Abstract
Composite polymer electrolyte (CPE) with ceramic fillers has gained great attention for lithium batteries with high energy density and safety. However, the agglomeration of ceramic fillers and weak polymer-ceramic interaction induces limited ionic conductivity and hinders its implementation. Here, hollow multishelled structure (HoMS) ZnO with a size range of 700 ~ 900 nm is designed as fillers for polyethylene oxide (PEO)-based CPE. Strong chemical and mechanical interaction between PEO and ZnO HoMS enable a high ionic conductivity and good electrochemical and mechanical stability. Wherein, double-shelled ZnO HoMS exhibits a good ionic conductivity of 1.04 × 10−4 S∙cm−1 and 1.2 × 10−3 S∙cm−1 at 30 ℃ and 60 ℃. Additionally, all-solid-state LiFePO4/Li full cell adopted with ZnO HoMS filled CPE exhibits a high initial specific capacity of 169 mAh∙g−1 and good cycling stability and withstands abuse test. The enhanced performance is due to that HoMS provides PEO with faster ion transport channels, more effective Lewis acid-based interaction sites, suppressed PEO crystallinity, and improved ionic conductivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) have been widely used for portable electronics and electric vehicles [1]. However, currently, LIBs mainly use liquid organic electrolyte, which suffer from a main issue of flammability. Besides, the ever-growing demand of higher energy density has driven the exploration of new electrode materials, such as lithium metal anode, sulfur cathode, etc [2, 3]. As for these newly-emerging materials, liquid electrolyte presents severe challenges, including accumulated solid electrolyte interphase (SEI), lithium dendrite, and polysulfide shuttle effect, which cause poor cycling life and bring safety issue. Solid-state electrolytes with higher mechanical strength, lower cost, and higher safety performance are expected to solve the problems fundamentally [4,5,6]. Particularly, solid polymer electrolytes (SPEs) which are based on long chains of organic polymer to transport lithium ions and feature the advantages of higher processability, flexibility, and lower contact resistance at the electrode\electrolyte interface, are promising for the fabrication of lithium batteries with high energy density, flexibility, and safety [7, 8]. Wherein, polyethylene oxide (PEO) with a high lithium salt solubility, high dielectric constant, and high stability to lithium metal is one of the most significant research fields in solid polymer electrolytes [9].
Despite the numerous advantages, the commercial use of PEO-based electrolytes is severely impeded by its much lower ionic conductivity (10−6−10−8 S∙cm−1 at room temperature), than the practically available level (~ 10−4 S∙cm−1) [10]. Through incorporation of ceramic fillers (ZnO, SiO2, Al2O3, etc.) with PEO solid electrolytes, to lower the internal crystallinity of PEO and accelerate the rate of polymer long-chains motion, has been proven effective to improve the ionic conductivity [11,12,13,14]. Compared to nanoparticle fillers, a more effective way to design a composite polymer electrolyte (CPE) is to fabricate a composite electrolyte by “PEO in ceramics” with a high-dimensional spatial structure material as the primary part. This electrolyte design ensures higher mechanical strength and energy density while limiting filler agglomeration, providing more effective Lewis acid-base interaction active sites, and increasing the proportion of amorphous regions in PEO-based electrolytes [15]. Nevertheless, considerable amount of crystallized polymer regions still exists, together with the agglomeration of ceramic fillers and the relatively weak polymer-ceramic interaction, making the further improvement of ionic conductivity a challenge.
Hollow multishelled structure (HoMS) materials are a specific category of hollow nanomaterials with multiple porous shells and interconnected closed cavities separated by shells [16,17,18,19]. Compared with single-shelled hollow structure, HoMS provide larger active interfaces and multi-level cavity structures, which are greatly beneficial for wide fields including energy conversion and storage, catalysis, and drug mitigation [20,21,22,23,24,25,26,27]. Herein, ZnO HoMSs are synthesized through a sequential templating approach (STA) and adopted in the “PEO-in-ceramic” composite electrolyte as hollow ceramic fillers [28,29,30]. ZnO HoMS are capable of offering abundant effective active sites for PEO long chains extending to its interior, suppressing PEO crystallinity, increasing ion migration number by adsorbing anions through surface positive charges, and enhancing the mechanical strength of electrolytes by acting as a backbone (Scheme 1 and Fig. S1). The composite electrolyte exhibits high ionic conductivity of 1.04 × 10−4 S∙cm−1 and 1.2 × 10−3 S∙cm−1 at 30 ℃ and 60 ℃, respectively. In the all-solid-state LiFePO4//Li full battery, it exhibits a high specific capacity of ~ 169 mAh∙g−1 in the first cycle and maintains ~ 121.8 mAh∙g−1 after 100 cycles.
Experimental section
Preparation of ZnO HoMS
First, the carbon microsphere template was prepared by the hydrothermal synthesis method. 250-mL deionized water was taken, and 130-g sucrose was stirred and dissolved in the liner of 500-mL reaction kettle at room temperature until it was completely dissolved. The solution was kept for 140 min in a drying oven at 200 ℃ and then cooled to room temperature. After dispersing and washing with ethanol for 12 h and then pumping and filtering again, drying for 12 h for later use. Taking the synthesis of single-shelled ZnO hollow spheres as an example, 60-mL deionized water was prepared into a zinc acetate dihydrate solution with a concentration of 144 mM·L−1. Then 1.2-g carbon microspheres were weighed and dispersed in the zinc acetate dihydrate solution. The microspheres were dispersed by ultrasound for 10 min, stirred and adsorbed at room temperature for 1 h, then pumped and filtered, and placed in a 60 ℃ air-blast drying oven for 24 h to dry. The massive material was ground and laid in a porcelain boat with a mortar in a Muffle furnace. The temperature was raised to 500 ℃ for 120 min at 4 ℃∙min−1, and the powder was collected after being cooled to room temperature naturally. ZnO HoMSs with more shells can be synthesized by controlling the adsorption time of carbonaceous microspheres or the type of solvent.
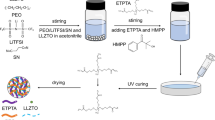
Preparation of ZnO HoMSs/PEO-LiTFSI CPE membranes
PEO (60 W molecular weight) and Li salt (LiTFSI) were weighed in accordance with the molar ratio of EO monomer and LiTFSI 10:1. Lithium salt was completely dissolved in anhydrous acetonitrile in the glove box. Then PEO powder was added into the prepared lithium salt solution and stirred for 24 h. The calculated weighing filler with the target filling ratio was dispersed in acetonitrile for 1 h by ultrasound, and then the dispersion was injected into the electrolyte solution and stirred for 24 h. After that, the acetonitrile solvent was volatilized in the vacuum oven at 60 ℃. Then pour the mixed electrolyte solution into the PTFE mold in the fume hood, place it in the oven at 60 ℃ to remove most of the acetonitrile volatilization, and then transfer it to the vacuum oven at 60 ℃ for 24 h to volatilize the remaining acetonitrile. After 24 h, the electrolyte was removed from the mold, and both sides were clamped with 100 μm polytetrafluoroethylene film at 80 ℃, pressed at 1.5 MPa for 1 min, then removed. The electrolyte film protected by polytetrafluoroethylene film on both sides was cut by a 15 mm punching machine for later use.
Characterizations
The crystal structure of the electrolyte films and ZnO HoMS were detected by a Philip X’Pert pro X-ray diffraction (XRD) analyzer (Cu Kα radiation, λ = 0.154 nm). The microstructure and morphology of the ZnO HoMS and electrolyte films were characterized by a JEM-2100 transmission electrolytes microscope (TEM) with an energy dispersive X-ray spectroscopy (EDS) and JSM-6700 scanning electron microscope (SEM). The applied voltages for taking SEM and EDS images were 5.0 and 20 kV. An AGS-X mechanical tester was applied to measure the mechanical tensile properties of the electrolyte films with a speed of 5 mm∙min−1. An analysis of thermodynamic behavior of electrolyte films by differential scanning calorimeter (DSC), model DSC-3 in argon flow with a heating speed of 10 ℃∙min−1.
Electrochemical measurements
The cell with different configurations of stainless steel (SS)/SPE/SS, Li/SPE/SS, Li/SPE/Li, and LiFePO4/SPE/Li was assembled in an argon-filled glove box for various electrochemical measurements. To fabricate LiFePO4 cathode, LiFePO4, poly(vinylidene fluoride) (PVDF), and carbon nanotubes were evenly mixed with a mass ratio of 7:2:1 in 1-methyl-2-pyrrolidinone (NMP). After grinding for 30 min, the obtained slurry was uniformly painted on an aluminum foil and dried in a vacuum at 60 °C for 24 h. The mass loading of LiFePO4 was controlled to be ~ 1.0 mg∙cm−2. Linear sweep voltammetry (LSV) measurements were conducted in a CHI760e electrochemical workstation with a scanning rate of 1 mV∙s−1 and obtained the electrochemical impedance spectroscopy (EIS) in a frequency range of 106–10−1 Hz. The ionic conductivity (σ) of the electrolytes was calculated based on the equation: σ = L/SR, where R, L, and S were the resistance, thickness, and surface area of the electrolyte films, respectively. The cells were kept at each measurement temperature (30–60 ℃) for 1 h for thermal equilibrium prior to the EIS measurements. Li+ ion transference number (t+) of the electrolytes was obtained by the combined measurements of EIS and chronoamperometry (CA) of the Li/SPE/Li cells at 60 ℃. Transference number t+ was calculated by using the classic equation: t+ = Is(U-I0R0)/I0(U-IsRs), where I0 and Is were the currents at the initial and steady states, respectively, U was the applied direct current (DC) polarization potential of 10 mV, and R0 and Rs were the electrolyte/Li metal interface resistance at the initial and steady states, respectively. Galvanostatic charge-discharge tests were conducted in a Land CT3001A multichannel battery tester between 2.8 and 4.0 V under 60 ℃ and 0.5 C rate (1 C = 170 mA∙g−1). As for the abuse test, soft-pack LiFePO4/SPE/Li cells with a size of 4 cm × 4 cm were assembled. The SPE is filled with 5% 2s-ZnO HoMS. After a full charge on a Land CT3001A multichannel battery tester, soft cell was connected with a LED lamp to light the lamp. Or soft cell was first folded in half and then connected with a LED lamp to see if it can light the lamp. Or soft cell was partially cut off and then connected with a LED lamp to see if it still can light the lamp.
Results and discussion
ZnO HoMSs were synthesized by a sequential templating approach, as we reported before [24, 31, 32]. The diameters of single-, double- and triple-shelled ZnO HoMSs (named as ZnO HS, 2s-, 3s-ZnO HoMS, respectively) were all estimated to be 700~900 nm, as shown in Fig. 1a–c and S2a-c. Elemental mapping indicates that Zn and O elements are uniformly distributed in the shells of ZnO HoMS (Fig. S3a). Lattice fringes with a spacing of 0.28 nm, 0.26 nm, and 0.24 nm corresponding to the (100), (002), and (101) crystal planes of ZnO are commonly observed through high-resolution transmission electron microscopy (HRTEM) (Fig. S3b). X-ray powder diffraction (XRD) patterns (Fig. S3c) demonstrate that ZnO HoMS has a hexagonal fibrillated zincite phase structure, the average particle size is 9.1 nm as calculated by the Scherrer equation [33], which is similar to that of commercial ZnO nanoparticles (NPs) (Fig. S4). The absorption peak located at 424 cm−1 in FTIR (Fourier transform infrared) corresponds to the Zn-O bond, while the other three at 3437, 1627, and 1384 cm−1 are assigned to the stretching and bending vibration absorption peaks of surface O-H or bridging O-H (Fig. S5) [34].
a–c TEM images of a ZnO HS, b 2s-ZnO HoMS, c 3s-ZnO HoMS. d–f SEM images of d pure PEO foil and PEO composite electrolytes filled e 5% ZnO nanoparticles and f 5% 2s-ZnO HoMS. g Cross-section SEM image of composite electrolyte membrane. h, i Optical photos of composite electrolyte membrane h after folded and i then unfolded
Characterization of PEO-ZnO composite electrolyte
PEO-based electrolyte membranes were prepared by the conventional solution casting method. The surface of the unfilled PEO electrolyte membrane presents a rough fibrous state (Fig. 1d). Comparatively, as for the composite one with 5% ZnO fillers, the surface is nonfibrous but smooth-like, which may be due to that ZnO fillers disrupts the crystalline state of PEO, causing a significant increase in the proportion of the amorphous phase (Fig. 1e,f). The cross-sectional photograph of the electrolyte film showed a thickness of about 80 μm (Fig. 1g). In addition, it is worth mentioning that with a molar ratio of EO to lithium salt being 10:1, soft, foldable, and mechanically strong pure electrolyte membranes were obtained (Fig. 1h, i).
The ionic conductivity of the composite electrolyte membranes filled with ZnO NP or ZnO HoMS is higher than that of the unfilled PEO solid electrolyte (Fig. 2a–c). It is worth noting that the ionic conductivity first increases and then decreases as the ratio of ZnO fillers keeps increasing, wherein the one filled with 5% 2s-ZnO HoMS achieved the highest ionic conductivity at both 30 ℃ and 60 ℃, being1.04 × 10−4 S∙cm−1 and 1.2 × 10−3 S∙cm−1, respectively. The composite electrolytes with 5% 2s-ZnO HoMS fillers exhibit the typical (Vogel–Fulcher–Tammann) VFT behavior (Fig. 2c), showing an excellent fit with the VFT equation, and the lithium-ion transfer activation energy of PEO-based electrolyte obtains a significant decrease [35].
a, b Calculation results of ionic conductivity of solid electrolytes under various composite conditions at 30 ℃ and 60 ℃. c Trend of ionic conductivity of pure PEO and filled 5% 2s-ZnO HoMS electrolytes with temperature. d, e XRD patterns and DSC curves of PEO electrolytes filled 5% ZnO nanoparticles, 5% hollow sphere, and 5% 2 s, 3s-ZnO HoMS. f Stress–strain curves of pure PEO and composite electrolytes filled 5% ZnO nanoparticles and 5% 2s-ZnO HoMS
The reason for the decreased ionic conductivity as the filler ratio increases to higher than 5%, may be due to that a too high filler ratio would lead to agglomeration of the filler, otherwise, an excessive filler would instead inhibit the movement of PEO chains segments and the migration of lithium ions between them. As shown in Fig. S6, ZnO nanoparticles in the composite electrolyte membranes underwent severer agglomeration and formed more clusters as the filling ratio increased from 5 to 10% and to 15%, which would cause a significant decrease in the effective contact area between PEO and filler and hinder the transport of lithium ions by the long polymer chains. In addition, it is worth noting that ZnO HoMS is uniformly dispersed in the electrolyte with almost no agglomeration observed (Fig. 1f), which may explain the higher ionic conductivity of the composite electrolyte with ZnO HoMS fillers than ZnO NP fillers. Additionally, the lower ionic conductivity of the composite electrolyte with 3s-ZnO HoMS fillers than that with 2s-ZnO NP fillers may be due to that the long chain of PEO is difficult to reach the third shell of 3s-ZnO HoMS, which may induce the decreased effective active sites for suppressing PEO crystallinity, and adsorbing anions through surface positive charges.
As obvious evidence, the sharp peaks at 19.2° and 23.3° of pure PEO electrolyte (Fig. S7) almost completely disappeared into a broad peak at 20° for the composite electrolyte with ZnO HS or HoMS fillers, wherein the one with 2s-ZnO HoMS shows the weakest peak at 20°, indicating the crystallinity of PEO is successfully suppressed (Fig. 2d) [36]. Comparatively, the composite electrolyte with ZnO NP fillers showed a weaker but sharp peak belonging to PEO at 19.2°, indicating that some crystalline regions still existed inside the composite electrolyte.
The behavior of the polymer electrolyte in terms of ion transport is analyzed by evaluating the glass transition temperature (Tg) and melting temperature (Tm) of the solid electrolyte [37, 38]. As shown in Fig. 2e, the unfilled PEO electrolyte exhibits obvious glass transition and melting heat absorption peaks with Tg of −47.1 ℃ and Tm of 70.7 ℃, while the Tg and Tm decrease significantly after filling with 5% ZnO fillers. The electrolyte films filled with 5% 2s-ZnO HoMS possess the lowest Tg and the melting heat absorption peak almost disappears, indicating that the interior of the composite electrolyte is almost completely composed of the amorphous phase, which could significantly benefits ionic conductivity.
The mechanical strength of the composite electrolyte film was characterized on a universal testing machine. The maximum stress of the unfilled PEO electrolyte film was only 0.02 MPa, and the elongation at break was low with poor toughness (~ 120%) (Fig. 2f). The addition of 5% ZnO NP increased the maximum stress of the electrolyte film to 0.45 MPa, and the elongation at break was improved and maintained at 327%. The addition of 5% 2s-ZnO HoMS electrolyte film further increased the maximum stress elongation at break to ~ 380%. The aforesaid results indicate that the addition of ZnO HoMS fillers enhances the mechanical properties and toughness of electrolyte films, which enables effective inhibition of lithium dendrite growth in solid-state batteries and reduces the risk of battery short circuit [39, 40].
Electrochemical performance of symmetric cell
The linear sweep voltammetry (LSV) curves of the composite electrolyte films (Fig. 3a) illustrate that the electrochemical stability window of the electrolyte is significantly enhanced by the introduction of ZnO filler. The electrolyte in the unfilled state showed a small reaction current in the voltage range of 4.0–4.2 V. This proves that there is a side reaction between the pure PEO electrolyte and the Li metal electrode when the contact reaches a certain potential [41]. The decomposition of PEO increases the current significantly when the applied voltage exceeds 4.5 V. The electrochemical stability of the electrolytes with the addition of 5% ZnO NP was significantly broadened, where the electrochemical stability of the 2s and 3s ZnO HoMS composite electrolytes reached the widest potential at 4.7 V level.
a, b a LSV curves of pure PEO and PEO electrolytes filled 5% ZnO NP, 5% HS, and 5% 2 s, 3s-ZnO HoMS. b I-t curve of electrolytes filled with 5% 2s-ZnO HoMS before and after polarization. c EIS curves at different time points of the symmetrical battery with PEO electrolytes filled 5% 2s-ZnO HoMS. d Constant current cycle curves of pure PEO and PEO electrolytes filled with 5% ZnO NP and 5% 2s-ZnO HoMS. e, g Area magnification of galvanostatic cycling curves of pure PEO and PEO electrolyte filled with 5% ZnO NP. f Rate cycling curves of symmetric cells with 5% 2 s ZnO HoMS
The i-t curve and pre- and post-impedance plots of the ion transference number test are shown in Fig. 3b, S8, and the calculation results are presented in Table S1. The ion transference number of unfilled electrolyte is about 0.11, while the number of the composite solid electrolyte with 5% 2s-ZnO HoMS fillers increased to 0.21, which was higher than that with the addition of 5% ZnO NP, HS, and 3s-HoMS.
The interface stability was verified by the constant current meter time potential method, cycling under 60 °C at a current density of 0.2 mA∙cm−2 (Fig. 3d, e, g) [42]. It turned out that the ZnO-free symmetric cell short-circuited after cycling for only 65 h, which may be due to that lithium dendrites pierce into the electrolyte membrane which has a weak mechanical strength. The cell with 5% ZnO NP was able to cycle for 420 h without short circuit, while the one with 5% 2s-ZnO HoMS stably cycled for 900 h without obvious voltage fluctuations. The reason may be that the excellent mechanical strength of 2s-ZnO HoMS inhibits the formation of lithium dendrites and punctures the electrolyte membrane to ensure the stable operation of the battery [43]. In addition, the initial impedance value of the symmetric cell with 5% 2s-ZnO HoMS is 155 Ω, which stabilizes around 105 Ω for 8 days (Fig. 3c). This indicates a stable interface between the electrolyte with 5% 2s-ZnO HoMS and lithium metal has formed, which benefits the good cycling stability of the cell.
The rate capability is also an important evaluation parameter of the cells. The ZnO-free symmetric cell was short-circuited when the current density increased to 0.4 mA∙cm−2 (Fig. S9), which may be due to the poor contact between electrolyte and lithium metal causes the uniform current distribution and drives severe lithium dendrites formation, especially at higher current densities; besides the inferior mechanical strength causes it to rapidly puncture through the electrolyte membrane, leading to the short-circuit of the symmetric cell. On the contrary, the cell with 5% ZnO fillers remained stably cycled at high current densities (Fig. 3f, S9), indicating that this strategy of composite electrolytes is effective in optimizing the interfacial stability between electrolyte membranes and electrodes.
Electrochemical performance of full cell
To examine the practical application of prepared solid polymer electrolyte, we fabricated and tested all-solid-state LiFePO4/SPE/Li cells (Fig. 4a). At 0.5C, the initial discharge specific capacities of full cells without filling, filled with 5% ZnO NP and 5% 2s-ZnO HoMS were 134, 153.2, and 169.3 mAh∙g−1, respectively. After 100 cycles, the discharge specific capacity of the cell with 5% 2s-ZnO HoMS maintained at ~ 121.8 mAh∙g−1 (71.9 %) with a stable charge/discharge plateau during the whole cycling process (Fig. 4b). The EIS test results revealed that the impedance value of the cell showed an increasing trend during the cycling process, the one with 2s-ZnO HoMS fillers maintained ~ 1000 Ω after 100 cycles while the one without ZnO fillers reached ~ 2000 Ω (Fig. 4c, S10), which confirms that the composite electrolyte could maintain a better contact interface with the cathode active material.
a Constant current charge–discharge cycle of full battery with pure PEO and PEO electrolytes filled 5% ZnO nanoparticles and 5% 2s-ZnO HoMS. b, c Cycle charge–discharge curves and EIS curve changes at different cycles of full battery with PEO electrolytes filled 5% 2s-ZnO HoMS. d Full cell rate cycling of PEO electrolytes filled 5% 2s-ZnO HoMS. (e) Display of soft pack battery with PEO electrolytes filled 5% 2s-ZnO HoMS, 1: voltage test, 2: driving LED light plate test, 3: driving LED while fold, 4: driving LED after partially cut off
The rate capability test (Fig. 4d) shows that the discharge specific capacity of the cell with 5% 2s-ZnO HoMS is significantly higher than that without ZnO fillers and with 5% ZnO NPs at both high and low current densities. The one with 2s-ZnO HoMS quickly recovers the low magnification to its initial level and exhibits stable charge/discharge curves at all magnification stages (Fig. S11), proved that 2s-ZnO HoMS has significantly enhanced the performance of the electrolyte in terms of charge/discharge stability.
To verify that the composite electrolyte with 5% 2s-ZnO HoMS is available for practical application in high-energy-density batteries, LiFePO4/SPE/Li cell were assembled in a soft pack (Fig. 4e). The soft-pack cell shows a stable voltage of 3.48 V, and is able to light up the LED sign and work stably. Moreover, after the soft-pack cell was folded in half or partially cut off, it could still light up the LED sign normally and no dangerous situation of leakage or spontaneous combustion occurred. These results demonstrate that this composite electrolyte could withstand abuse test and provides reliable safety in high-energy storage devices.
Conclusion
In summary, ZnO HoMS is designed and synthesized as fillers for PEO-based composite polymer electrolyte. The composite electrolyte with 5% 2s-ZnO HoMS fillers exhibits a higher ionic conductivity of 1.2 × 10−3 S∙cm−1 at 60 ℃, larger ionic transference number, wider electrochemical stability window, and stronger mechanical properties than other electrolytes without fillers or filled with different structural ZnO particles. The reason may be that 2s HoMS could provide more effective active sites to form more Lewis acid-base interaction with PEO chains as well as to absorb more anions of lithium salt, thus significantly inhibiting the entanglement and crystallization of PEO long chains, promoting the dissociation of lithium salt and improving the ionic conductivity. In addition, the skeleton role served by HoMS enhances the mechanical strength of the electrolyte film effectively and reinforces the maximum deformation under the action of dendrite stress, resulting in a stable interface between 2s-ZnO HoMS-based composite electrolyte and lithium metal anode. As a result, both the all-solid-state asymmetric cell and full cell based on this composite electrolyte exhibited a good cycling performance, maintaining a specific discharge capacity of 121.8 mAh∙g−1 after 100 cycles. This study provides a simple yet effective strategy to develop solid polymer electrolyte with high ionic conductivity and good mechanical and electrochemical stability for safe and high-energy-density lithium batteries.
Data availability
The original data are available from corresponding authors upon reasonable request.
References
Fan LZ, He H, Nan CW (2021) Tailoring inorganic-polymer composites for the mass production of solid-state batteries. Nat Rev Mater 6:1003–1019. https://doi.org/10.1038/s41578-021-00320-0
Li BQ, Peng HJ, CX et al (2019) Polysulfide electrocatalysis on framework porphyrin in high-capacity and high-stable lithium-sulfur batteries. CCS Chem 1: 128–137. https://doi.org/10.31635/ccschem.019.20180016
Rameez R, Nana Z, Ying X et al (2020) Electrocatalytic conversion of lithium polysulfides by highly dispersed ultrafine Mo2C nanoparticles on hollow N-doped carbon flowers for Li-S batteries. EcoMat 2:e12020. https://doi.org/10.1002/eom2.12020
Dias FB, Plomp L, Veldhuis JBJ (2000) Trends in polymer electrolytes for secondary lithium batteries. J Power Sources 88:169–191. https://doi.org/10.1016/S0378-7753(99)00529-7
Guo Y, Wu SC, He YB et al (2022) Solid-state lithium batteries: safety and prospects. eScience 2: 138–163. https://www.sciencedirect.com/science/article/pii/S2667141722000209?via%3Dihub. Accessed 2 Jan 2023
Hong Y, Jia L, Yang L et al (2020) Toward practical all-solid-state batteries with sulfide electrolyte: a review. Chem Res Chinese Universities 36:377–385. https://doi.org/10.1007/s40242-020-0103-5
Kairui G, Shao QL, Gong C et al (2022) One-pot synthesis of polyester-based linear and graft copolymers for solid polymer electrolytes. CCS Chem 4:3134–3149. https://doi.org/10.31635/ccschem.021.202101364
Li L, De CZ, Xi JX et al (2021) Challenges and development of composite solid electrolytes for all-solid-state lithium batteries. Chem Res Chinese Universities 37:210–231. https://doi.org/10.1007/s40242-021-0007-z
Xu SJ, Sun ZH, Sun CG (2020) Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature. Adv Funct Mater 30:2007172. https://doi.org/10.1002/adfm.202007172
Zhang XD, Yue FS, Liang JY et al (2020) Structure design of cathode electrodes for solid-state batteries: challenges and progress. Small Struct 1:2000042. https://doi.org/10.1002/sstr.202000042
Croce F, Appetecchi GB, Persi L et al (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458. https://doi.org/10.1038/28818
Jia Z, Yuan W, Zhao H et al (2014) Composite electrolytes comprised of poly (ethylene oxide) and silica nanoparticles with grafted poly (ethylene oxide)-containing polymers. RSC Adv 4:41087–41098. https://doi.org/10.1039/C4RA07262F
Wan J, Xie J, Kong X et al (2019) Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat Nanotechnol 14:705–711. https://doi.org/10.1038/s41565-019-0465-3
Xiong HM, Zhao X, Chen JS (2001) New polymer-inorganic nanocomposites: PEO-ZnO and PEO-ZnO-LiClO4 films. J Phys Chem B 105:10169–10174. https://doi.org/10.1021/jp0103169
Ding WQ, Lv F, Xu N et al (2021) Polyethylene oxide-based solid-state composite polymer electrolytes for rechargeable lithium batteries. ACS Appl Energy Mater 4:4581–4601. https://doi.org/10.1021/acsaem.1c00216
Li B, Bi R, Yang M et al (2022) Coating conductive polypyrrole layers on multiple shells of hierarchical SnO2 spheres and their enhanced cycling stability as lithium-ion battery anode. Appl Surf Sci 586:152836. https://doi.org/10.1016/j.apsusc.2022.152836
Li B, Wang J, Bi RY et al (2022) Accurately localizing multiple nanoparticles in a multishelled matrix through shell-to-core evolution for maximizing energy-storage capability. Adv Mater 34:2200206. https://doi.org/10.1002/adma.202200206
Wang J, Cui Y, Wang D (2020) Hollow multishelled structures revive high energy density batteries. Nanoscale Horiz 5:1287–1292. https://doi.org/10.1039/D0NH00311E
Dong Z, Lai X, Halpert JE et al (2012) Accurate control of multishelled ZnO hollow microspheres for dye-sensitized solar cells with high efficiency. Adv Mater 24:1046–1049. https://doi.org/10.1002/adma.201104626
Wang J, Wang Z, Mao D et al (2022) The development of hollow multishelled structure: from the innovation of synthetic method to the discovery of new characteristics. Sci China Chem 65:7–19. https://doi.org/10.1007/s11426-021-1097-9
Wang J, Yang M, Wang D (2022) Progress and perspectives of hollow multishelled structures. Chinese J Chem 40:1190–1203. https://doi.org/10.1002/cjoc.202100932
Wang L, Wan J, Wang J et al (2020) Small structures bring big things: performance control of hollow multishelled structures. Small Struct 2:2000041. https://doi.org/10.1002/sstr.202000041
Yang M, Bi R, Wang J et al (2022) Decoding lithium batteries through advanced in situ characterization techniques. Int J Min Met Mater 29:965–989. https://doi.org/10.1007/s12613-022-2461-0
Zhang X, He Y, Wei Y et al (2021) Carving the shell thickness of tungsten trioxide hollow multi-shelled structures for enhanced photocatalytic performance. Mater Chem Front 5:8010–8017. https://doi.org/10.1039/D1QM01124C
Zhao D, Yang N, Wei Y et al (2020) Sequential drug release via chemical diffusion and physical barriers enabled by hollow multishelled structures. Nature Commun 11:1–7. https://doi.org/10.1038/s41467-020-18177-2
Wang J, Tang H, Zhang L et al (2016) Multi-shelled metal oxides prepared via an anion-adsorption mechanism for lithium-ion batteries. Nat Energy 1:16050. https://doi.org/10.1038/nenergy.2016.50
Cong W, Jiang YW, Wen PH et al (2020) Controllable synthesis of hollow multishell structured Co3O4 with improved rate performance and cyclic stability for supercapacitors. Chem Res Chinese Universities 36:68–73. https://doi.org/10.1007/s40242-019-0040-3
Zhao J, Wang J, Bi R et al (2020) General synthesis of multiple-cores@ multiple-shells hollow composites and their application to lithium–ion batteries. Angew Chem Int Ed 60:25719–25722. https://doi.org/10.1007/s40242-019-0040-3
Zhao J, Yang M, Yang N et al (2020) Hollow micro-/nanostructure reviving lithium-sulfur batteries. Chem Res Chinese Universities 36:313–319. https://doi.org/10.1007/s40242-020-0115-2
Wang J, Wan J, Yang N et al (2020) Hollow multishell structures exercise temporal-spatial ordering and dynamic smart behaviour. Nat Rev Chem 4:159–168. https://doi.org/10.1038/s41570-020-0161-8
Wei Y, Wan J, Yang N et al (2020) Efficient sequential harvesting of solar light by heterogeneous hollow shells with hierarchical pores. Natl Sci Rev 7:1638–1646. https://doi.org/10.1093/nsr/nwaa059
Mao D, Wan J, Wang J et al (2018) Sequential templating approach: a groundbreaking strategy to create hollow multishelled structures. Adv Mater 31:1802874. https://doi.org/10.1002/adma.201802874
Holzwarth U, Gibson N (2011) The Scherrer equation versus the’Debye-Scherrer equation’. Nat Nanotechnol 6:534–534. https://doi.org/10.1038/nnano.2011.145
ElBellihi AA, Bayoumy WA, Masoud EM (2012) Preparation, characterizations and conductivity of composite polymer electrolytes based on PEO-LiClO4 and Nano ZnO filler. B Korean Chem Soc 33:2949–2954. https://doi.org/10.5012/bkcs.2012.33.9.2949
Paranjape N, Mandadapu PC, Wu G et al (2017) Highly-branched cross-linked poly (ethylene oxide) with enhanced ionic conductivity. Polymer 111:1–8. https://doi.org/10.1016/j.polymer.2017.01.014
Appetecchi GB, Henderson W, Villano P et al (2001) PEO-LiN(SO2CF2CF3)2 Polymer electrolytes: I. XRD, DSC, and ionic conductivity characterization. J Electrochem Soc 148:A1171–A1178. https://doi.org/10.1149/1.1403728
Li Y, Yu YC, Han CY et al (2020) Sustainable blends of poly (propylene carbonate) and stereocomplex polylactide with enhanced rheological properties and heat resistance. Chinese J Polym Sci 38:1267–1275. https://doi.org/10.1007/s10118-020-2408-8
Stolwijk NA, Heddier C, Reschke M et al (2013) Salt-concentration dependence of the glass transition temperature in PEO-NaI and PEO-LiTFSI polymer electrolytes. Macromolecules 46:8580–8588. https://doi.org/10.1021/ma401686r
Homann G, Stolz L, Nair J et al (2020) Poly (ethylene oxide)-based electrolyte for solid-state-lithium-batteries with high voltage positive electrodes: evaluating the role of electrolyte oxidation in rapid cell failure. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-61373-9
Ma Y, Wan J, Yang Y et al (2022) Scalable, ultrathin, and high-temperature-resistant solid polymer electrolytes for energy-dense lithium metal batteries. Adv Energy Mater 12:2103720. https://doi.org/10.1002/aenm.202103720
Qiu J, Liu X, Chen R et al (2020) Enabling stable cycling of 4.2 V high-voltage all-solid-state batteries with PEO-based solid electrolyte. Adv Funct Mater 30:1909392. https://doi.org/10.1002/adfm.201909392
Jiao Y, Li F, Jin X et al (2021) Engineering polymer glue towards 90% zinc utilization for 1000 hours to make high-performance Zn-Ion batteries. Adv Funct Mater 31:2107652. https://doi.org/10.1002/adfm.202107652
Liu Y, Zhao Y, Lu W et al (2021) PEO based polymer in plastic crystal electrolytes for room temperature high-voltage lithium metal batteries. Nano Energy 88:106205. https://doi.org/10.1016/j.nanoen.2021.106205
Acknowledgements
We are grateful to Professor Kai Liu’s laboratory of Tsinghua University for the technical guidance of sample preparation provided in this study.
Funding
This work was financially supported by the Natural Science Foundation of China (Grant No.: 51932001, 51872024, 21820102002, 21931012, 22111530178, 51972305, 51972306), the National Key R&D Program (Grant No.: 2018YFA0703503, 2021YFC2902500, 2022YFA1504100), the Cooperation Fund of the Institute of Clean Energy Innovation, Chinese Academy of Sciences (Grant No.: DNL202020), the Zhongke-Yuneng Joint R&D Center Program (No.: ZKYN2022008).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. D.W., R.Y., and J.W. conceived the idea and supervised the research. Y.M. performed experiments and basic characterizations. D.W., R.Y., J.W., Y.M., R.B., M.Y., J.Q., and P.W. analyzed and discussed the experimental data. Y.M. and J.W. drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the topical collection: Self-assembled Functional Nanomaterials and Devices in Asia
Guest Editosr: Zhixiang Wei and Yong Yan
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Bi, R., Yang, M. et al. Hollow multishelled structural ZnO fillers enhance the ionic conductivity of polymer electrolyte for lithium batteries. J Nanopart Res 25, 14 (2023). https://doi.org/10.1007/s11051-022-05661-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05661-7