, having a higher antitumour activity than MWNTs, GEM and MWNT-GEM [99].
In another study conducted by Yang et al., the potential therapeutic effect of GEM loading magnetic multiwalled carbon nanotubes (mMWNTs) was compared with that of GEM-loaded magnetic-activated carbon particles (mACs). mMWNT-GEM and mAC-GEM both had high antitumour activity in vitro similar to free drug. Subcutaneous administration of GEM loading magnetic nanoparticles resulted in successful regression and inhibition of lymph node metastasis under the magnetic field, with mMWNT-GEM superior to mAC-GEM, and more effectively in the high-dose versus low-dose groups [100].
Gemcitabine hydrochloride-based quantum dots in cancer therapeutics
The GEM-based quantum dots (QDs) have captured the interest of researchers due to its unique optical and electrical properties. The QD surface can be decorated with molecular species, making it suitable for bioimaging samples and optical sensor applications. It also has excellent photo-bleaching resistance and luminescence characteristics like large absorption and narrow, symmetrical emission bands. They not only retain almost all of the aforementioned benefits, but they also have clear advantages such as a longer half-life of doping emissions and possibly reduced cytotoxicity [101, 102]. Table 4 shows examples of nanotherapeutics based on quantum dots that have been investigated for cellular/tumour delivery of GEM.
Pancreatic cancer is considered to be the deadliest of all cancers due to its poor prognosis and resistance to conventional therapies. In this study, the potential of hyaluronic acid functionalized and green fluorescent graphene quantum dot (GQD)-labelled human serum albumin nanoparticles for pancreatic cancer-specific drug delivery and bioimaging was explored. The study adopted lawsone (2-hydroxy-1,4-naphthoquinone) as a novel reducing agent for the synthesis of quantum dots and, in addition to excellent fluorescence of the synthesized GQDs, a good quantum yield of ∼14% was also obtained. GEM, the most preferred drug for pancreatic cancer treatment, was encapsulated in albumin nanoparticles, and it was observed that our nanoformulation significantly enhanced the bioavailability and sustained release property of the drug to pancreatic cancer cells in vitro. Moreover, the GQD-mediated bioimaging was excellent and enhanced the efficacy of our system as a drug delivery vehicle [103].
Carbon quantum dots (CQDs) have undergone extensive research and proved to have the potential to act both as drug delivery vehicles and as imaging agents. Quinic acid is an antioxidant that has shown anticancer activity through apoptosis-mediated cytotoxicity in breast cancer cells. Besides, it has demonstrated a strong affinity for selectins , which are angiogenesis factors increased in breast cancer tissue. In this study, nitrogen-doped CQDs were prepared via hydrothermal method . The resulting nanoparticles were conjugated with quinic acid as targeting agent towards breast cancer cells. Characterization of the resulting nanoparticles included TEM , SEM, zeta potential , FTIR, EDX, MAP, UV–Visual and fluorescent spectroscopy. GEM was loaded on the resulting nanoparticles through electrostatic interactions. Cell viability was evaluated via MCF7 cell line. In vivo imaging and biodistribution studies were conducted in breast cancer cells growing in mice. Taken together, quinic acid-conjugated N-CQDs exhibited promising properties such as excellent luminescent properties and high tumour accumulation, suggesting that they could be excellent candidates as multifunctional theranostic agents [104].
GEM-based liposomes
Liposomes are biocompatible and biodegradable self-assembled vesicles with the same supramolecular lipidic organization as living cell membranes. This is advantageous in terms of biocompatibility and biodegradability because it causes no side effects or accumulation. Liposomal formulations are the only nanomedicine platforms that have been approved by the US Food and Drug Administration for the treatment of cancer. It has been well established that the use of liposomes in the treatment of solid tumours, in particular, protects the incorporated molecule from being inactivated following intravenous administration, reducing anticancer drug accumulation in healthy tissues before it reaches the desired site of action. As a result, liposomes allow for a reduction in nonspecific toxicity while increasing the concentration of the encapsulated drug in specific body compartments. Because of their structure, they can encapsulate both hydrophilic and hydrophobic drugs. The size of the liposome is determined by its composition and the method of preparation used, and it influences drug loading capacity [106].
Because of the lipid composition of the colloidal formulation, liposomes can improve the in vitro antitumoural activity of GEM. Many studies have been conducted over the last few decades in order to identify a suitable lipid composition for systemic administration of anticancer drugs, particularly for the treatment of solid tumours. The physicochemical and technological characterization of various liposomal formulations revealed that a combination of distearoyl phosphatidylethanolamine (DSPE)-mPEG2000, cholesterol and dipalmitoyl phosphatidylcholine (DPPC) provided the best GEM delivery results. The presence of cholesterol provided rigidity to the bilayer as well as colloidal stability, whereas the PEGylated agent allowed for a long circulation time due to its shielding effect on the polar heads of DPPC, resulting in low zeta potential values, a characteristic that influences circulation time in the bloodstream, opsonization, uptake by the reticuloendothelial system and interaction within biological compartments [107].
Many studies have been conducted to evaluate the activity of GEM-loaded liposomes using various cell lines. Vono et al. investigated the antitumour activity of GEM-loaded PEGylated unilamellar liposomes in anaplastic thyroid cancer cells in vitro in terms of dose-dependent antitumour effect and incubation time. After 12 h of incubation, the colloids significantly improved the drug’s cytotoxicity at a concentration of 1 M, whereas the free drug only showed significant pharmacological activity after 72 h. This trend was confirmed by prolonging the anaplastic thyroid cancer cells’ exposure to liposomal GEM during incubation; in this case, the liposomal formulation resulted in 100% cell mortality at the aforementioned drug concentration after only 24 h [108].
To confirm the superior antitumoural activity of GEM-loaded PEGylated liposomes with respect to free GEM, the aforementioned formulation was compared with the commercial product, Gemzar®, using in vivo models of anaplastic thyroid carcinoma in NOD-SCID mice bearing human anaplastic thyroid xenograft tumours. After 4 weeks of treatment, the antitumour activity of the colloidal formulation was similar to that of Gemzar at a drug dose which was ten times higher (5 mg/kg of liposomal GEM versus 50 mg/kg of the commercial form), in terms of average tumour size and volume [109].
Furthermore, Affram et al. formulated GEM-loaded PEGylated thermosensitive liposomal nanoparticles (GEM-TSLnps) to increase residence time and deliver high payload of GEM to pancreatic cancer cells using mild hyperthermia (mHT). The cytotoxic effects of GEM and GEM-TSLnps were evaluated against human pancreatic cancer cell lines. In vitro release of GEM by TSLnps was determined at temperatures from 26 to 50°C. Cell viability studies, clonogenic assay, flow cytometry and confocal imaging were performed on pancreatic cancer cell lines using GEM and GEM-TSLnps + mHT. In vitro cytotoxicity of GEM-TSLnps + mHT-treated pancreatic cancer cell lines was significantly higher than GEM treated. The IC50 values for PANC-1, MiaPaCa-2 and BxPC-3 cells GEM-TSLnps + mHT treated were 1.2 to 3.5-fold higher than GEM treated. Among the cell lines, GEM-TSLnps + mHT-treated PANC-1 and MiaPaCa-2 cells show significantly reduced reproductive viability compared with the GEM-treated cells. Flow cytometric and confocal images revealed high Rho-TSLnps cellular uptake [110]. Furthermore, Kim and co-workers introduced a photosensitizer-conjugated lipid into the bilayer of GEM-loaded liposomes, which gave encouraging results in a biliary tract cancer model [111].
Triggered drug release is a promising strategy for delivering anticancer drugs to cancer cells and tissues. Fuse et al. designed liposomes co-encapsulating calcein (a water-soluble model drug and fluorescence marker) and talaporfin sodium (TPS, a water-soluble photosensitizer) that released the drug upon irradiation with a near-infrared (NIR) laser. The liposomes were composed of phospholipid (DSPC)/helper lipid (DOPE)/cholesterol/PEG-lipid (PEG2000-DSPE) at a molar ratio of 85/10/5/5 and released a large amount of drug (70%<, within 10 min) upon irradiation, but no drug in the absence of NIR-laser irradiation and/or TPS. NIR-laser-triggered drug release was facilitated by the incorporation of DOPE into the liposomes, and the amount of DOPE incorporated affected drug leakage in the absence of NIR-laser-irradiation at 37 °C (body temperature). Drug leakage was tuned by incorporating cholesterol into the liposomes. NIR-laser-triggered drug release from the liposomes was confirmed using the anticancer drug GEM. NIR-laser treatment of liposomes co-encapsulating GEM and TPS provided the maximum cytotoxic effect towards EMT6/P cells. The obtained results suggest that these novel light-sensitive liposomes may be useful for drug delivery to cancer cells [112].
Later, in a study conducted by Emamzadeh et al., they reported a thermoresponsive polymer-coated liposome nanocarrier that is capable to cocarry two potent anticancer drugs and release them via a thermally triggered mechanism. A synthetic polymer ([poly(diethylene glycol) methacrylate-co-poly(oligoethylene glycol) methacrylate]-b-poly(2-ethylhexyl) methacrylate) was synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization and was used as a thermoresponsive polymer coating shell on thermosensitive liposome carriers. The formulations were tested in vitro against two pancreatic cancer cell lines, MiaPaCa-2 and BxPC-3, and their cytotoxic potency was studied with respect to their targeted release properties as well as their biological interactions with cellular organelles. The polymer-modified liposomes (PMTLs) could cocarry and release GEM and cisplatin (Cis) in a thermally controlled rate and were also found to exhibit specific hydrophobic interactions with the cell membranes above the temperature transition of the formulations. In addition, the nanocarriers were found to induce more than 10-fold improvement of the IC50 of both drugs, either as monotherapies or in combination, in both cell lines tested, in a temperature-dependent manner [113].
GEM delivery to pancreatic ductal adenocarcinoma is limited by poor pharmacokinetics , dense fibrosis and hypo-vascularization. Activatable liposomes , with drug release resulting from local heating, enhance serum stability and circulation, and the released drug retains the ability to diffuse within the tumour. A limitation of liposomal GEM has been the low loading efficiency. To address this limitation, Tucci et al. used the superior solubilizing potential of copper (II) gluconate to form a complex with GEM at copper:GEM (1:4). Cryo transmission electron microscopy confirmed the presence of a liquid crystalline GEM-copper mixture. The optimized GEM liposomes released 60% and 80% of the GEM within 1 and 5 min, respectively, at 42°C. Liposomal encapsulation resulted in a circulation half-life of ~2 h in vivo (compared to reported circulation of 16 min for free GEM in mice), and free drug was not detected within the plasma. The resulting GEM liposomes were efficacious against both murine breast cancer and pancreatic cancer in vitro. Three repeated treatments of activatable GEM liposomes plus ultrasound hyperthermia regressed or eliminated tumours in the neu deletion model of murine breast cancer with limited toxicity, enhancing survival when compared to treatment with GEM alone. With 5% of the free GEM dose (5 rather than 100 mg/kg), tumour growth was suppressed to the same degree as GEM. Additionally, in a more aggressive tumour model of murine pancreatic cancer , liposomal GEM combined with local hyperthermia induced cell death and regions of apoptosis and necrosis [114].
Liposomal GEM has also been investigated in conjunction with gene therapy. Wang and colleagues [109] reported on the co-encapsulation of GEM and anti-KRAS small interfering RNA (siRNA) in apolipoprotein E3-based liposomes. The combination of the siRNA, which downregulated the expression of the KRAS oncogene by the endogenous mechanism of RNA interference (RNAi), and GEM improved pancreatic cancer cell apoptosis when compared with single-agent treatment [115].
As an alternative to conventional drug loading, GEM conjugate was combined with cholesterol and phospholipids to form liposomes. The liposome inhibited tumour growth to a greater extent than free GEM at less than 6 % of the normal dose, without systemic toxicity in a mouse model of pancreatic cancer [116].
GEM-based niosomes in cancer therapeutics
Niosomes are formed in an aqueous phase by the self-association of cholesterol and non-ionic surfactants. These nanostructures can be optimized for drug delivery by varying their composition, size, number of lamellae and surface charge. Due to their biocompatibility, lack of immunogenicity, high degree of stability and lengthy shelf life, they have become popular for use in medicine [117]. In a study reported by Seleci et al., niosomes loaded with GEM were prepared with cholesterol, Span 60 and D--tocopheryl polyethylene glycol 1000 to improve in vitro efficacy in pancreatic cancer cells [118]. In another study, Saimi and colleagues developed an aerosolized GEM and cisplatin co-loaded niosome to treat lung cancer. The developed niosomes demonstrated controlled drug release for both drugs for up to 24 h and were found to be safe with growth inhibitory effects in non-small cell lung cancer [119].
GEM-based solid lipid nanoparticles in cancer therapeutics
Solid lipid nanoparticles (SLNPs) are created by combining lipids that remain solid at physiological temperatures with emulsifiers. SLNPs are biocompatible and biodegradable, can protect the encapsulated drug from harsh conditions and have emerged as viable drug carrier alternatives to liposomes. As a drug delivery system, solid lipid nanoparticles (SLN) hold great promise for improving the therapeutic effectiveness and safety profile of conventional cancer chemotherapeutic agents. A number of SLNs or SLN-based nano-delivery systems have also been developed and studied for the delivery of cytotoxic drugs. SLNs are nano-sized particles (100-700 nm) that can diffuse out of blood vessels and accumulate within tumours. In fact, compelling evidence has recently been provided to support the view that nanocarriers (such as SLNs, polymeric nanoparticles) can be used to improve chemotherapeutic drug delivery to the tumour site in human studies [120,121,122].
Lung cancer is one of the leading causes of mortality worldwide. A significant proportion of patients with this disease have lymph node metastasis. In a study conducted by Wauthoz et al., they investigated the use of lipid nanocapsules, loaded with the lipophilic prodrug GEM for targeting tumours in lymph nodes after subcutaneous injection. The delivery method was shown to be effective in controlling tumour progression and may be useful in future clinical use [123]. Nandini and co-workers used a double emulsification technique to prepare GEM-loaded SLNPs from stearic acid, soy lecithin and sodium taurocholate. The SLNPs showed controlled drug release and increased cellular uptake in several organs compared with the free drug [124]. Soni and co-workers attached mannose to the surface of GEM-loaded SLNPs to target the mannose receptor on lung macrophages [125]. Wang and co-workers investigated the possibility of oral administration in mice with pre-established lung tumours. SLNPs loaded with a lipophilic amide prodrug of GEM, 4-(N)-stearoyl GEM, significantly inhibited tumour cell growth and angiogenesis, induced apoptosis and extended survival time [126]. Lysosomes are reportedly beneficial for the attenuation of GEM resistance by stearoyl GEM-SLNPs. It was put forward that the SLNP enters the cell via clathrin-mediated endocytosis and is fated for the lysosome where degradation of the SLNP allows for the release of the GEM conjugate and its hydrolysis to free GEM, and this is subsequently exported to the cytoplasm by nucleoside transporters [127]. Affram and colleagues evaluated the cytotoxic effects on patient-driven primary pancreatic cancer cell lines 48 (PPCL-46) and MiaPaCa-2 (GEM-SLN). With the aid of the cold-homogenization process, a number of SLN formulations containing polysorbate 80, poloxamer 188, glyceryl monostearate (GMS) and other 50 surfactants were created. The particle size and load distribution, trap efficiency and loading capacity were investigated for GEM-SLN [128]. Table 5 presents recent examples of research studies on lipid-based systems for the release of GEM.
Clinical status of GEM-based nanotherapeutics
Numerous GEM-based nanotherapeutics are either in clinical use or currently undergoing clinical trials. Polymeric nanoparticles, polymer micelles, liposomes, metal nanoparticles, nanogels, nanocrystals, dendrimers, carbon nanotubes and hybrid nanoparticles are currently being developed and used extensively in various pre-clinical studies to reverse cancer drug resistance. Some examples are listed below:
-
Gemzar
Gemzar® is a hydrochloride salt of GEM produced by Eli Lilly that is widely used against pancreatic cancer as a single therapeutic agent as well as in combination with many other anticancer agents. Gemzar is available as intravenous injection. Gemzar®, in combination with another chemotherapeutic agent, cisplatin, was approved by the Food and Drug Administration in 1996 for the treatment of inoperable stage III or IV non-small cell lung cancer. It has since been applied to the treatment of a wide range of solid tumours, usually in combination with other drugs. Currently, GEM is introduced intravenously in 3 or 4-week cycles. It is approved by the FDA to treat advanced ovarian cancer in combination with carboplatin , metastatic breast cancer in combination with paclitaxel , non-small cell lung cancer in combination with cisplatin and pancreatic cancer as monotherapy. It is also being investigated in other cancer and tumour types [22, 23, 25].
Gemzar has a number of side effects, including pale skin, easy bruising or bleeding, numbness, tingling, weakness, nausea, vomiting, stomach upset, diarrhoea, constipation, headache, swelling in the hands, ankles and feet, a skin rash, drowsiness and hair loss, even though they are not immediately life-threatening. The primary drawback of Gemzar administration is the inactive metabolite difluorodeoxyuridine that is produced when the liver and blood’s abundant supply of the enzyme deoxycytidine deaminase is present. Clinical data from 126 patients with significant symptoms of pancreatic cancer were collected throughout the experiment. GEM 1000 mg/m2 weekly 7, then weekly 3 every 4 weeks after that (for 63 patients), or 5-FU 600 mg/m2 once weekly (for 63 patients) were the two drug dosages used. The anticipated outcome was to reduce at least one symptom-related parameter, which might increase patient survival rates without having any negative side effects.
-
HPMA copolymer-based gemcitabine formulation
Poly(N-(2-hydroxypropyl) methacrylamide) (PHPMA) is a copolymer that is commonly used in the formulation of anticancer drugs. The PHPMA formulation extends GEM by utilising enhanced permeability and retention effects that localize the drug at the tumour site. A-GEM and B-GEM are the two versions of the PHPMA-GEM formulation that were produced. B-GEM was created using glycyl-phenyl-alanyl-leucyl-glycine (GFLG) spacers with the ability to get cleaved by lysosomal cysteine protease cathepsin, while A-GEM was created using uncleavable amino hexanoic acid spacers. It was suggested that HPMA formulations be used in conjunction with radiation therapy. In particular, the B-GEM formulation, which outperformed GEM alone, showed a 100% drug release within 6 h while radiotherapy was present [27, 29].
-
Gemlip
Gemlip is a GEM formulation (hydrogenated egg phosphatidylcholine/cholesterol) based on liposomes that contains the same amount of drug on both the inside and outside of the liposomal shell. This composition allows GEM to be present in a constant portion between the vesicle cores and the aqueous space. The therapeutic efficacy of this formulation was up to 35 times greater than GEM alone, and the maximum tolerable dose was lowered from 360 to 6–9 mg/kg. It also increased the drug’s half-life to 13 h while protecting it from deamination [134].
-
In co-delivery
In co-delivery, Abraxane, comprising of paclitaxel encapsulated within the albumin nanoparticles, indicated improved paclitaxel water solubility and a 28% reduction in death risk in metastatic pancreatic cancer patients when employed in a combination therapy with GEM during phase III clinical trials.
In another study, miR-1291 delivery along with GEM and nab-paclitaxel to pancreatic cancer reported induced DNA damage, mitotic block, induced apoptosis and significant inhibition of tumour cell growth by upregulating the AT-rich interactive domain-containing protein 3B (ARID3B) gene [135].
Conclusions and future trends
Nanotechnology, as a multidisciplinary and interdisciplinary field, opens up new avenues for patient treatment. The introduction of nanomaterials as nanocarriers for conventional medications in the context of cancer is expanding the potential for their use by enhancing their efficacy and safety. This is the case with GEM, a common anthracycline used to treat cancer that has been linked to the occurrence of serious side effects. A few GEM-based nanotherapeutics are currently in the clinical setting, and others are undergoing various stages of clinical trials, despite the fact that there is still a long way to go before a nanotherapeutic is commercially available Interestingly, these GEM nanotherapeutics are evolving, not only by exploring the EPR effect to accumulate and exert their action in the tumour site, but also by becoming smarter over time and equipping themselves with new tools that allow them to overcome physiological barriers, respond to environmental stimuli and reach specific cells/molecular targets. Research on GEM-based nanotherapeutics is still going strong, and the findings are very promising. In this review, only illustrative examples of the many publications that can be found in the literature are presented. Future GEM delivery techniques are likely to become more sophisticated, with the potential to be applied to other drug delivery techniques as well.
Data availability
Not applicable.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Rl S, Kd M, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):21254
Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie S (2013) Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu Rev Anal Chem (Palo Alto, Calif) 6(1):143
Nandini PT, Doijad RC, Shivakumar HN, Dandagi PM (2015) Formulation and evaluation of gemcitabine-loaded solid lipid nanoparticles. Drug Deliv 22(5):647–651
Aslan B, Ozpolat B, Sood AK, Lopez-Berestein G (2013) Nanotechnology in cancer therapy. J Drug Target 21(10):904–913
Benko A, Medina-Cruz D, Vernet-Crua A, O’Connell CP, Świętek M, Barabadi H, Saravanan M, Webster TJ (2021) Nanocarrier drug resistant tumor interactions: novel approaches to fight drug resistance in cancer. Cancer Drug Resist 4(2):264
Abdul Razak M, Devi Prasad Boggupalli S, Viswanath B (2015) Drug-loaded nanocarriers in tumor targeted drug delivery. Current. Biotechnology 4(3):319–344
Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM (2008) The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105:11613–11618
Khosravi-Darani K, Pardakhty A, Honarpisheh H, Rao VS, Mozafari MR (2007) The role of high-resolution imaging in the evaluation of nanosystems for bioactive encapsulation and targeted nanotherapy. Micron 38:804–818
Choi CH, Alabi CA, Webster P, Davis ME (2010) Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci USA 107(3):1235–1240
Lei W, Yang C, Wu Y, Ru G, He X, Tong X, Wang S (2022) Nanocarriers surface engineered with cell membranes for cancer targeted chemotherapy. J Nanobiotechnol 20(1):1–21
Singhvi G, Rapalli VK, Nagpal S, Dubey SK, Saha RN (2020) Nanocarriers as potential targeted drug delivery for cancer therapy. In: Nanoscience in Medicine, vol 1. Springer, Cham, pp 51–88
Kairdolf BA et al (2013) Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu Rev Anal Chem 6:143–162
Dadwal A, Baldi A, Narang RK (2018) Nanoparticles as carriers for drug delivery in cancer. Artif Cells Nanomed Biotechnol 46(sup2):295–305
Kumari P, Ghosh B, Biswas S (2016) Nanocarriers for cancer-targeted drug delivery. J Drug Target 24(3):179–191
Edis Z, Wang J, Waqas MK, Ijaz M, Ijaz M (2021) Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives. Int J Nanomed 16:1313–1330
Din FU, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S, Zeb A (2017) Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed 12:7291–7309
Lammers F, Kiessling WE, Hennink G (2012) Storm, Drug targeting to tumors principles, pitfalls and (pre-) clinical progress. J Control Release 161:175–187
Tian T, Ruan J, Zhang J, Zhao CX, Chen D, Shan J (2022) Nanocarrier-based tumor-targeting drug delivery systems for hepatocellular carcinoma treatments: enhanced therapeutic efficacy and reduced drug toxicity. J Biomed Nanotechnol 18(3):660–676
Yan L, Shen J, Wang J, Yang X, Dong S, Lu S (2020) Nanoparticle-based drug delivery system: a patient-friendly chemotherapy for oncology. Dose-Response 18(3):1559325820936161
Abdul Razak M, Devi Prasad Boggupalli S, Viswanath B (2015) Drug-loaded nanocarriers in tumor targeted drug delivery. Curr Biotechnol 4(3):319–344
Han H, Li S, Zhong Y, Huang Y, Wang K, Jin Q, Ji J, Yao K (2021) Emerging pro-drug and nano-drug strategies for gemcitabine-based cancer therapy. Asian J Pharm Sci
Samanta K, Setua S, Kumari S, Jaggi M, Yallapu MM, Chauhan SC (2019) Gemcitabine combination nano therapies for pancreatic cancer. Pharmaceutics 11(11):574
Shetty A, Nagesh PK, Setua S, Hafeez BB, Jaggi M, Yallapu MM, Chauhan SC (2020) Novel paclitaxel nanoformulation impairs de novo lipid synthesis in pancreatic cancer cells and enhances gemcitabine efficacy. ACS Omega 5(15):8982–8991
Emamzadeh M, Pasparakis G. Polymer coated gold nanoshells for combinational photochemotherapy of pancreatic cancer with gemcitabine. Sci Rep 2021;11(1):1-5.
Bhattacharya S, Anjum MM, Patel KK (2022) Gemcitabine cationic polymeric nanoparticles against ovarian cancer: formulation, characterization, and targeted drug delivery. Drug Deliv 29(1):1060–1074
Stylianopoulos T, Wong C, Bawendi MG, Jain RK, Fukumura D (2012) Multistage nanoparticles for improved delivery into tumor tissue. Methods Enzymol 508:109–130
Chidambaram M, Manavalan R, Kathiresan K (2011) Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci 14(1):67–77
Chen AM, Zhang M, Wei D et al (2009) Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small 5(23):2673–2677
Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, Wu S, Deng Y, Zhang J, Shao A (2020) Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci 7:193
Ranganathan R, Madanmohan S, Kesavan A et al (2012) Nanomedicine: towards development of patient-friendly drug-delivery systems for oncological applications. Int J Nanomed 7:1043–1060
Hossen S, Hossain MK, Basher MK, Mia MN, Rahman MT, Uddin MJ (2019) Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res 15:1–8
Sahoo SK, Parveen S, Panda JJ (2007) The present and future of nanotechnology in human health care. Nanomedicine 3(1):20–31
Dong X, Mumper RJ (2010) Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine 5(4):597–615
Rosenblum D, Joshi N, Tao W, Karp JM, Peer D (2018) Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun 9(1):1–2
Davis ME, Chen Z, Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7:771–782
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nano 2:751–760
Chidambaram M, Manavalan R, Kathiresan K (2011) Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci 14(1):67–77
Dahiya S, Dahiya R, Hernández E (2021) Nanocarriers for anticancer drug targeting: recent trends and challenges. Crit Rev Ther Drug Carrier Syst 38(6)
Cho K, Wang X, Nie S, Chen Z, Shin DM (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14:1310–1316
Tapasya K, Kumar AS, Dharmarajan A, Parvathi VD (2022) Nanocarriers: the promising future to cancer diagnostics and treatment. Biomed Pharmacol J 15(2)
Kaushik N, Borkar SB, Nandanwar SK et al (2022) Nanocarrier cancer therapeutics with functional stimuli-responsive mechanisms. J Nanobiotechnol 20:152. https://doi.org/10.1186/s12951-022-01364-2
Kenchegowda M, Rahamathulla M, Hani U, Begum MY, Guruswamy S, Osmani RAM, Gowrav MP, Alshehri S, Ghoneim MM, Alshlowi A, Gowda DV (2021) Smart nanocarriers as an emerging platform for cancer therapy: a review. Molecules 27(1):146
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148(2):135–146
Peer D, Karp J, Hong S et al (2007) Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech 2:751–760. https://doi.org/10.1038/nnano.2007.387
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63(3):136–151
Dastidar DG, Ghosh D, Das A (2022) Recent developments in nanocarriers for cancer chemotherapy. OpenNano:100080
Huda S, Alam MA, Sharma PK (2020) Smart nanocarriers-based drug delivery for cancer therapy: an innovative and developing strategy. J Drug Deliv Sci Technol 60:102018
Fang Z, Shen Y, Gao D (2021) Stimulus-responsive nanocarriers for targeted drug delivery. New J Chem 45(10):4534–4544
Neerooa BN, Ooi LT, Shameli K, Dahlan NA, Islam JM, Pushpamalar J, Teow SY (2021) Development of polymer-assisted nanoparticles and nanogels for cancer therapy: an update. Gels. 7(2):60
Gagliardi A, Giuliano E, Venkateswararao E, Fresta M, Bulotta S, Awasthi V, Cosco D (2021) Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front Pharmacol 12:601626
Resen AK, Atiroğlu A, Atiroğlu V, Eskiler GG, Aziz IH, Kaleli S, Özacar M (2022) Effectiveness of 5-Fluorouracil and gemcitabine hydrochloride loaded iron based chitosan-coated MIL-100 composite as an advanced, biocompatible, pH-sensitive and smart drug delivery system on breast cancer therapy. Int J Biol Macromol 198:175–186
İçhedef Ç, Teksöz S, Çetin O, Aydın B, Sarıkavak İ, Parlak Y, Bilgin BE (2021) Design of 99mTc radiolabeled gemcitabine polymeric nanoparticles as drug delivery system and in vivo evaluation. Mater Chem Phys 263:124380. https://doi.org/10.1016/j.matchemphys.2021.12
García-García G, Fernández-Álvarez F, Cabeza L, Delgado ÁV, Melguizo C, Prados JC, Arias JL (2020) Gemcitabine-loaded magnetically responsive poly (ε-caprolactone) nanoparticles against breast cancer. Polymers 12(12):2790
Emre Yalcin T, Ilbasmis-Tamer S, Takka S (2020) Antıtumor actıvıty of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNS) ın vıtro and ın vıvo. Int J Pharm:119246. https://doi.org/10.1016/j.ijpharm.2020.119246
Kush P, Bajaj T, Kaur M et al (2020) Biodistribution and pharmacokinetic study of gemcitabine hydrochloride loaded biocompatible iron-based metal organic framework. J Inorg Organomet Polym 30:2827–2841. https://doi.org/10.1007/s10904-019-01417-4
Jamil A, Mirza MA, Anwer K, Thakur PS, Alshahrani S, Alshetaili AS, Iqbal Z (2019) Co-delivery of gemcitabine and simvastatin through PLGA polymeric nanoparticles for the treatment of pancreatic cancer: in-vitro characterization, cellular uptake and pharmacokinetic studies. Drug Dev Ind Pharm:1–34. https://doi.org/10.1080/03639045.2019.1569040
Yalcin TE, Ilbasmis-Tamer S, Takka S (2018) Development and characterization of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs) using central composite design. Int J Pharm 548(1):255–262
Vandana M, Sahoo SK (2010) Long circulation and cytotoxicity of PEGylated gemcitabine and its potential for the treatment of pancreatic cancer. Biomaterials 31(35):9340–9356
Alibolandi M, Ramezani M, Abnous K, Hadizadeh F (2016) AS1411 aptamer-decorated biodegradable polyethylene glycol–poly (lactic-co-glycolic acid) nanopolymersomes for the targeted delivery of gemcitabine to non–small cell lung cancer in vitro. J Pharm Sci 105(5):1741–1749
Yalcin TE, Ilbasmis-Tamer S, Ibisoglu B, Özdemir A, Ark M, Takka S (2018) Gemcitabine hydrochloride-loaded liposomes and nanoparticles: comparison of encapsulation efficiency, drug release, particle size, and cytotoxicity. Pharm Dev Technol 23(1):76–86
Ghosh B, Biswas S (2021) Polymeric micelles in cancer therapy: state of the art. J Controll Release 332:127–147. https://doi.org/10.1016/j.jconrel.2021.02.016
Di Y, Gao Y, Gai X, Wang D, Wang Y, Yang X, Zhang D, Pan W, Yang X (2017) Co-delivery of hydrophilic gemcitabine and hydrophobic paclitaxel into novel polymeric micelles for cancer treatment. RSC Adv 7(39):24030–24039
Norouzi P, Amini M, Dinarvand R, Arefian E, Seyedjafari E, Atyabi F (2020) Co-delivery of gemcitabine prodrug along with anti NF-κB siRNA by tri-layer micelles can increase cytotoxicity, uptake and accumulation of the system in the cancers. Mater Sci Eng C 116:111161. https://doi.org/10.1016/j.msec.2020.111161
Wu LP, Ficker M, Christensen JB, Trohopoulos PN, Moghimi SM (2015) Dendrimers in medicine: therapeutic concepts and pharmaceutical challenges. Bioconjug Chem 26(7):1198–1211
Esfand R, Tomalia DA (2001) Poly (amidoamine)(PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today 6(8):427–436
Kesharwani P, Banerjee S, Gupta U, Amin MC, Padhye S, Sarkar FH, Iyer AK (2015) PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater Today 18(10):565–572
Almutairi A, Rossin R, Shokeen M, Hagooly A, Ananth A, Capoccia B, Guillaudeu S, Abendschein D, Anderson CJ, Welch MJ, Fréchet JM (2009) Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc Natl Acad Sci USA 106(3):685–690
Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL (2013) Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev 113(3):1904–2074
Singh S, Hassan D, Aldawsari HM, Molugulu N, Shukla R, Kesharwani P (2020) Immune checkpoint inhibitors: a promising anticancer therapy. Drug Discov Today 25(1):223–229
Hanurry EY, Mekonnen TW, Andrgie AT, Darge HF, Birhan YS, Hsu WH, Chou HY, Cheng CC, Lai JY, Tsai HC (2020) Biotin-decorated PAMAM G4. 5 dendrimer nanoparticles to enhance the delivery, anti-proliferative, and apoptotic effects of chemotherapeutic drug in cancer cells. Pharmaceutics 12(5):443
Zhang C, Pan D, Li J, Hu J, Bains A, Guys N, Zhu H, Li X, Luo K, Gong Q, Gu Z (2017) Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater 55:153–162
Zhang C, Pan D, Li J, Hu J, Bains A, Guys N, Zhu H, Li X, Luo K, Gong Q, Gu Z (2017) Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater 55:153–162. https://doi.org/10.1016/j.actbio.2017.02.047
Bhargava S. 2018 Mannosylated poly (propylene imine) dendrimer mediated lung delivery of anticancer bioactive. In Pediatric Blood & Cancer (Vol. 65, pp. S417-S418). 111 River St, Hoboken 07030-5774, NJ USA: Wiley.
Öztürk K, Esendağlı G, Gürbüz MU, Tülü M, Çalış S (2017) Effective targeting of gemcitabine to pancreatic cancer through PEG-cored Flt-1 antibody-conjugated dendrimers. Int J Pharm 517(1-2):157–167
Parsian M, Mutlu P, Yalcin S, Tezcaner A, Gunduz U (2016) Half generations magnetic PAMAM dendrimers as an effective system for targeted gemcitabine delivery. Int J Pharm 515(1-2):104–113
Soni N, Jain K, Gupta U, Jain NK (2015) Controlled delivery of gemcitabine hydrochloride using mannosylated poly (propyleneimine) dendrimers. J Nanopart Res 17(11):1–7
Pourjavadi A, Tehrani ZM, Moghanaki AA (2016) Folate-conjugated pH-responsive nanocarrier designed for active tumor targeting and controlled release of gemcitabine. Pharm Res 33(2):417–432
Yalçın S, Erkan M, Ünsoy G, Parsian M, Kleeff J, Gündüz U (2014) Effect of gemcitabine and retinoic acid loaded PAMAM dendrimer-coated magnetic nanoparticles on pancreatic cancer and stellate cell lines. Biomed Pharmacother 68(6):737–743
Sztandera K, Gorzkiewicz M, Klajnert-Maculewicz B (2018) Gold nanoparticles in cancer treatment. Mol Pharm 16(1):1–23
Emamzadeh M, Pasparakis G (2021) Polymer coated gold nanoshells for combinational photochemotherapy of pancreatic cancer with gemcitabine. Sci Rep 11:9404
Lin L, Fan Y, Gao F, Jin L, Li D, Sun W, Li F, Qin P, Shi Q, Shi X, Du L (2018) UTMD-promoted co-delivery of gemcitabine and miR-21 inhibitor by dendrimer-entrapped gold nanoparticles for pancreatic cancer therapy. Theranostics 8(7):1923
Huai Y, Zhang Y, Xiong X, Das S, Bhattacharya R, Mukherjee P (2019) Gold nanoparticles sensitize pancreatic cancer cells to gemcitabine. Cell Stress 3(8):267–279. https://doi.org/10.15698/cst2019.08.195
Devi L, Gupta R, Jain SK, Singh S, Kesharwani P (2020) Synthesis, characterization and in vitro assessment of colloidal gold nanoparticles of gemcitabine with natural polysaccharides for treatment of breast cancer. J Drug Deliv Sci Technol 56:101565
Guemei AA, Dessouky E, Shalaby TI, Amer SK, Nassra R (2021) Study of the efficacy and multidrug resistance using gold nanoparticles-based drug delivery versus conventional chemotherapy in non-small-cell lung cancer cell line. Res Oncol 17(2):42–50
Waghmare MN, Qureshi TS, Krishna CM, Pansare K, Gadewal N, Hole A, Dongre PM (2022) β-Lactoglobulin-gold nanoparticles interface and its interaction with some anticancer drugs–an approach for targeted drug delivery. J Biomol Struct Dyn 40(13):6193–6210
Waghmare MN, Qureshi TS, Shaikh AN, Khade BS, Murali Krishna C, Dongre PM (2020) Functionalized alpha-lactalbumin conjugated with gold nanoparticle for targeted drug delivery. Chem Select 5(6):2035–2049
Huai Y, Zhang Y, Xiong X, Das S, Bhattacharya R, Mukherjee P (2019) Gold nanoparticles sensitize pancreatic cancer cells to gemcitabine. Cell Stress 3(8):267
Wang Z, Chen L, Chu Z, Huang C, Huang Y, Jia N (2018) Gemcitabine-loaded gold nanospheres mediated by albumin for enhanced anti-tumor activity combining with CT imaging. Mater Sci Eng C 89:106–118
Santiago T, DeVaux RS, Kurzatkowska K, Espinal R, Herschkowitz JI, Hepel M (2017) Surface-enhanced Raman scattering investigation of targeted delivery and controlled release of gemcitabine. Int J Nanomed 12:7763–7776. https://doi.org/10.2147/IJN.S149306
Pal K, Al-Suraih F, Gonzalez-Rodriguez R, Dutta SK, Wang E, Kwak HS, Caulfield TR, Coffer JL, Bhattacharya S (2017) Multifaceted peptide assisted one-pot synthesis of gold nanoparticles for plectin-1 targeted gemcitabine delivery in pancreatic cancer. Nanoscale 9(40):15622–15634
Rhamani S, Chaix A, Aggad D, Hoang P, Moosa B, Garcia M, Gary-Bobo M, Charnay C, Almalik A, Durand JO, Khashab NM. Gold core mesoporous organosilica shell degradable nanoparticles for two-photon imaging and gemcitabine monophosphate delivery 2017.
Pooja D, Panyaram S, Kulhari H, Reddy B, Rachamalla SS, Sistla R (2015) Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int J Biol Macromol 80:48–56
Raoof M, Corr SJ, Zhu C, Cisneros BT, Kaluarachchi WD, Phounsavath S, Wilson LJ, Curley SA (2014) Gold nanoparticles and radiofrequency in experimental models for hepatocellular carcinoma. Nanomed Nanotechnol Biol Med 10(6):1121–1130
Singh R, Deshmukh R (2022) Carbon nanotube as an emerging theranostic tool for oncology. J Drug Deliv Sci Technol:103586
Tang L, Xiao Q, Mei Y et al (2021) Insights on functionalized carbon nanotubes for cancer theranostics. J Nanobiotechnol 19:423. https://doi.org/10.1186/s12951-021-01174-y
Das S, Desai JL, Thakkar HP (2013) Gemcitabine hydrochloride-loaded functionalised carbon nanotubes as potential carriers for tumour targeting. Indian J Pharm Sci 75(6):707–715
Razzazan A, Atyabi F, Kazemi B, Dinarvand R (2016) Influence of PEG molecular weight on the drug release and in vitro cytotoxicity of single-walled carbon nanotubes-PEG-gemcitabine conjugates. Curr Drug Deliv 13(8):1313–1324
Zhang P, Yi W, Hou J, Yoo S, Jin W, Yang Q (2018) A carbon nanotube-gemcitabine-lentinan three-component composite for chemo-photothermal synergistic therapy of cancer. Int J Nanomed 13:3069–3080
Yang F, Jin C, Yang D, Jiang Y, Li J, Di Y, Hu J, Wang C, Ni Q, Fu D (2011) Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur J Cancer 47(12):1873–1882
Devi S, Kumar M, Tiwari A, Tiwari V, Kaushik D, Verma R, Bhatt S, Sahoo BM, Bhattacharya T, Alshehri S, Ghoneim MM (2022) Quantum dots: an emerging approach for cancer therapy. Front Mater 8:798440. https://doi.org/10.3389/fmats
Matea CT, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia C, Iancu C, Mocan L (2017) Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomed 12:5421–5431. https://doi.org/10.2147/IJN.S138624
Nigam P, Waghmode S, Louis M, Wangnoo S, Chavan P, Sarkar D (2014) Graphene quantum dots conjugated albumin nanoparticles for targeted drug delivery and imaging of pancreatic cancer. J Mater Chem B 2(21):3190–3195
Samimi S, Ardestani MS, Dorkoosh FA (2021) Preparation of carbon quantum dots-quinic acid for drug delivery of gemcitabine to breast cancer cells. J Drug Deliv Sci Technol 61:102287
Habib S, Singh M (2021) Recent advances in lipid-based nanosystems for gemcitabine and gemcitabine–combination therapy. Nanomaterials 11(3):597. https://doi.org/10.3390/nano11030597
Campbell E, Hasan MT, Gonzalez Rodriguez R, Akkaraju GR, Naumov AV (2019) Doped graphene quantum dots for intracellular multicolor imaging and cancer detection. ACS Biomater Sci Eng 5(9):4671–4682
Federico C, Morittu VM, Britti D, Trapasso E, Cosco D (2012) Gemcitabine-loaded liposomes: rationale, potentialities and future perspectives. Int J Nanomed 7:5423–5436. https://doi.org/10.2147/IJN.S34025
Vono M, Cosco D, Celia C et al (2010) In vitro evaluation of the activity of gemcitabine-loaded pegylated unilamellar liposomes against papillary thyroid cancer cells. Open Drug Deliv J 4:55–62
Paolino D, Cosco D, Racanicchi L et al (2010) Gemcitabine-loaded PEGylated unilamellar liposomes vs GEMZAR: biodistribution, pharmacokinetic features and in vivo antitumor activity. J Control Release 144(2):144–150
Affram K, Udofot O, Agyare E (2015) Cytotoxicity of gemcitabine-loaded thermosensitive liposomes in pancreatic cancer cell lines. Integr Cancer Sci Ther 2(2):133
Kim DH, Im BN, Hwang HS, Na K (2018) Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials 183:139–150
Fuse T, Tagami T, Tane M, Ozeki T (2018) Effective light-triggered contents release from helper lipid-incorporated liposomes co-encapsulating gemcitabine and a water-soluble photosensitizer. Int J Pharm 540(1-2):50–56. https://doi.org/10.1016/j.ijpharm.2018.01.040
Emamzadeh M, Emamzadeh M, Pasparakis G (2019) Dual controlled delivery of gemcitabine and cisplatin using polymer-modified thermosensitive liposomes for pancreatic cancer. ACS Appl Bio Mater 2(3):1298–1309
Tucci ST, Kheirolomoom A, Ingham ES, Mahakian LM, Tam SM, Foiret J, Hubbard NE et al (2019) Tumor-specific delivery of gemcitabine with activatable liposomes. J Controll Release 309:277–288. https://doi.org/10.1016/j.jconrel.2019.07.014
Wang F, Zhang Z (2020) Nanoformulation of apolipoprotein E3-tagged liposomal nanoparticles for the co-delivery of KRAS-siRNA and gemcitabine for pancreatic cancer treatment. Pharm Res 37:1–11
Bulanadi JC, Xue A, Gong X, Bean PA, Julovi SM, De Campo L, Smith RC, Moghaddam MJ (2020) Biomimetic gemcitabine–lipid prodrug nanoparticles for pancreatic cancer. ChemPlusChem 85:1283–1291
Seleci DA, Seleci M, Walter J-G, Stahl F, Scheper T (2016) Niosomes as nanoparticular drug carriers: fundamentals and recent applications. J Nanomater 2016:1–13
Maniam G (2019) Preparation, characterization and anti-pancreatic cancer effects of gemcitabine-tocotrienols entrapped niosomes. Ph.D. Thesis, International Medical University, Kuala Lumpur, Malaysia
Saimi NM, Salim N, Ahmad N, Abdulmalek E, Rahman MA (2021) Aerosolized niosome formulation containing gemcitabine and cisplatin for lung cancer treatment: optimization, characterization and in vitro evaluation. Pharmaceutics 13:59
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 16(1):71. https://doi.org/10.1186/s12951-018-0392-8
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8(1):102. https://doi.org/10.1186/1556-276X-8-102
Mohammadi-Samani S, Ghasemiyeh P (2018) Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci 13:288–303
Wauthoz N, Bastiat G, Moysan E, Cieslak A, Kondo K, Zandecki M, Moal V, Rousselet MC, Hureaux J, Benoit JP (2015) Safe lipid nanocapsule-based gel technology to target lymph nodes and combat mediastinal metastases from an orthotopic non-small-cell lung cancer model in SCID-CB17 mice. Nanomed Nanotechnol Biol Med 11(5):1237–1245
Nandini PT, Doijad RC, Shivakumar HN, Dandagi PM (2015) Formulation and evaluation of gemcitabine-loaded solid lipid nanoparticles. Drug Deliv 22:647–651
Soni N, Soni N, Ramteke PW, Pandey H (2018) A validated RP-HPLC assay method for determination of gemcitabine loaded nanosized solid lipid nanoparticles. J Drug Deliv Ther 8:308–313
Wang C, Zheng Y, Oval MAS, Valdes SA, Chen Z, Lansakara-P DS, Du M, Shi Y, Cui Z (2017) Oral 4-(N)-stearoyl gemcitabine nanoparticles inhibit tumor growth in mouse models. Oncotarget 8:89876–89886
Chen Z, Zheng Y, Shi Y, Cui Z (2018) Overcoming tumor cell chemoresistance using nanoparticles: lysosomes are beneficial for (stearoyl) gemcitabine-incorporated solid lipid nanoparticles. Int J Nanomedicine 13:319–336
Affram KO, Smith T, Ofori E, Krishnan S, Underwood P, Trevino JG, Agyare E (2020) Cytotoxic effects of gemcitabine-loaded solid lipid nanoparticles in pancreatic cancer cells. J Drug Deliv Sci Technol 55:101374
Unnam S, Panduragaiah VM, Sidramappa MA, Muddana Eswara BR (2019) Gemcitabine-loaded folic acid tagged liposomes: improved pharmacokinetic and biodistribution profile. Current Drug Deliv 16(2):111–122
Al-Mutairi AA, Alkhatib MH (2022) Antitumour effects of a solid lipid nanoparticle loaded with gemcitabine and oxaliplatin on the viability, apoptosis, autophagy, and Hsp90 of ovarian cancer cells. J Microencapsul 39(5):467–480. https://doi.org/10.1080/02652048.2022.2109218
Wang C, Zheng Y, Sand Oval MA, Valdes SA, Chen Z, Lansakara-P DS, Du M, Shi Y, Cui Z (2017) Oral 4-(N)-stearoyl gemcitabine nanoparticles inhibit tumor growth in mouse models. Oncotarget 8(52):89876–89886
Soni N, Pandey H, Maheshwari R, Kesharwani P, Tekade RK (2016) Augmented delivery of gemcitabine in lung cancer cells exploring mannose anchored solid lipid nanoparticles. J Colloid Interface Sci 481:107–116. https://doi.org/10.1016/j.jcis.2016.07.020
Mokashi AS, Jadhav NR, Rooge SB (2012) Design, development and characterization of solid lipid nanoparticles of gemcitabine hydrochloride. Drug Deliv Lett 2(4):262–269
Bukhari SNA (2022) Emerging nanotherapeutic approaches to overcome drug resistance in cancers with update on clinical trials. Pharmaceutics 14:866. https://doi.org/10.3390/pharmaceutics14040866
Hoy SM (2014) Albumin-bound paclitaxel: a review of its use for the first-line combination treatment of metastatic pancreatic cancer. Drugs 74:1757–1768
Acknowledgements
The authors are thankful to the Faculty of Pharmacy, Integral University, Lucknow, for providing all the necessary facilities related to the present work (Manuscript Communication Number: IU/R&D/2022-MCN0001421).
Author information
Authors and Affiliations
Contributions
PK made substantial contribution to conception and design. LD, TMA and AK participated in the analysis and interpretation of data. LD carried out acquisition of data and wrote the paper with input from all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, L., Ansari, T.M., Kumar, A. et al. Assessment of gemcitabine hydrochloride-based nanotherapeutics in cancer: a proof of concept study. J Nanopart Res 25, 112 (2023). https://doi.org/10.1007/s11051-023-05764-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05764-9