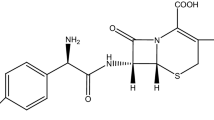
Abstract
The rapid increase in antibacterial resistance of pathogenic bacteria poses a key threat to human health. This has triggered initiatives worldwide to discover more potent antimicrobial agents. Hence, cefixime was conjugated to gold and silver nanoparticles (NPs) and resultant nano-conjugates (Cfm-AuNPs and Cfm-AgNPs) were evaluated against Staphylococcus aureus (S. aureus) ATCC 25923. The synthesized nano-conjugates were characterized by using UV–visible, IR spectroscopy, and atomic force microscopy (AFM). The bactericidal potential of Cfm-AuNPs and Cfm-AgNPs was compared with the efficacy of non-conjugated cefixime served as control. Experimentally obtained results confirmed that the bactericidal potential of cefixime was enhanced by 8 and 3 times upon conjugation with gold and silver NPs respectively. Moreover, the improved antibacterial activity and kinetics of the conjugated cefixime were observed under AFM. Surface topography analysis of the controlled and treated S. aureus cells revealed that effective treatment time (8 h) of cefixime also gets reduced to one-half(4 h) upon conjugation with both gold and silver NPs. Furthermore, the toxicity assay demonstrated that the cytocompatibility of gold and silver NPs was improved by conjugation with cefixime. Consequently, Cfm-AuNPs and Cfm-AgNPs offer the technical benefits of improved bactericidal effects of cefixime at lower dosages and serve to overcome antibiotic resistance in bacteria.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of bacterial resistance toward existing antibiotics is a global issue for human health. This has challenged the clinical worth of some existing antimicrobial drugs and emphasized the need for comparatively more potent antibiotics (Fair and Tor 2014). Several practices have been developed to overcome this issue; however, bacteria always adapt a manner to adjust to the situation and become resistant. Hence, they are continuously developing resistance toward the conventional antibiotics. Beta-lactam antibiotics are the widely used antibacterial drugs; though, due to bacterial resistance, some members of this class are no longer clinically active (Drawz and Bonomo 2010; Shaikh et al. 2015). This growing resistance to antibiotics (particularly beta-lactam antibiotics) led to look for possible alternatives that can overcome this issue.
The extensive use of antibiotics in the hospitals and community has fueled this crisis. Consequently, bacteria such as Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococci, and S. aureus, and some members of the Pseudomonas and Enterobacteriaceae families, are now resistant to nearly all the conventional antibiotics (Neu 1992). Mechanisms responsible for the development of antibiotic resistance include transfiguration of the target site of the drug, transformation of antibiotics by bacteria, decreased assimilation of these antibiotics, and alteration of the metabolic pathway as a preventive measure (Kumar and Schweizer 2005; Stewart 2002). S. aureus can destroy beta-lactam antibiotics by means of lactamase and hence show resistance to these antibiotics (Lyon and Skurray 1987).
Cefixime is an active beta-lactam antibiotic, used for the treatment of bacterial disorders such as the ear, throat, lung, and urinary tract infections, bronchitis, gonorrhea, and pneumonia. (Zhang et al. 2014). Unfortunately, the growing increase in the antibiotic resistance poses a threat to the use of this life-saving drug. Hence, mechanisms such as better hygiene, antibiotic control programs, and fabrication of the drug to enhance its antibacterial activity need to be implemented in order to control bacterial resistance. Fabrication of antibiotics is the ultimate solution to circumvent the resistance mechanisms. Particularly, nano-antibiotics are fascinating substitutes as their mechanism of action is totally different than conventional antibiotics.
Reports have shown that noble metal-based nanomaterials have great potential in the biomedical field (Rai et al. 2016; Ali et al. 2020a, b), and silver and gold NPs have emerged as promising candidates for antimicrobial application because their large surface area enabling high synergy for antimicrobial action (Gordon et al. 2010; Li et al. 2014; Naqvi et al. 2013). Silver and gold NPs can target bacterial structures (Ali et al. 2019), and evidences have shown that the synergistic action of NPs and antibiotics caused an upsurge in the antibacterial potential of antibiotics (Allahverdiyev et al. 2011). Previously, various approaches have been used to improve the bactericidal activity of antibiotics. For example, antibiotics were used in combination with gold and silver nano-alloys to enhance their bactericidal activity (Dos Santos et al. 2012). Silver and gold NPs of antibiotics such as cefadroxil, ceftriaxone, vancomycin, penicillin, and amoxicillin have been used against S. aureus and E. coli(Ali et al. 2019; Gu et al. 2003; Shah et al. 2014). Cefixime metal complexes have been synthesized for antibacterial applications (Anacona and Estacio 2006). Furthermore, the improved solubility of cefixime by converting into NPs through antisolvent precipitation technique (Kuang et al. 2015), and the mutual effects of gold ions and gold NPs with fourteen antibacterial drugs including cefixime against Pseudomonas aeruginosa strain (Nazari et al. 2012) have been studied. In another study, the antibacterial activities of antibiotics like amoxicillin, penicillin G, erythromycin, vancomycin, and clindamycin have been increased in the presence of Ag-NPs, but no enhancement was observed in the activity of cefixime (Shahverdi et al. 2007). These are important conclusions; however, there are still shortcomings such as the complex synthetic procedure, the use of toxic reagents, and a very less enhancement in the bacterial potential of cefixime. Hence, there is a need to develop a simple, nontoxic procedure for the significant enhancement of antibacterial potential of cefixime that could be used against S. aureus which is emerging as a resistive strain of bacteria.
In this study, we demonstrate the simple and one-step conjugation of cefixime with gold and silver NPs (hereafter abbreviated as Cfm-AuNPs and Cfm-AgNPs respectively). The nano-conjugates were characterized and their stability under various physiological conditions (temperature, pH, and salt) was examined. The antibacterial potential of cefixime, Cfm-AuNPs, and Cfm-AgNPs was evaluated against S. aureus ATCC 25923 by agar well diffusion method. Then, minimum inhibitory concentrations (MICs) of Cfm-AuNPs and Cfm-AgNPs were compared with unconjugated cefixime and bare gold and silver NPs. Furthermore, the cytotoxicity of Cfm-AuNPs and Cfm-AgNPs was examined to know their biocompatibility. Since AFM is a valuable tool to collect more detailed information about interface and surface properties compared with other microscopic techniques (Ali et al. 2020a, b; Bolshakova et al. 2001). Furthermore, AFM is gaining an additional worth as a useful instrument for analysis of living structures because it is giving the prospect to perform a wide range of sample studies without any complex sample preparation procedures (Madl et al. 2006; Yamashita et al. 2012). Hence, to demonstrate the influence of conjugation on the efficacy of cefixime, morphological analysis of controlled and treated S. aureus was observed at different time intervals under AFM.
Materials and methods
Materials
Tetra-chloroauric-acid-trihydrate (HAuCl4.3H2O) and silver nitrate (AgNO3) were bought from Merck, triethylamine (TEA) was purchased from Scharlau, and cefixime was provided by Pharmagen Limited, Lahore, Pakistan. S. aureus ATCC 25923 were provided by ICCBS University of Karachi, Pakistan. Deionized water was used throughout the experiment.
Synthesis of Cfm-AuNPs
Gold solution (HAuCl4.3H2O, 1 mM) was reacted with 1 mM cefixime solution in the ratio of 13:1 (Au:cefixime). The reaction mixture was stirred for 30 min and then 0.1 ml of TEA was added to it. The color of the reaction mixture gradually turned into violet; the reaction was monitored by UV-visible spectroscopy. The reaction mixture was stirred for 2 h then the suspensions were centrifuged to collect NPs. The NPs were frequently washed with deionized water to remove reaction by-products and unreacted precursors.
Synthesis of Cfm-AgNPs
Silver salt solution (AgNO3, 1 mM) and cefixime solution (1 mM) were prepared in deionized water. From the stock solutions, two volumes in the ratio 10:1 (Ag:cefixime) were mixed and the reaction mixture was stirred for 30 min. Then, TEA (0.1 mL) was added to the reaction mixture. The color of the reaction mixture gradually turned into yellowish red; the reaction was carefully monitored through UV-visible spectroscopy. The reaction mixture was stirred for 2 h then the suspensions were centrifuged to collect NPs. The NPs were frequently washed with deionized water to remove reaction by-products and unreacted precursors.
Characterization
Cfm-AuNPs and Cfm-AgNPs were characterized by UV-Vis spectroscopy; a Thermo Scientific Evolution 300 spectrophotometer was used for this purpose. Fourier-transform infrared (FT-IR) spectra were obtained with a “Bruker Victor 22 spectrophotometer” using KBr pellets. The shape and size of the NPs were determined by AFM “Agilent Technologies AFM 5500, USA” in ACAFM mode. Si cantilever (of force constant 42 N/m, length 125 μm, and resonance frequency 330 kHz) was kept during the study (Rafiq et al. 2014).
Quantification of the amount of cefixime in Cfm-AuNPs and Cfm-AgNPs
The amount of cefixime in Cfm-AuNPs and Cfm-AgNPs was quantified by the previously described method (Shah et al. 2014). Known volumes of the Cfm-AuNPs and Cfm-AgNPs suspensions were centrifuged separately and the precipitated NPs were collected respectively. The supernatant was frequently centrifuged to collect the NPs completely. Then, the supernatant was freeze-dried and weighed the residues. Using this manner, the quantity of cefixime was determined, to be 7.2 wt% for Cfm-AuNPs and 20.85 wt% for Cfm-AgNPs.
Stability of Cfm-AuNPs and Cfm-AgNPs
UV-visible spectroscopy was used to describe the pH, temperature, and salt stability of Cfm-AuNPs and Cfm-AgNPs because the coagulation of NPs usually accompanied by a change in color and shift of the surface plasmon (Ali et al. 2020a, b). The effect of temperature was observed by heating the Cfm-AuNPs and Cfm-AgNPs separately up to 100 °C, followed by UV-visible analysis. To study pH stability, the NPs were stored in different pH medium (from pH 1 to 14) and after 24 h, UV-visible spectra were recorded. For the study of stability in salt, NPs were mixed quantitatively with NaCl solutions. These mixtures were kept for 24 h followed by UV-visible spectroscopy.
Antibacterial evaluation by agar well diffusion method
Antibacterial activities of Cfm-AuNPs and Cfm-AgNPs were studied against the standard strain of S. aureus (ATCC 25923 strain). The activity was evaluated by agar well diffusion process. In brief, a lawn of S. aureus was grown on Nutrient agar. A concentration of 106cells/mL was preserved, and corresponding dilutions were utilized to measure minimum inhibition zones. By using a borer, wells (60 mm) were made and 500 μg mL−1 stock solutions of cefixime, Cfm-AuNPs, and Cfm-AgNPs were used distinctly to avoid nonspecific mixed zones of inhibition. Different concentrations (ranging from 500 to 5 μg mL−1) were added into each well. The plates were incubated at 25 °C for 2 h to allow the dispersion process to take place and then were incubated at 37 °C ± 1 for 24–48 h.
Antibacterial evaluation by broth dilution method
MICs of cefixime, Cfm-AgNPs, Cfm-AuNPs, and bare silver and gold NPs were determined against S. aureus by standard broth dilution (Panáček et al. 2006). In detail, bacterial cells were grown for 12 h on TSB (Oxoid, UK) at 37 °C to obtain the transferable dose of bacterial cells. Two-fold serial dilutions of cefixime and its NPs were arranged in Muller Hinton broth (Oxoid, UK) in the concentration range from 250 to 5 μg. An inoculum containing 5 × 105 CFU/mL cells was inoculated in each well except negative control. Into the plates, two controls were also added such as positive control (bacterial cells) and negative control (containing only medium). MICs were measured as the minimum concentration which inhibited the growth of bacteria.
AFM analysis of the antibacterial activity
AFM was used to evaluate the antimicrobial potential and action mechanism of Cfm-AuNPs and Cfm-AgNPs. Stock bacterial culture was produced using tryptic soy agar (Oxoid, UK) at 37 °C. Polylysine was added on a freshly cleaved mica surface and subsequently, a newly incubated culture of bacteria was inoculated to make 106 CFU of bacteria. Then, 5–10 μL from the bacterial culture were transferred onto a freshly cleaved (polylysine coated) mica surface and dried in the ascertained environment. The prepared samples were studied under AFM to record the morphology of bacteria before treatment. Calculated concentrations (MICs) of cefixime, Cfm-AuNPs, and Cfm-AgNPs were taken and incubated at 37 °C for 1 h. Drops of each sample (5–10 μL) were placed separately onto freshly cleaved (polylysine added) mica slides and were dried for 12 h at a relative humidity of 65% and at a temperature of 25 °C. Similarly, the samples were taken in quantified amount (MICs) and incubated for different time intervals such as 2, 4, and 8 h to study their antimicrobial action. By using the same method, the antimicrobial activity of the bare silver and gold NPs was also evaluated, for consideration as the negative control. Consequently, we recorded AFM images of S. aureus before treatment (control) and after treatment (with bare Ag and Au NPs, cefixime, Cfm-AuNPs, and Cfm-AgNPs) under similar condition.
Toxicological assay against human macrophages cells: ex vivo cell viability assay
Human mononuclear macrophage cells were isolated and the salt colorimetric assay was performed to evaluate the cytotoxicity of Cfm-AgNPs and Cfm-AuNPs(Singh et al. 2006). Cells were cultured in 24-well plates in an amount of 106cells/well with the volume of 0.3 mL. The stock solutions of 500 μg mL−1 of analyzing compounds such as bare Ag and Au NPs, Cfm-AuNPs, Cfm-AgNPs, and cefixime were prepared. Added different concentrations ranged from 5 to 200 μg in duplicate wells and incubated at 37 °C in the moistened CO2 (5%) incubator for 24 h. After this, 50 μL MTT solution (5 mg/mL) in sterile water was added to each well and left for 2 h at 37 °C; then, 0.3 mL of dimethyl sulfoxide (DMSO) was added to each well for 5 min followed by centrifugation. Subsequently, the optical density of supernatant was recorded and percentage inhibition was described (Barbasz et al. 2015).
Statistical analysis
All the experiments were carried out in triplicate form and results were expressed as mean ± SEM. Statistical analyses were performed by GraphPad Prism (Version 5.01, GraphPad Software Inc., San Diego, CA, USA) and comparisons were made using unpaired t test and one-way ANOVA, as appropriate. P values < 0.05 were considered statistically significant.
Results
Synthesis and characterization of Cef-AuNPs and Cef-AgNPs
As shown in Scheme 1, Cfm-AuNPs and Cfm-AgNPs were prepared by reacting cefixime with metal precursors (HAuCl4.3H2O/AgNO3) in the presence of TEA. The UV-visible spectra of Cfm-AuNPs and Cfm-AgNPs showed plasmonic peaks at 532 nm and 397 nm respectively (Fig. 1 a and b), which were in agreement with the distinguishing plasmonic absorption bands of Ag and Au NPs (Chen et al. 2020; Li et al. 2019). The formation of Cfm-AgNPs and Cfm-AuNPs was further confirmed by FT-IR spectroscopy. The FT-IR spectra of free and conjugated cefixime are shown in Fig. 1 c and d. Characteristic absorption bands noticed in the spectrum of cefixime were at 3296 cm−1, 3559 cm−1, 1669 cm−1, and 1771 cm−1; these peaks could be associated to the stretching vibrations of NH, OH, amide carbonyl, and lactam carbonyl groups respectively (Kuang et al. 2015). On the other hand, the merging of bands and the reduction in the absorbance intensities of N-H (3296 cm−1), O-H (3559 cm−1), and C=O (1771 cm−1 and 1669 cm−1) could be ascribed to the conjugation of cefixime to the NPs (Anacona and Estacio 2006).To investigate the morphology and size, AFM was performed. Figure 2 shows the AFM images of Cfm-AgNPs and Cfm-AuNPs. The Cfm-AgNPs and Cfm-AuNPs were spherical in shapes with size range of 35–60 nm and 25–50 nm, respectively.
UV-vis and FTIR spectra of Cefixime, Cfm-AgNPs, and Cfm-AuNPs. UV-vis absorption spectrum of Cfm-AgNPs exhibited surface plasmon band at 397 nm (a) and UV-vis spectrum of Cfm-AuNPs exhibited surface plasmon band at 532 nm (b). FTIR spectra of cefixime and Cfm-AgNPs (c), FTIR spectra of cefixime and Cfm-AuNPs (d), significant bands for functional groups involved in the synthesis of NPs are labeled
Stability of Cfm-AuNPs and Cfm-AgNPs
For the biomedical applications of nano-conjugates, it is important to examine their stability under certain physiological conditions. Hence, the nano-conjugates were studied against different parameters such as pH, temperature, and salinity. UV-visible spectroscopy was employed to observe the stability of the NPs. From the spectroscopic analysis, it was found that Cfm-AuNPs and Cfm-AgNPs could maintain their morphology in a pH range from 3 to 11, at 100 °C temperature, and salt concentration up to 500 mM and 100 mM respectively (Fig. 3).
Antibacterial evaluation
The antimicrobial potential of Cfm-AuNPs and Cfm-AgNPs was evaluated by agar well diffusion method and broth dilution method. The MICs of the cefixime, Cfm-AuNPs, and Cfm-AgNPs were calculated to be 25 ± 0.32 μg mL−1, 45 ± 0.12 μg mL−1 (correspond to a 3.24 μg cefixime), and 35 ± 0.13 μg mL−1 (corresponds to a 7.29 μg cefixime) respectively, while the MICs of bare Au and Ag NPs were found to be 60 ± 0.52 μg mL−1 and 50 ± 0.31 μg mL−1 respectively (Fig. 4). Similarly, the MICs of the abovementioned samples were found 25 ± 0.20 μg mL−1, 46 ± 0.40 μg mL−1, 38 ± 0.70 μg mL−1, 61 ± 0.50 μg mL−1, and 52 ± 0.40 μg mL−1 based on the broth dilution method (Table 1).
MICs of cefixime, Cfm-AgNPs, Cfm-AuNPs, and bare silver and gold NPs. Cfm-AgNPs and Cfm-AuNPs contain a small weight fraction of the cefixime (7.20% for Cfm-AuNPs and 20.85% for Cfm-AgNPs); this established that the efficacy of cefixime is enhanced up to 8 and 3 times respectively. Comparisons were made using unpaired t test and one-way ANOVA, as appropriate. P values < 0.05 were considered statistically significant
AFM analysis of the antibacterial activity
AFM analysis verified the enhancement in the antibacterial activity of cefixime. Images of the S. aureus ATCC 25923 before treatment showed round cells with smooth membranes and spherical shape as shown in Fig. 5. Bacteria from the stock culture were taken and treated with cefixime, Cfm-AuNPs, Cfm-AgNPs, and bare Au and Ag NPs. After treatment under optimized conditions, the samples were visualized under AFM to study the effects on the bacterial cell morphology. Samples from 1 h treatment (with MICs dose of cefixime) exposed a slight effect and the only scratch on the bacterial cell surface was noticed (Fig. 6a). Increased roughness and morphological degradation were observed with a 2 h treatment as shown in Fig. 6d. Further significant damages of the bacterial cells were detected after 4 h treatment (Fig. 7a). Eventually, completely distorted and degraded cells were observed after 8 h treatment (Fig. 7d). Then, the culture was treated with Cfm-AgNPs (MIC dose) for 1 h, and it is clear from Fig. 6b that the cells are comparatively more affected than those treated with cefixime. Here, we assumed that this is due to the alterations produced in the bacterial membrane by Cfm-AgNPs that may lead to changes in osmolarity without the incidence of lysis of the cells. Much more effect was observed in 2 h treatment, as the lysis of the membranes started (Fig. 6e) and a 4 h treatment led to the complete death of the cells (Fig. 7b). Likewise, Cfm-AuNPs have exhibited potent antibacterial effect in 1 and 2 h treatment compared with the cefixime alone (Fig. 6 c and f), and a 4 h treatment caused the destruction of the bacterial cells (Fig. 7c).
AFM images of S. aureus ATCC 25923 after treatment (at 37 °C) with cefixime (a), Cfm-AgNPs (b), and Cfm-AuNPs (c) for 4 h. Bacteria from the same culture was treated with cefixime (d), bare silver NPs (e), and bare gold NPs (f) for 8 h. The images were recorded in the ACAFM mode using silicon nitride cantilever
Unconjugated Au and Ag NPs of MICs doses did not show characteristic effects, but only slight morphological alterations were detected even after treatment for comparatively long time (8 h)(Fig. 7 e and f).
Toxicological assay against human macrophages cells: ex vivo cell viability assay
The toxicity of the Cfm-AgNPs and Cfm-AuNPs was evaluated with human mononuclear macrophages cells via cell viability assay. The Cfm-AgNPs and Cfm-AuNPs exerted no substantial cytotoxic effect up to 50 μg/mL and 75 μg/mL concentrations respectively (Table. 2). The NPs were lethal even at low concentrations for the bacteria tested in this study, indicating that the Cfm-AgNPs and Cfm-AuNPs can show antibacterial potential without being toxic to human cells. However, treatment with a higher dose (100 μg/mL) caused about a 0.50% decrease in cell viability.
Discussion
S. aureus is a human pathogen and can cause various infections such as food poisoning and skin and respiratory diseases (McCaig et al. 2006). This strain of bacteria rapidly developing resistance toward the prevailing antibiotics and has produced a global trouble in clinical cure (Chambers and DeLeo 2009). In this work, cefixime was conjugated with gold and silver NPs and their enhanced antimicrobial activity and kinetics against a sensitive strain (S. aureus ATCC 25923) were studied. Cfm-AgNPs and Cfm-AuNPs were synthesized through a facile single-step reduction method by reacting cefixime solution separately with the precursor solutions (of Ag and Au) under optimized conditions. The conjugation of cefixime with silver and gold NPs was confirmed by UV-visible, FT-IR, and AFM. The synthesized NPs were found to be stable under certain physiological conditions. Experimental findings showed that the cefixime conjugated with silver and gold NPs have the highest antimicrobial activity compared with the free cefixime. Though the MICs of Cfm-AuNPs and Cfm-AgNPs were not less than pure cefixime, but the NPs contain a small weight fraction of the cefixime (7.20% for Cfm-AuNPs and 20.85% for Cfm-AgNPs), this established that the efficacy of cefixime in the form of Cfm-AuNPs and Cfm-AgNPs was enhanced up to 8 and 3 times respectively. Hence, the as-synthesized bactericidal agents could be effectively used against S. aureus which is a resistive strain of bacteria. Furthermore, this study gives an insight into the improved bactericidal action of cefixime in the form of NPs and the approach can be exploited for the devising of potent antimicrobial agents. The method could be used for the enhancement of the bactericidal action of the conventional antibiotics.
Cytotoxicity assay is important because it gives suggestions on metabolic activities survival and cell death. Gold and silver NPs have been examined for their cytotoxicity and researchers have exposed their varying toxicity on mammalian cells. These variations were largely ascribed to individual NPs, preparation procedures, and target cells (Aurore et al. 2018; de Souza et al. 2019; Jia et al. 2017). In this work, the synthesized Cfm-AgNPs and Cfm-AuNPs were biocompatible in their MICs and even at higher concentrations compared with the bactericidal dose. Though, at a very higher concentration (100 μg/mL), a little reduction in cell viability was noticed. Our findings are in agreement with other research reports (de Lima et al. 2012; Hauck et al. 2008), and hence suggest that the synthesized nano-conjugates are biocompatible for human cells. The enhanced antibacterial activity and kinetics of Cfm-AgNPs and Cfm-AuNPs were validated by AFM while studying the morphological changes occurred in the bacteria. The benefits of using AFM were easy sample preparation, clear surface profile, and safe analysis (not require coating or extra treatments). Furthermore, AFM does not need the specimens to be stained or metal-coated, and under near-physiological conditions, non-invasive imaging of the samples can be done in their natural states. This technique is predominantly effective for bio-samples such as whole cells or bacteria without harsh or damaging procedures. Keeping these in view, we employed AFM to study the morphological changes in the bacterial cells after treatment with cefixime, Cfm-AgNPs, and Cfm-AuNPs. AFM exposed the noticeable examination of bacteria by recording topographic presentation of shape, surface, and phase images. The probable mechanism for interaction of Cfm-AgNPs and Cfm-AuNPs with the cell wall of bacteria could be explained by a three-step pathway which resulted in the enhancement of the antibacterial effect. These steps are the interaction of cefixime in conjugation with Ag and Au (in the form of Cfm-AgNPs and Cfm-AuNPs), the release of Au/Ag ions or Au/Ag NPs from conjugates which are attached to a bacterium and the toxicity caused by the NPs through binding with a sulfur atom of protein and phosphorous of DNA (Jin et al. 2018). Thus, we assumed that cefixime increased the membrane’s permeability by acting on the peptidoglycan cover of S. aureus, and subsequently, the NPs got into the bacterial cell and acted on the DNA and protein; induced death of the cell by disturbing vital functions and metabolism (Nair et al. 2009; Rai et al. 2009; Sondi and Salopek-Sondi2004). Also, the production of reactive oxygen species by the conjugates could result in oxidative stress which facilitated further cell damage; consequently, cefixime conjugated with gold and silver NPs led to enhanced antibacterial potential (Rai et al. 2010).
Previous investigations have also discovered that metal NPs first interact with the bacterial cell due to high responsiveness of metals toward sulfur in the bacterial structures (Ali et al. 2020a, b) and get into the cell, creating holes, and led to the expiration of the cellular matrix (Chakravarty and Banerjee 2008; Morones et al. 2005).
In conclusion, an organized approach was planned to enhance the antimicrobial activity and kinetics of cefixime by conjugation with gold and silver NPs. The synthesized Cfm-AuNPs and Cfm-AgNPs were characterized through UV-visible, FTIR, and AFM. Cfm-AgNPs and Cfm-AuNPs showed good pH, salt, and thermal stability. The antimicrobial evaluation showed that Cfm-AgNPs and Cfm-AuNPs killed the bacteria with better efficacy compared with the cefixime. Beside AFM analysis of the action mechanism on bacterial cell morphology, the toxicity with human mononuclear macrophages cells demonstrated that the synthesized NPs exhibited no toxic effect. The strategy explored in this work could provide medications for infections caused by resistant bacteria.
References
Ali S et al (2019)Nano-conjugates of Cefadroxil as efficient antibacterial agent against Staphylococcus aureus ATCC 11632. J Clust Sci:1–11
Ali S et al (2020a) Noble metals based bimetallic and trimetallic nanoparticles: controlled synthesis, antimicrobial and anticancer applications. Crit Rev Anal Chem:1–28
Ali S, Perveen S, Ali M, Jiao T, Sharma AS, Hassan H, Devaraj S, Li H, Chen Q (2020b) Bioinspired morphology-controlled silver nanoparticles for antimicrobial application. Mat Sci Eng C-Mater 108:110421
Allahverdiyev AM, Kon KV, Abamor ES, Bagirova M, Rafailovich M (2011) Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev Anti-Infect 9:1035–1052
Anacona JR, Estacio J (2006) Synthesis and antibacterial activity of cefixime metal complexes. Transit Metal Chem 31:227–231
Aurore V, Caldana F., Blanchard M., Kharoubi Hess S., Lannes N., Mantel P.Y., Filgueira L., Walch M. (2018)Silver-nanoparticles increase bactericidal activity and radical oxygen responses against bacterial pathogens in human osteoclasts.Nanomed Nanotechnol 14:601–607
Barbasz A, Oćwieja M, Barbasz J (2015) Cytotoxic activity of highly purified silver nanoparticles sol against cells of human immune system. Appl Biochem Biotechnol 176:817–834
Bolshakova A, Kiselyova O, Filonov A, Frolova OY, Lyubchenko YL, Yaminsky I (2001) Comparative studies of bacteria with an atomic force microscopy operating in different modes. Ultramicroscopy. 86:121–128
Chakravarty R, Banerjee PC (2008) Morphological changes in an acidophilic bacterium induced by heavy metals. Extremophiles 12:279–284
Chambers HF, DeLeo FR (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641
Chen Q et al (2020) Pre etched Ag nanocluster as SERS substrate for the rapid quantification of AFB1 in peanut oil via DFT coupled multivariate calibration. Spectrochim Acta A Mol Biomol Spectrosc:118411
de Lima R, Seabra AB, Durán N (2012) Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol 32:867–879
de Souza TAJ, Souza LRR, Franchi LP (2019) Silver nanoparticles: an integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol Environ Safe 171:691–700
Dos Santos MM, Queiroz MJ, Baptista PV (2012) Enhancement of antibiotic effect via gold: silver-alloy nanoparticles. J Nanopart Res 14:1–8
Drawz SM, Bonomo RA (2010) Three decades of β-lactamase inhibitors. Clin Microbiol Rev 23:160–201
Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st century.Persp Med Chem 6:25
Gordon O et al (2010) Silver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob Agents Chemother 54:4208–4218
Gu H, Ho P, Tong E, Wang L, Xu B (2003) Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett 3:1261–1263
Hauck TS, Ghazani AA, Chan WC (2008) Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small 4:153–159
Jia Y-P, Ma B-Y, Wei X-W, Qian Z-Y(2017) The in vitro and in vivo toxicity of gold nanoparticles. Chin Chem Lett 28:691–702
Jin C et al (2018)Ag/AgBr-loaded mesoporous silica for rapid sterilization and promotion of wound healing. Biomater Sci 6:1735–1744
Kuang Y-Y, Zhang Z-B, Xie M-L, Wang J-X, Le Y, Chen J-F(2015)Large-scale preparation of amorphous cefixime nanoparticles by antisolvent precipitation in a high-gravity rotating packed bed. Ind Eng Chem Res 54:8157–8165
Kumar A, Schweizer HP (2005) Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev 57:1486–1513
Li X, Robinson SM, Gupta A, Saha K, Jiang Z, Moyano DF, Sahar A, Riley MA, Rotello VM (2014) Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8:10682–10686
Li H et al (2019) Rapid quantitative analysis of Hg2+ residue in dairy products using SERS coupled with ACO-BP-AdaBoost algorithm. Spectrochim Acta A Mol Biomol Spectrosc 223:117281
Lyon BR, Skurray R (1987) Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev 51:88
Madl J, Rhode S, Stangl H, Stockinger H, Hinterdorfer P, Schütz GJ, Kada G (2006) A combined optical and atomic force microscope for live cell investigations. Ultramicroscopy. 106:645–651
McCaig LF, McDonald LC, Mandal S, Jernigan DB (2006)Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis 12:1715
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology. 16:2346–2353
Nair S, Sasidharan A, Rani VD, Menon D, Nair S, Manzoor K, Raina S (2009) Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J Mater Sci Mater Med 20:235–241
Naqvi S, Kiran U, Ali MI, Jamal A, Hameed A, Ahmed S, Ali N (2013) Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomed 8:3187–3195
Nazari ZE, Banoee M, Sepahi AA, Rafii F, Shahverdi AR (2012) The combination effects of trivalent gold ions and gold nanoparticles with different antibiotics against resistant Pseudomonas aeruginosa. Gold Bull 45:53–59
Neu HC (1992) The crisis in antibiotic resistance. Science. 257:1064–1073
Panáček A et al (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem Bull 110:16248–16253
Rafiq Z, Nazir R, Shah MR, Ali S (2014) Utilization of magnesium and zinc oxide nano-adsorbents as potential materials for treatment of copper electroplating industry wastewater. J Environ Chem Eng 2:642–651
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Rai A, Prabhune A, Perry CC (2010) Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J Mater Chem 20:6789–6798
Rai M, Ingle AP, Birla S, Yadav A, Santos CAD (2016) Strategic role of selected noble metal nanoparticles in medicine. Crit Rev Microbiol 42:696–719
Shah MR, Ali S, Ateeq M, Perveen S, Ahmed S, Bertino MF, Ali M (2014) Morphological analysis of the antimicrobial action of silver and gold nanoparticles stabilized with ceftriaxone on Escherichia coli using atomic force microscopy. New J Chem 38:5633–5640
Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S (2007) Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed Nanotechnol 3:168–171
Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA (2015) Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci 22:90–101
Singh RP, Kumar S, Nada R, Prasad R (2006) Evaluation of copper toxicity in isolated human peripheral blood mononuclear cells and it's attenuation by zinc: ex vivo. Mol Cell Biochem 282:13
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J Colloid Interf Sci 275:177–182
Stewart PS (2002) Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol 292:107–113
Yamashita H, Taoka A, Uchihashi T, Asano T, Ando T, Fukumori Y (2012)Single-molecule imaging on living bacterial cell surface by high-speed AFM. J Mol Biol 422:300–309
Zhang T, Liu Q, Xie Z, Song X, Gong J (2014) Determination and correlation of solubility data and dissolution thermodynamic data of cefixime trihydrate in seven pure solvents. J Chem Eng Data 59:1915–1921
Acknowledgments
The authors are thankful to the ICCBS, HEJ Research Institute of Chemistry, University of Karachi for instrumental availability.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, S., Perveen, S., Shah, M.R. et al. Bactericidal potentials of silver and gold nanoparticles stabilized with cefixime: a strategy against antibiotic-resistant bacteria. J Nanopart Res 22, 201 (2020). https://doi.org/10.1007/s11051-020-04939-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-020-04939-y