Abstract
We present a study on the chemical and structural transformations in highly porous monolitic materials consisting of the nanofibrils of aluminum oxyhydroxides (NOA, Al2O3·nH2O) in the temperature range 20–1700 °C. A remarkable property of the NOA material is the preservation of the monolithic state during annealing over the entire temperature range, although the density of the monolith increases from ~0.02 up to ~3 g/cm3, the total porosity decreases from 99.3 to 25% and remains open up to 4 h annealing at the temperature ~1300 °C. The physical parameters of NOA monoliths such as density, porosity, specific area were studied and a simple physical model describing these parameters as the function of the average size of NOA fibrils—the basic element of 3D structure—was proposed. The observed thermally induced changes in composition and structure of NOA were successfully described and two mechanisms of mass transport in NOA materials were revealed. (i) At moderate temperatures (T ≤ 800 °C), the mass transport occurs along a surface of amorphous single fibril, which results in a weak decrease of the length-to-diameter aspect ratio from the initial value ~24 till ~20; the corresponding NOA porosity change is also small: from initial ~99.5 to 98.5%. (ii) At high temperatures (T > 800 °C), the mass transport occurs in the volume of fibrils, that results in changes of fibrils shape to elliptical and strong decrease of the aspect ratio down to ≤ 2; the porosity of NOA decreases to 25%. These two regimes are characterized by activation energies of 28 and 61 kJ/mol respectively, and the transition temperature corresponds to the beginning of γ-phase crystallization at 870 °C.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oxidation of liquid-metal alloys resulting in the formation of porous oxides has been discovered in Al-Hg system over a century ago (Wislicenus 1908). Since that time, different ways of obtaining aerogels consisting of nanofibrous alumina organized in 2D and 3D nanostructures have been proposed and investigated (Noordin and Liew 2010). Among the various methods for preparing aerogels, the growth of porous NOA monolith on the surface of the liquid metal alloys has considerable advantage because it provides 3D nanomaterials preparation with highly reproducible chemical and physical properties. Further development of this technique results in the laboratory technology for the synthesis of nanofibrous amorphous alumina grown on deoxidized metallic aluminum plates wetted by mercury upon exposure of humid air (Pinnel and Bennett 1972). This method has been improved by doping a mercury amalgam with silver, which permitted better control of growth rate of NOA monolith and provide high homogeneity of the samples grown. The raw NOA monoliths obtained in this way can be annealed to obtain oxide materials with a given porosity, density, and specific surface area (Vignes et al. 1997; Di Costanzo et al. 2004; Vignes et al. 2008). It was shown that annealing of NOA materials does not affect their integrity and porous structure, leading only to an isotropic decrease only in the linear dimensions of the samples. At the temperature range ~25–1700 °C, the structural transformations were observed in NOA materials—from amorphous state to γ-, θ-, and α-aluminas (Vignes et al. 1997; Di Costanzo et al. 2004; Vignes et al. 2008; Askhadullin et al. 2008). The corresponding characteristics of the NOA samples also are changing: the density increased from 0.02 to ~3 g/cm3; porosity and specific surface area decreasing from 99.3 to 25% and from ~300 m2/g till 1 m2/g respectively. It should also be noted that a similar method of making porous oxides was proposed recently, which is based on the use of different of liquid alloys: Ga-Al and Bi-Al (Askhadullin et al. 2008; Bouslama et al. 2011).
Thermally treated NOA samples were used to create new oxide nanocomposites, functional nanomaterials, and hybrid structures (Bouslama et al. 2011; Asadchikov et al. 2015; Bouslama et al. 2012; Mukhin et al. 2012; Stepanenko O et al. 2015). In particular, potential applications of NOA materials in catalysis (Asadchikov et al. 2015; Bouslama et al. 2012) and THz optics (Mukhin et al. 2012; Stepanenko et al. 2015) have been demonstrated and discussed. In the same time, an adequate theoretical model describing the morphological changes, structural, and phase transformations in NOA materials has not been proposed until now.
The present report proposes a physical model that describes quantitatively the morphological changes of NOA induced by the diffusion transport, chemical, and phase transformations in the temperature range between 20 and 1700 °C. Experimental results obtained in this study are discussed along with the data reported earlier. Within the proposed model, it is shown that the changes in the physical properties of NOA can be described as the evolution of the key structural element—an average fibril and its aspect ratio (length/diameter).
Experimental
The samples of NOA monoliths were grown in the chamber filled with an atmospheric air with a humidity ~70–80% at room temperature ~25 °C. The primary layers with the aerosol structure are formed as a result of the oxidation reaction of the surface of a mercury-silver liquid solution deposited as a thin layer on a foil 99.9% aluminum. Under these conditions, a monolithic sample of NOA is growing with the rate of ~1 cm/h (Vignes et al. 2008), Fig. 1a.
Complex studies of morphological and structural features of the NOA samples have been carried out using powder X-ray diffraction (INEL XRG 3000 and Bruker D8 Advance diffractometers with X-ray source Cu-Kα (λ = 1.5418 Å), transmission electron microscopy (JEOL2011 equipped with 200 keV LaB6 gun), and scanning electron microscopy (Zeiss Supra 40 VP SEM-FEG operated at low acceleration voltage). Neutron scattering studies were carried out using small-angle KWS-2 and ultra-small-angle KWS-3 diffractometers in Research Neutron Source Heinz Maier-Leibnitz, reactor FRM II (Khodan et al. 2018).
The specific surface area and pore size distribution were determined by low-temperature nitrogen adsorption using a Coulter SA3100 and QuantaChrome Nova 4200B analyzers. Before the measurements, the samples were degassed in vacuum at 120 °C for 16 h. Specific surface area was calculated using the Brunauer-Emmett-Teller (BET) model.
The thermogravimetric (TG) analysis and differential scanning calorimetry (DSC) studies were performed using the SETARAM TG92 and Intertech TGA Q500 equipment. The sample with mass about 20 mg was heated in argon flow (~50 ml/min) at the increment rates between 0.1 and 50 °C/min up to the maximum temperature of 1000 °C.
Results and discussion
Effect of annealing on the structure and chemical composition of NOA
The characteristic structure of raw NOA is filamentary-nodular that is clearly visible on TEM images (Fig. 1b). The monolithic structure of NOA is formed by entangled fibrils of hydrated alumina possessing an average diameter of ~4–10 nm and length within 150–300 nm. The chemical and phase composition of raw NOA сan be described as an amorphous hydrated aluminum oxyhydroxide with water content of 40–43 wt.%, having very high porosity ≥ 99%, and specific surface area about 250–300 m2/g; the latter can be increased two times or more by applying a freeze drying.
Thermal treatment of raw NOA at temperatures below 800 °C does not affect significantly an amorphous structure, while at 1150 °C and higher the initial structure of fibrils begins to change, what is accompanied by a significant reducing of the specific surface area and the volume of mesopores. Crystallization and subsequent phase transitions to γ-, θ-, and α-alumina takes place in NOA samples under 4 h isochronous annealing at 870, 1100, and 1200 °C respectively (Table 1). The characteristic diameter of the fibrils and the corresponding values of specific surface area of polymorphs are 7 nm and 150 m2/g for γ-alumina, 10 nm and 100 m2/g for θ-alumina, and 250 nm and 10 m2/g for α alumina phases. Raw NOA possesses a very low mass density ~0.025 g/cm3 which increases up to 3 g/cm3 at 1700 °C—the maximum treatment temperature applied. As shown at SEM image of the sample treated at 1300 °C (Fig. 1d), the fibrils are transformed to well-crystallized α-Al2O3 particles. The respective size of ellipsoidal particles varies from 150 to 250 nm with the average value of ~200 nm.
Chemical and structural transformations
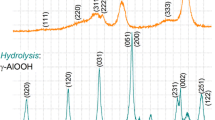
The XRD patterns of NOA treated at different temperatures are shown in Fig. 2. Up to ~650 °C, they do not reveal any significant change in the amorphous structure. Our experimental data and those of previous studies (Vignes et al. 1997; Di Costanzo et al. 2004; Vignes et al. 2008) show that the fibrils passes four structural transitions upon isochronous annealing in the temperature range 800–1600 °С going from the native amorphous state, consisting of chains of Al+3 cations in the octahedral surrounding of anions (Frappart 2000), to the stable α-crystalline state as summarized in Table 1. Aluminum oxyhydroxides obtained by the chemical precipitation are also included in this table for comparison.
We noticed that the very beginning of γ-Al2O3 crystallization from the amorphous alumina obtained by chemical precipitation can be detected at temperatures as low as ~400 °C (see Table 1), which follows from our detailed analysis of electron diffraction patterns. The crystallization is a kinetically limited process and its rate is extremely low in this range of temperatures. Moreover, crystallization of single (non-aggregated) nanofibrils can be delayed to higher temperatures due to a contribution of surface energy, in agreement with previous observations in oxide nanoparticles (Zhang and Banfield 1998). In particular, an assessment of the enthalpy of formation for γ-Al2O3 with the surface area exceeding ~125 m2/g predicts its decreasing lower compared to that of α-Al2O3 (Frappart 2000; Navrotsky 2003). This effect also concerns crystallization from the amorphous phase (Khatim et al. 2013). Amorphous fibrils begins to crystallize at a temperature above 870 °C what is in agreement with the results published earlier (Vignes et al. 2008; Frappart 2000). An additional confirmation of the dominant contribution of surface transport to the kinetics of crystallization can be a significant slowdown of γ-, θ-, and α-alumina phase transformation in NOA materials, when the surface of fibrils are covered with a few monolayers of silica (Di Costanzo et al. 2004). It should be noted that silica layer also prevents the elimination of the structural water from NOA what inhibits the phase transformation. Under the 4 h isochronous annealing, the overall effect appears as a “phase transition temperature shift up” within ~150–300 °C (Table 1).
Key changes in the chemical composition of NOA upon annealing are related to the losses of the adsorbed and structural water. Results of TG measurements in the temperature range of 25–950 °C are presented at Fig. 3 as the relative mass losses and related water content in raw and thermally treated NOA samples. At the given temperature T °C, we can present a relative molecular composition n(T) as the sum of the two types of water state:
where nstr(T) and nads(T) are the relative fractions of the structural water (Al2O3·nstrH2O) and the adsorbed water at the NOA surface. With an aim to separate and define nstr(T) and nads(T) values, the studies were performed using two groups of samples. For the first group of samples, a weight loss was measured in relation to the initial raw state of NOA; the samples of second group passed preliminary annealing at the temperature Tp = 100; 380; 400; 520; 660 °C during 4 h and were measured after cooling down to room temperature in air (Fig. 3).
From the TG results, it follows that the raw NOA contains about 3.6 water molecules per Al2O3, which exceeds the water content in the stoichiometric hydroxide. This confirms that the raw NOA fibrils consist of aluminum oxyhydrates with excess of adsorbed molecular water. The loss of structural water nstr(T) starts below 400 °C and is accompanied with partial dehydration of the amorphous aluminum oxyhydroxide. Stable compositions Al2O3·1.5H2O and Al2O3·0.9H2O are formed at ~100 and 380 °C respectively. The last composition can be interpreted as amorphous phase of partially dehydrated boehmite Al2O3·H2O, that is in an agreement with the data on the beginning of γ-Al2O3 crystallization (see Table 1). The further increase in temperature up to 700 °C moderately affects the Al2O3·xH2O composition with x decreasing down to 0.4, which completes the formation of γ-phase at 870 °C in agreement with data presented by J-L. Vignes et al. 2008.
The values nstr(T) and nads(T) are plotted in Fig. 4 versus preannealing temperature Tp. The main changes in n(T) are caused with the loss of water bound in NOA structure nstr(T) and can be associated with hydroxide decomposition, whereas nads(T) only weakly depend on the preliminary annealing temperature (Fig. 4). It should be noted the change in the properties of NOA adsorption surface at annealing temperatures T ≥ 400 °C indicated by the arrow in Fig. 4a.
Variation of the water molecular ratio per Al2O3 for structural ( ) and adsorbed (
) and adsorbed ( ) components in NOA samples with the temperature of pre annealing Tp (Table 2)
) components in NOA samples with the temperature of pre annealing Tp (Table 2)
Analysis of the Table 2 data for the dependence of nstr(T) = f(Tp) allowed to estimate the activation energy of oxyhydroxide decomposition (ED = 22 ± 5 kJ/mol using least square fit for Arrhenius equation:
where n0 is the composition of raw NOA (Al2O3·3.6H2O), and kB is the Boltzmann constant.
An increase in the annealing temperature activates the diffusive mass transport in NOA, which leads to the material densification due to morphological changes in nanofibrils and beginning of nucleation of new crystalline phases (Fig. 2). The annealing for 4 h at temperatures ranging from 100 to 1700 °C does not affect the integrity of NOA samples while their dimensions decreases and mass density increases, as confirmed by the experimental data presented in Fig. 5a. However, below 450 °C, the mass density changes are negligible. This is related to the water desorption from NOA fibrils as explained in the previous discussion. Indeed, taking into account the water losses, (1) we evaluated the mass density of NOA fibrils as shown in Fig. 5b. The comparison between the measured and calculated mass densities indicates major surface water losses below 450 °C. Based on this result, we conclude that the fibril densification is continuous in the whole temperature range and its mechanism appreciably changes only at temperatures > 800 °C, when the mass transport mechanism changes from surface diffusion to sintering. These processes are quantitatively described in the framework of the 3D model proposed.
Intermediate metastable phases
Five relatively stable phases can be identified in NOA upon annealing in the temperature range from 20 °C up to 1700 °C.
-
(I)
The NOA synthesized in ambient air with relative humidity ~80% at 25 °C has the chemical composition Al2O3·nH2O with n ≥ 3. The NMR studies have shown that Al+3 cations have anionic surroundings including octahedral (83%), pentahedral (16%), and tetrahedral (1%) (Frappart 2000). Consequently, the NOA structure consists of polynuclear aqua-hydroxide complexes of aluminum cation and water molecules connected with hydrogen bonds in different spatial alignments.
-
(II)
The native structure of NOA starts loosing water and decomposes when heated to moderate temperatures ~100 °C. It progressively transforms into an amorphous oxide of Al2O3·nH2O composition with n ≤ 1.5. A further increase in temperature does not promote significant changes in the amorphous structure, while the structural water content continuously reduces until n ≈ 0.1 at 450–500 °C.
-
(III)
At ~450 °C, the crystallization into γ-Al2O3 phase begin, which preserves a small amount of structural water n < 0.1. This small amount of water is necessary for stabilization of the transition phase (McHale et al. 1997a, b). However, the crystallization in nanofibrous alumina is delayed to 870 °C (Vignes et al. 1997), and NOA monoliths remains amorphous in the extended temperature range between 100 and 800 °C and converts to γ-Al2O3 at 870 °C.
-
(IV)
After 4 h annealing at T ≥ 1000 °C, γ-Al2O3 converts to θ-Al2O3, which is accompanied by a further reduction of the structural water content to n < 0.04.
-
(V)
The temperature increase to 1200 °C leads to the formation of well-crystallized stable α-Al2O3 polymorph, which does not contain any appreciable amount of structural water. This phase transition is completed at 1250 °C with the annealing time of 4 h.
The main changes in chemical composition, phase structure, and morphology of the nanofibrils of NOA materials during annealing can be described in the framework of the physical model presented in the next chapter.
3D model of NOA materials
We assume that monolithic structure of NOA materials consist of entangled hydrated alumina fibrils with an average diameter d and length a (Figs. 1b–d). These fibrils form the porous structure as a network with multiple connections at the cross points. Upon annealing the aspect ratio a/d decreases and elementary fibrils progressively shorten and transform into ellipsoidal-shape particles, when the stable α-Al2O3 polymorph is formed at temperatures above 1200 °C. Even in this case, the interconnected deformed fibrils can be recognized (Fig. 1d). Therefore, we relate the mass density and specific area of NOA materials to the evolution of the shape of elementary fibril. A relevant 3D geometrical model of the raw NOA material is presented in Fig. 6, where the primary volume a3 contains nf fibers that interconnect arbitrary and are surrounded with a free volume Vp. The total volume of fibrils is \( {V}_f={n}_f\frac{\pi }{4}a{d}^2 \) and their mass \( {M}_f={n}_f\bullet {\rho}_f\bullet \frac{\pi }{4}{d}^2a \), where ρf is a specific density of the fibril’s material. Corresponding specific surface area of Mf shall comply with Ssp = nf ∙ sf ≈ n ∙ πda, where sf is the surface of a single fibril. It is evident that Mf and Ssp depend on the aspect ratio a/d.
Mass density
We estimated a specific density of the fibril’s material close to ρf = 2.4 g/cm3. This reasonably agrees with the data for different crystalline oxyhydrates and aluminum oxides phases with different water content: 2.42 g/cm3 for gibbsite Al2O3∙3H2O, 2.53 g/cm3 for bayerite Al2O3∙3H2O, 3.01 g/cm3 for boehmite Al2O3∙H2O, 3.5 and up to 3.9 g/cm3 in γ-Al2O3 phase stabilized by the structural water. At temperatures below 1200 °C, the maximum density 3.67 g/cm3 can be obtained for γ-Al2O3, and 3.99 g/cm3 for α-Al2O3 phase at the high sintering temperatures Т > 1200 °C.
The mass density of raw NOA material ρ0 can be calculated as the mass of nanofibrils located in the volume a3:
or an average number n of the fibrils in the volume a3 can be estimated:
Using measured mass density ρ0 ≈ 0.025 g/cm3 of raw NOA and aspect ratio variations a0/d0 between 4 and 16, the number of fibrils in the volume a3 can be estimated: nf ≈ 10 ± 6. We notice that the knowledge of the true pore geometry is not necessary for calculation of the NOA-specific density with the rise of annealing temperature; the proposed model assumes that it scales with ρ ∝ (d/a)2.
Porosity and specific surface area
The porosity of NOA can be determined as a fraction of the volume free from the nanofibers. From the definitions for nf and Mf follows:
The total surface of all the nanofibrils located inside a primary volume a3 can be presented as follows:
and specific surface area of raw NOA material can be calculated:
In the general case, d ≅ a and expression (7) becomes:
Equation (5) establishes the relationship between the specific surface area and the average size of nanofibrils in NOA materials, which is consistent with the experimental results within 5% accuracy. Equation (7) was used to describe the evolution of aspect ratio a/d in the entire temperature range. Evolution of the mean diameter d(T) of NOA fibrils calculated from (7) using fibril’s density ρf(T) and specific surface area Ssp(T) obtained from isochronous annealing data is presented in Fig. 7. One more expression relating the specific density and specific surface area in NOA materials can be obtained from (2) and (7):
Mean diameter of NOA fibrils, estimated from (7), versus temperature of isochronous annealing during 4 h
Taking into account the limited range of nf variation, the estimate of the “effective size of NOA fibril” can be obtained as δ≈ 0.02(a2d).
We notice that the mean fibril diameter observed in TEM images of raw NOA (Fig. 1b) is about 5 nm, that fits the value obtained from (7) using the initial mass density 0.025 g/cm3 and specific area 320 m2/g. The similar estimations of the effective diameter of nanofibrils result in values about 7 nm in γ-alumina (ρ = 0.04 g/cm3, Ssp = 150 m2/g), 9 nm in θ-alumina (ρ = 0.045 g/cm3, Ssp = 100 m2/g) and 120 nm in α-phase (ρ = 0.65 g/cm3, Ssp = 5 m2/g) which are in agreement with the TEM and SEM observations. We conclude that the model proposed successfully describes 3D transformations in NOA materials during thermal treatment.
Evolution of the morphology of NOA fibrils under annealing
Three main temperature regions that change the mass transfer mechanism and activation of sintering process in NOA materials should be highlighted.
Low annealing temperatures T < 300 °C
In this temperature range, the structure of the raw Al2O3·nH2O material is preserved in the fibrils and the changes of the shape or in aspect ratio are small. Changes in the material density also can be neglected despite the fact that the value of n(T) varies between 3.6 and 1.5 (Figs. 3 and 4, Table 2). We assume that water losses do not lead to any significant modification of the material morphology at nano- and micro-level.
Moderate annealing temperatures T ≤ 870 °C
The NOA density changes upon annealing in this temperature range are below 0.01 g/cm3. We assume that the shape modifications of amorphous fibrils are limited by the diffusion transport and takes place at the fibril surface. This process leads to a decrease in the surface-to-volume ratio. Since both agglomeration and sintering of fibrils are not observed under these conditions, we can express the reduction in fibril length a(t) and increase in its diameter d(t) simply as a result of surface diffusion. We assumed that:
where a0 is the initial fibril length and D is “efficient diffusion coefficient” which is related mostly to the surface. We also neglect mass transport between fibrils and consider the modification of a single fibril. On the basis of conservation of the fibrils volume, we can write:
From (9) and (10), one can obtain the shape variation of the fibril during annealing:
where \( D={D}_0\bullet \mathit{\exp}\left(-\frac{E_a}{k_BT}\right) \) is the “effective” diffusion coefficient with an activation energy Ea. Using (2), (7), (10), and (11), we can describe the changes in density and specific surface area of NOA materials at temperatures below 870 °C:
The “effective” diffusion coefficient: D0 = 6.0 × 10−18 m2/s and activation energy Ea = 28 kJ/mol were estimated by applying the least-squares fit to the all experimental data for ρ(t) and Ssp(t) with the set of parameters: a0 = 140 nm, d0 = 5 nm, ρf = 3.1 g/cm3. Figure 8 show a good agreement between the experimental data and the model plot for a/d. The value of activation energy Ea is close to the activation energy of oxyhydroxide decomposition ED = 22 ± 5 kJ/mol obtained from TG data. Therefore, we assume that surface diffusion and chemical decomposition of the oxyhydroxide are intrinsically connected and possess a common activation mechanism for these processes.
Evolution of NOA fibrils aspect ratio a/d of NOA with annealing temperature. The results obtained both in this work and published earlier (Vignes et al. 1997; Di Costanzo et al. 2004; Vignes et al. 2008; Askhadullin et al. 2008) are presented. The scatter of the a/d ratio near the temperature ~20–25 °C are caused by variation of humidity and air temperature during the measurements of raw NOA samples
The applied formalism is relevant to the conditions when the diffusion mass transport is limited within a single fibril: \( {a}_0\ge \sqrt{Dt} \). The single fibril of raw NOA has the mean volume Vf ≈ (2.5–3.0) × 103 nm3 and cylindrical shape with the aspect ratio a/d~30. The surface mass transport results in the shape changes described by Eqs. (9)–(11). Consequently, the aspect ratio decreases by minimizing the surface energy reaching a minimum when a/d→1 and lmax(ax ≈ dx) ≈ 15 nm, but it does not take place due to diffusion limitations at temperatures below 870 °C and the aspect ratio remains larger than 20 (Fig. 8).
Thus, at moderate temperatures ≤ 870 °C with the short annealing times ≤ 10 h, when high porosity is remaining, the parameters of mesoporous NOA materials such as specific surface area, density, porosity, evolution of fibrils shape, and aspect ratio can be described using Eqs. (11–13).
High annealing temperatures T ≥ 870 °C
An annealing of the NOA materials at temperatures higher 870 °C leads to significant coarsening of the alumina particles and those attains the sizes of 100–500 nm that is much larger than the maximum estimates ~10 nm for the average size of the fibril in raw NOA. The mass transport progressively involves the overall volume of fibrils and the area of contacts between adjacent fibrils. The annealing at temperatures above 1000 °C leads to a significant shrinkage of NOA materials, which is related to an increase in the samples density and decrease in free volume. The initial porosity of raw NOA sample is ≥ 99% and only slightly decreases to 98.8% after 4 h annealing at 1000 °C. Further increase in the temperature results in a sharp decrease in porosity down to 71% at 1400 °C, 56% at 1500 °C, and to 26% at 1650 °C. It is important to notice significant changes in the fibril morphology, which becomes spherical or ellipsoidal with the aspect ratio (a/d) ≤ 2.
The structural evolution of NOA materials at high temperatures was described using a simplified version of Ivensen equation (Ivensen 1995). We assume that the changes of free volume in time follow the relationship:
and V is a free space (volume) between the fibrils. Coefficient B comprises two constants: the activation energy of the mass transport Eb, and the constant of free volume B0. The solution of Eq. (14) allows obtaining the dependence of free volume Vfree(t, T) from the sintering temperature T and time t:
where V0 is the free volume in NOA sample at t = 0. It is easy to get similar relationship for the NOA density ρ(t, T):
From (16), we can define the initial density of NOA at t = 0, and from (16) we can define the initial density of NOA at t = 0 as ρ0 = ρf/(1 + V0 ∙ Vf−1) ≈ (ρfVf)/V0, and in combination with expression (15), we get:
From the definition of nf can be obtained the relationship:
and after some transformations and substitutions in (16), we get the function describing an aspect ratio a/d evolution during sintering of NOA materials:
The latter expression was obtained assuming that ρf ≫ ρ0.
After applying the least-squares fit to the experimental data shown in Fig. 8, the following values of the parameters in Eqs. (16') and (17) were obtained: ρf = 3.1 g/cm3, B0 = 2.3 × 10−2 s−1, and Eb = 61 kJ/mol. The determined parameters B0 and Eb were used for a/d and ρ(t, T) curve plotting shown in Figs. 8 and 9. Obviously, that Eqs. (16') and (17) describe fairly well the results obtained for NOA samples after annealing at the temperatures T ≥ 870 °C.
A characteristic feature of this temperature range is the process of sintering covering the whole volume and contact areas of the adjacent nanofibrils, which is accompanied by profound restructuring of the mesoporosity as well as with the processes of crystallization and phase transformations. Since the γ → θ → α phase transformations are characterized by very close values of the activation energy Eb, the relevant process can be considered as permanent and progressive ordering in the crystalline structure due to release of residual structural water from the fibrils. A complete water removal from the NOA structure takes place at the formation of the α-phase. The activation energy of the sintering Eb = 61 kJ/mol is considerably higher than Ea = 28 kJ/mol—the assessment made for moderate annealing temperatures T ≤ 870 °C at which the changes of fibrils shape can be associated with the surface transport effects.
Conclusions
Chemical and structural modifications of mesoporous nanofibrous alumina Al2O3·nH2O were studied in the temperature range of 20–1700 °C. Two mechanisms of the morphological and structural transformations in NOA materials are evidenced and the physical model was proposed allowing describing the changes in physical parameters such as density, porosity, and specific surface area as morphological evolution of the averaged structural element—the NOA fibril. At temperatures < 300 °C, the change in composition of amorphous Al2O3·nH2O is insignificant and is mainly due to desorption of molecular water; amorphous structure of raw NOA is preserved. At moderate temperatures between 300 and 870 °C, the mass transport is limited by surface diffusion along the single fibrils and characterized by activation energy Ea = 28 kJ/mol; this cause shrinkage of the fibril size about 20% and aspect ratio a/d decreasing from ≈24 till 20; the onset of crystallization of amorphous NOA is not observed upon 4 h annealing. At high temperatures, the mass transport characterized by activation energy Ea = 61 kJ/mol and involves the overall volume of fibrils followed by the material sintering and growth of particles with the aspect ratio a/d ≤ 2 and elliptical shape. The transition temperature from moderate to high temperatures is close to 870 °C, which corresponds to the beginning of the crystallization of amorphous alumina to γ-phase. Applying 3D model for the experimental results analysis, we explain the phenomenon of preservation of the integrity of monoliths of NOA during thermal annealing by the important role of surface processes and first of all high mobility of atoms on the surface of nanofibrils. The surface diffusion provides an isotropic reduction in the 3D structure parameters without a significant increase in internal stresses over a wide range of temperatures. The model proposed allow а quantitative description of the morphological and structural properties of mesoporous aluminas and may be useful for further development of the technology of 3D nanomaterials.
References
Asadchikov VE, Askhadullin RS, Volkov VV, Dmitriev VV, Kitaeva NK, Martynov PN, Osipov AA, Senin AA, Soldatov AA, Chekrygina DI, Yudin AN (2015) Structure and properties of “nematically ordered” aerogels. JETP Lett 101:556–561. https://doi.org/10.1134/S0021364015080020
Askhadullin RS, Martynov PN, Yudintsev PA, Simakov AA, Chaban AY, Matchula EA, Osipov AA (2008) Liquid metal based technology of synthesis of nanostructured materials (by the example of oxides). These materials properties and applications areas. J Phys: Conf Ser 98:1–6. https://doi.org/10.1088/1742-6596/98/7/072012
Bouslama M, Amamra MC, Tieng S, Brinza O, Chhor K, Abderrabba M, Vignes JL, Kanaev A (2011) Isolation of titania nanoparticles in monolithic ultraporous alumina: effect of nanoparticle aggregation on anatase phase stability and photocatalytic activity. Appl Catal A 402:156–161
Bouslama M, Amamra MC, Jia Z, Ben Amar M, Brinza O, Chhor K, Abderrabba M, Vignes JL, Kanaev A (2012) Nanoparticulate TiO2-Al2O3 photocatalytic media: Effect of particle size and polymorphism on photocatalytic activity. ASC Catal 2:1884–1892
Di Costanzo T, Fomkin AA, Frappart C, Khodan AN, Kuznetsov DG, Mazerolles L, Michel D, Minaev AA, Sinitsin VA, Vignes JL (2004) New method of porous oxide synthesis: alumina and alumina based compounds. Mater Sci Forum 453-454:315–322
Frappart C (2000) Elaboration et caractérisation de monolithes poreux d’alumine obtenus par oxydation d’aluminium. Insertion d’oxydes nanométriques, PhD Dissertation, University Paris 11
Ivensen VA (1995) Use of a mathematical model for pore volume shrinkage over a wide temperature range. Powder Metall Met Ceram 34:528–533. https://doi.org/10.1007/BF00559962
Khatim O, Amamra M, Chhor K, Bell T, Novikov D, Vrel D, Kanaev A (2013) Amorphous-anatase phase transition in single immobilised TiO2 nanoparticles. Chem Phys Let 558:53–56
Khodan AN, Kopitsa GP, Yorov KE, Baranchikov AE, Ivanov VK, Feoktystov A, Pipich V (2018) Structural analysis of aluminum oxyhydroxide aerogel by small angle X-ray scattering. J SURF INVESTIG-X-RA 12:287–296
McHale JM, Auroux A, Perrotta AJ, Navrotsky A (1997a) Surface energies and thermodynamic phase stability in nanocrystalline aluminas. Science 277:788–791
McHale JM, Navrotsky A, Perrotta AJ (1997b) Effects of increased surface area and chemisorbed H2O on the relative stability of nanocrystalline γ-Al2O3 and α-Al2O3. J Phys Chem B 101:603–613
Mukhin VI, Khodan AN, Nazarov MM, Shkurinov AP (2012) Study of the properties of nanostructured aluminum oxyhydroxide in the terahertz frequency range. Radiophys Quant Electron 54:591–599
Navrotsky A (2003) Energetics of nanoparticle oxides: interplay between surface energy and polymorphism. Geochem Trans 4:34–37
Noordin MR, Liew KY (2010) Synthesis of alumina nanofibers and composites, in: Kumar A (ed.) Nanofibers, InTech
Pinnel MR, Bennett JE (1972) Voluminous oxidation of aluminium by continuous dissolution in a wetting mercury film. J Mater Sci 7:1016–1026
Stepanenko O, Tartari A, Amamra M, Nguyen THN, Piat M, Favero I, Ducci S, Khodan A, Boinovich LB, Emelyanenko AM, Kanaev A, Leo G (2015) Ultra-porous alumina for microwave planar antennas. Adv Device Mater 1:93–99. https://doi.org/10.1080/20550308.2015.1120442
Vignes JL, Mazerolle L, Michel D (1997) A novel method for preparing porous alumina objects. Key Eng Mater 132-136:432–435
Vignes JL, Frappart C, Di Costanzo T, Rouchaud JC, Mazerolles L, Michel D (2008) Ultraporous monoliths of alumina prepared at room temperature by aluminium oxidation. J Mater Sci 43:1234–1240
Wislicenus H (1908) Über die faserähnliche gewähsene Tonerde (Fasertonerde) und ihre Oberflächenwirkungen (Adsorption). Zeitschrift für Chemie und Industrie der Kolloide 2:XI-XX
Zhang H, Banfield JF (1998) Thermodynamic analysis of phase stability of nanocrystalline titania. J Mater Chem 8:2073–2076
Acknowledgments
ANR (Agence Nationale de la Recherche) and CGI (Commissariat à l’Investissement d’Avenir) are gratefully acknowledged for their financial support of this work through Labex SEAM (Science and Engineering for Advanced Materials and devices) ANR 11 LABX 086, ANR 11 IDEX 05 02.
Funding
This work was supported by the French-Russian collaboration project DRI CNRS No. EDC26176, and part of this work was carried out with the financial support of the Russian Foundation for Basic Research (Project 17-53-150007 CNRS_a).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khodan, A., Nguyen, T.H.N., Esaulkov, M. et al. Porous monoliths consisting of aluminum oxyhydroxide nanofibrils: 3D structure, chemical composition, and phase transformations in the temperature range 25–1700 °C. J Nanopart Res 20, 194 (2018). https://doi.org/10.1007/s11051-018-4285-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4285-4









 ,
,  , and
, and  . b Measured density of NOA (
. b Measured density of NOA ( ) and NOA density after correction for the water adsorbed at the surface (
) and NOA density after correction for the water adsorbed at the surface ( )
)


