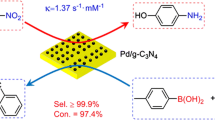
Abstract
Ligand-free palladium nanoparticles supported on multi-walled carbon nanotubes (Pd/MWCNT) were prepared by the supercritical carbon dioxide (scCO2) deposition method using a novel scCO2-soluble Pd organometallic complex as a precursor. The precursor with the perfluoroalkyl chain group was synthesized and identified by microanalytic methods. The deposition was carried out at the temperature of 363.15 K and pressure of 27.6 MPa CO2. The prepared metallic nanoparticles were obtained with an average size of 2 nm. Pd/MWCNT was utilized as a heterogeneous catalyst in Suzuki cross-coupling reaction. The nanocatalyst was found very effective in Suzuki reaction and it could also be recovered easily from the reaction media and reused over several cycles without significant loss of catalytic activity under mild conditions.

Pd/MWCNT was prepared by the scCO2 deposition method using a new synthesized perfluroalkylated vic-dioxime Pd complex as the precursor. The prepared nanoparticle was very effective as catalyst and reusable for Suzuki cross coupling reaction under mild conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preparation of supported metal nanoparticles (NPs), especially Pd nanoparticles, is gaining increasing importance in catalysis of numerous organic synthesis (Campelo et al. 2009; Hunt et al. 2014). These nanocatalysts provide a lot of advantages such as stability to thermal changes and recovery from the reaction media and may be the most important one with higher surface area (Narayanan 2010; Pérez-Lorenzo 2012). There are several methods typically used for preparation of NPs including impregnation (GAO et al. 2016), deposition-precipitation (Skaf et al. 2014; Xiong et al. 2014), chemical vapor deposition (Krisyuk et al. 2015), electrochemical deposition (Vilana et al. 2014), sol-gel (Koli et al. 2017), and microemulsion deposition (Perez-Coronado et al. 2016). The supercritical fluid deposition method which has become more attractive because of its environmentalist approach since the early 2000s is a demonstrated technology for deposition of metals (Erkey 2009; Wu et al. 2013). Especially, supercritical carbon dioxide (scCO2) is the most favorable fluid because of its critical temperature and pressure (31.1 °C and 7.38 MPa) parameters. ScCO2 is non-toxic, cheap, non-flammable, and inert in almost every reaction (Jiao et al. 2009). All these advantages produce a clean, successful process using the deposition method (Ulusal et al. 2017).
In the past decades, there has been limited number of work about the development of precursor for scCO2 deposition methods. Solubility, magnetic properties, and stability play significant roles to choose the right precursor for the supercritical carbon dioxide deposition method (SCD). For preparing the supported metal nanoparticles by the SCD method generally, cyclooctadiene, β-diketonate, dithiocarbamate, amine, and their derivatives are used as a precursor (Erkey 2009). Also, it is well known that fluorine groups attached to ligands increase their solubility in scCO2 (Teoh et al. 2013; Škerget et al. 2011). Even though the attachment of long perfluoroalkyl chains to conventional ligand has been widely utilized for the synthesis of metal-based catalysts compatible with scCO2 and fluorous solvents, there have not been any report on the use of the perfluoroalkylated precursor in a scCO2 deposition (Erkey 2009; Škerget et al. 2011). Vic-dioximes are among the most useful and popular ligands because of the preparation of metal complexes with a wide variety of transition metals. In our previous work, Pd complexes of dimethylglyoxime and phenanthrenequinonedioxime were used as a precursor to prepare γ-alumina-supported nanocatalyst and the results showed that the vic-dioxime metal complexes are alternative precursors for the scCO2 deposition method. (Ulusal et al. 2017). In this work, an unusual precursor, perfluoroalkylated vic-dioxime metal complex, was selected and utilized in deposition.
Biaryl compounds are very important intermediates for various industrial areas such as pharmaceutical, agrochemicals, fine materials, and natural products (Torborg and Beller 2009; Magano and Dunetz 2011; Keleş and Ylmaz 2014). Pd-catalyzed Suzuki C-C cross-coupling reaction is the most powerful option to synthesize biaryl intermediates (Sobhani and Zarifi 2015; Zhang et al. 2015; Yaşar et al. 2008). Suzuki reaction is generally catalyzed by both homogeneous and heterogeneous Pd catalysts; however, heterogeneous Pd nanocatalysts are more preferred over the homogeneous ones because of their ease of reusability (Pérez-Lorenzo 2012; Zhang et al. 2015; Liew et al. 2015). This type of C-C coupling reactions requires higher energy due to their reaction temperature. To occur the coupling reactions under milder conditions is a promising research area. Developing more eco-friendly new nanocatalyst for Suzuki reaction highlights the importance of our work.
In this work, a new scCO2-soluble organometallic compound, perfluoroalkylated vic-dioxime Pd complex, was synthesized and used as a precursor in the supercritical deposition method for preparing a MWCNT-supported heterogeneous catalyst. The catalytic efficiency of synthesized palladium nanocatalyst was evaluated in Suzuki C-C cross-coupling reaction.
Experimental
Characterization of precursor and catalyst
IR spectra of the precursor were obtained by Thermo FT-IR spectrometer and Smart ITR diamond attenuated total reflection (ATR). Elemental analyses (C, N, H) were conducted by Thermo Scientific Flash 2000 and CHNS elemental analysis apparatus. 1H and 19F NMR spectra were recorded on a Bruker AVANCE-500 (in CHCl3 and DMSO).
The amount of Pd metal content was determined using an inductively coupled plasma-optical emission spectrometer (ICP-OES) (ICAP 6300). The size and composition of supported NPs were analyzed by powder XRD diffraction (Rigaku Miniflex CuKα, λ = 0.154 nm) measurements, using Scherrer equation to determine the metal NP crystallite size. Morphology and distribution were studied by transmission electron microscopy (TEM) (JEOL 2100F, 200 kV). The crushed powders for TEM examination were suspended in ethanol and ultrasonicated to obtain a uniform suspension. One drop of this suspension was deposited by syringe onto a copper mesh grid coated with a holey carbon film. The sample was dried at least one night to get ready for TEM analysis. Scanning electron microscopy (SEM) images and energy dispersion spectrum (EDS) mapping were obtained with a ZEISS SUPRA-55 which was used to analyze material composition.
Synthesis of organometallic precursor
All chemicals and solvents were purchased from commercial suppliers and used without purification. For the synthesis of 4-perfluoro-vic-dioxime [4PFVD], a modified procedure reported by E. Taş was used. The ligand of precursor was synthesized using anti-monochloroglioxime and fluorinated aniline in absolute ethanol (Taş et al. 1999) (Scheme 1). Yield: 94%, m.p. 205–207 °C, 1H NMR (CDCl3), δ ppm 11.5 (d, 2H, –OH), 8.5 (s, 1H, –NH), 7.6–6.8 (m, 4H, Ph), 3.3 (s, 1H, =CH); 19F NMR (CDCl3), δ ppm − 80.8 (−(CF2)7CF 3 ), − 109.7, − 112.2, − 120.9, − 121.1, − 122, − 122.6, − 126 (−(CF 2 ) 7 CF3), FT-IR (ATR, mmax/cm−1); 3408 (N–H), 3255 (O–H), 1642 (C=N), 1608–1527 (C=C), 1299 (N–O), 1144–1196 (C–F), 953 (N–O); anal. calcd for [C16H8F17N3O2]: C 32.18, H 1.35, N 7.04; found C 31.06, H 1.29, N 6.11.
Subsequently, the organometallic precursor was synthesized with PdCl2 as metal salt according to the same reported paper (Taş et al. 1999) (Scheme 1). Yield: 70.0%, m.p. > 330 °C, 1H NMR (DMSO-d6, δ ppm): 3.9 (s, 2H, H–C=NOH), 7.9 (s, 2H, Ph–N–H), 6.8–7.6 (m, 8H, Ph–H). 19F NMR (CDCl3), δ ppm − 84 (−(CF2)7CF 3 ), − 112, − 122.5, − 124.5, − 125, − 125.5, − 126, − 129.5 (−CF 2 ) 7 CF3), FT-IR (ATR, mmax/cm−1); 3218 (N–H), 3058 (C–H, Ph), 1678 (O•••H–O, w), 1612 (C=N), 1297 (N–O), 1196–1144 (C–F), 955 (N–O); anal. calcd for [C32H14F34N6O4Pd]: C 29.59, H 1.09, N 6.47; found C 28.88, H 1.05, N 6.25.
Preparation of catalyst (Pd/MWCNT)
MWCNTs were obtained from Sigma Aldrich and used and had the following average dimensions: O.D. × L (6–9 nm × 5 μm) and diameter (mode, 5.5 nm; median, 6.6 nm). Carbon dioxide (99.98%) and hydrogen (99.999%) gases were purchased from Linde Gas. The precursor and support material amounts were placed in a 100-mL inner volume stainless steel reactor (Amar brand) (Fig. 1.) in order to achieve 8% of Pd/MWCNT ratio.
Subsequently, the cell was sealed and heated up to 363.15 K and pressurized with CO2 by using a syringe pump (ISCO, 260D). Following this step, the cell was filled with H2 (0.5 MPa) and CO2 (27.6 MPa) and stirred 4 h at desired pressure and temperature. At the end of 4 h, the reactor was stopped and cooling until room temperature and the system was depressurized slowly by using vent valve. Finally, obtained palladium nanoparticles were washed with THF several times to remove unreduced organometallic complex and then dried in an oven overnight. ICP-OES, XRD, and TEM analysis techniques were used to confirm the presence of metal nanoparticles, the size of metal nanoparticles, and their distribution on MWCNT.
General procedure for Suzuki reaction
Bromobenzene (0.25 mmol), phenylboronic acid (0.30 mmol), base (0.50 mmol), Pd/MWCNT (0.5 mol%, 1.85 mg), and organic solvent water (1:1, 4.0 mL) were allowed to react at 60 °C. An aliquot of the reaction mixtures was withdrawn periodically and extracted with CH2Cl2 (3 × 10 mL). The extracts were washed with brine (10 mL) and dried over Na2SO4 and solvent was evaporated. The crude was directly analyzed by gas chromatography (GC) (Agilent 6850 with HP-5 column) to get the reaction yield. In the calculation of the percent conversions of the products, the consumption of bromobenzene determined from GC was based on.
Results and discussion
Catalyst preparation and characterization
Differently from the other preparation procedures, Pd/MWCNT nanoparticles were prepared by an environmentally friend deposition method. Using scCO2 as a solvent and absence of by-products and residue after the deposition makes this method more advantageous than the others. Utilizing synthesized scCO2-soluble organometallic compound as a precursor provides a new perspective on the scCO2 deposition method. The synthesized precursor was characterized by various micro-analytical and spectrometric methods and the results were in agreement with literature data (Taş et al. 1999). In the FT-IR spectrum of ligand, the peaks that appeared between 1144 and 1196 cm−1 represent ν(C–F) frequencies of perfluoroalkylated vic-dioxime. For the Pd complex, the shift of the (C=N) vibration from 1642 cm−1 to lower frequency to 1612 cm−1 is due to N,N-metal coordination. Also, the loss of (O–H) peak and appearance of a new peak that belongs to (O•••H–O) indicate that the vic-oxime group takes part in complexation with Pd metal. FT-IR spectra (Fig. 2) of the Pd precursor and Pd/MWCNT samples were obtained to show whether there is non-reduced Pd precursor that remained in the sample or not. After the reduction process in scCO2, the characteristic peaks of the precursor disappeared and the new peaks have occurred which belong to Pd/MWCNT. So, it can be said that there is not non-reduced form of Pd precursor in the prepared catalyst according to these comparative results.
It was known that fluorinated precursors are more soluble in scCO2 media; the ligand was chosen with perfluoroalkylated tail accordingly (Teoh et al. 2013; Škerget et al. 2011). The solubility of synthesized precursor was determined qualitatively (at 28 MPa and 363.15 K) with a sapphire glass of reactor cell. The significant color change has verified its solubility clearly.
The deposition was carried out at the pressure of 27.6 MPa and temperature of 363.15 K in supercritical CO2 media as shown in Fig. 1. The metal loading amount of the prepared catalyst was determined by the ICP-OES technique and the amount was found to be 7.0 wt%. At the beginning of the deposition, the calculated ratio was 8.0 wt%. It shows that the yield of deposition is almost 90% which is a very satisfactory yield for the scCO2 deposition method.
Distribution and size of palladium nanoparticles onto MWCNT were analyzed by TEM. Figure 3 displays the TEM images of the Pd/MWCNT catalyst. As can be seen, circled areas in the figure represent some of the biggest Pd nanoparticles. On the other hand, the smaller nanoparticles were distributed on MWCNTs more uniformly with an average diameter of 2 nm. In order to verify the chemical composition of the obtained Pd/MWCNT, energy-dispersive X-ray spectroscopy (EDS) analysis was used. The typical SEM result is shown in Fig. 4a. Since the average size of the nanoparticles is less than 10 nm, it is very difficult to use this image for size analysis due to the resolution. Therefore, mapping scanning was employed. Figure 4b indicates that the blue background is the MWCNT and it is decorated with red dots corresponding to the Pd nanoparticles. Figure 4c, d shows the distribution of the elements carbon (C) and palladium (Pd) respectively. All results show that Pd nanoparticles are distributed uniformly onto MWCNT.
The XRD patterns of the MWCNT as a support material and Pd/MWCNT as a catalyst are shown in Fig. 5. The difference between two patterns obviously shows the presence of metallic Pd nanoparticles. The metals on the MWCNT surface are polycrystalline. The observed main diffraction peaks of the Pd nanoparticles on the MWCNTs were as expected at 2θ values of 39.6° for Pd(111); 46.5°, Pd(200); and 68.1°, Pd(220) (Rather et al. 2007). Pd(111) peak showed the highest intensity. According to N. Narayanan, those with (111) facets and sharp edges and corners were known to be the most catalytically active sites of nanoparticles for Suzuki reaction (Narayanan and El-Sayed 2005). The average particle size of the Pd nanoparticles was calculated according to the Scherrer equation taking Pd(111) as the main peak and it was calculated to be 1.4 nm. The calculated particle size from the XRD peak was similar with the size obtained by the TEM image. It was known that the size of nanoparticles plays a key role in the activity of Suzuki C-C cross-coupling reactions (Collins et al. 2014).
In a typical scCO2 deposition method, the mechanism occurs in three steps: the dissolution of the metallic precursor in the scCO2, adsorption of the metallic precursor on the substrate, and reduction of the precursor to its metal form (Erkey 2009). Although all of these steps affect the size of the nanoparticles, the most effective one is the final step in this process. The reduction that occurred in the scCO2 deposition method is generally preferred in a thermal or chemical way. In this study, the chemical reduction was carried out with H2 gas in scCO2 media. After the adsorption of the decomposed precursor molecules onto the active sites of MWCNT, the chemical reduction with H2 gas occurs autocatalytically. The reduction takes place over all surfaces of the support when the hydrogen gas is injected. Then, Pd nanoparticles at the surface of MWCNT continue to grow until the entire metal precursor or hydrogen in scCO2 phase is consumed (Ulusal and Güzel 2018). In addition, the selected metal precursor has a large molecular structure and it was suggested that this type of huge molecules comes close to the surface planarly and then adsorbs onto the support. This approach prevents agglomeration of Pd nanoparticles at the surface and allows the size of smaller nanoparticles to be obtained.
Catalysis
For Suzuki C-C cross-coupling reaction, bromobenzene and phenylboronic acid were chosen as the model reactants. The influence of solvents and bases on the cross-coupling reaction was investigated. To optimize the conditions of cross-coupling reaction, the reaction was held on in the presence of 0.5 mol% Pd/MWCNT at 60 °C in air and conversion ratio of C-C coupling reaction is summarized in Table 1. Conversion ratio clearly shows that the best solvent-base combination was methanol and K2CO3 for prepared catalyst on bromobenzene-phenylboronic acid coupling reaction.
G. Collins (Collins et al. 2014) reported that the presence of dissolved O2 plays a key role in the catalytic activity of Suzuki reaction. O2 affects Pd leaching concentration and it also correlated with reactivity. The higher conversion was observed in the aerobic media. It means that molecular O2 is beneficial for catalytic activity. So, in this paper, the catalytic reaction medium selected was air atmospheric.
The Suzuki cross-coupling reactions between phenylboronic acid and iodobenzene catalyzed by MWCNT-supported Pd in methanol under reflux conditions were reported by Pan et al. (2006). The conversion yield in Suzuki C-C coupling reaction was reported to be 96% when the most active aryl halide, iodobenzene, was used. However, the activity with other aryl halides was not noticed.
In Suzuki coupling reactions, due to the high activation barriers of the mechanism steps, heterogeneous Pd nanocatalysts require high temperatures (Li et al. 2013; Molnar 2011; Dumbre et al. 2016). By increasing the reaction temperature, the product yield increases automatically. Improving new Pd nanocatalysts, which are active at room temperature, is one of the most rising research areas. In our work, the temperature effect was investigated at three different temperatures: room temperature and 60 and 90 °C, and the results are given in Fig. 6. As can be seen, the reaction could be catalyzed by Pd/MWNT catalyst at room temperature with 100% percent of yield. Therefore, less energy is required to perform the reaction which is a critical point for industrial applications.
The reusability of Pd/MWCNT was tested at the same reaction conditions except for the reaction time and temperature parameters. This time, the room temperature was selected as reaction temperature and the reaction time automatically changed with 24 h. The results are summarized in Table 2. From the results, the catalyst was reused three times without significant loss of activity. After the third run, the efficiency decreased, but it is still satisfying.
Conclusions
A highly efficient, eco-friendly, and recyclable nanocatalyst was prepared by a scCO2 deposition method. Firstly, the scCO2 soluble organometallic precursor was synthesized and it shows that vic-dioxime is a viable option for use in a supercritical deposition. The synthesized perfluoroalkylated vic-dioxime ligand can be utilized with various transition metals and different application areas.
Using an environmentally friendly method, the deposition was carried out. Prepared catalyst was analyzed by ICP-OES, SEM-EDS, TEM, and XRD. The metal nanoparticles formed were evenly dispersed over the substrate with particle sizes as small as almost 2 nm and they showed polycrystalline shape on the support. The catalytic activity of the prepared catalyst was investigated on Suzuki C-C cross-coupling reaction under mild conditions and 100% of yield was obtained at room temperature. The catalyst was reused three times without any significant loss of catalytic activity.
References
Campelo JM, Luna D, Luque R, Marinas JM, Romero AA (2009) Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2:18–45
Collins G, Schmidt M, O’Dwyer C, Holmes JD, McGlacken GP (2014) The origin of shape sensitivity in palladium-catalyzed Suzuki-Miyaura cross coupling reactions. Angew Chem Int Ed Engl 53:4142–4145
Dumbre D, Choudhary VR, Selvakannan PR (2016) Cu–Fe layered double hydroxide derived mixed metal oxide: environmentally benign catalyst for Ullmann coupling of aryl halides. Polyhedron 120:180–184
Erkey C (2009) Preparation of metallic supported nanoparticles and films using supercritical fluid deposition. J Supercrit Fluids 47:517–522
GAO X-h, Sheng WANG, Dian-nan GAO, Wei-gang LIU, Zhi-ping CHEN, Ming-zhe WANG, Shu-dong WANG (2016) Catalytic combustion of methane over Pd/MWCNTs under lean fuel conditions. J Fuel Chem Technol 44:928–936
Hunt AJ, Budarin VL, Comerford JW, Parker HL, Lazarov VK, Breeden SW, Macquarrie DJ, Clark JH (2014) Deposition of palladium nanoparticles in SBA-15 templated silica using supercritical carbon dioxide. Mater Lett 116:408–411
Jiao J, Liu X, Gao W, Wang C, Feng H, Zhao X, Chen L (2009) Synthesis of PbS nanoflowers by biomolecule-assisted method in the presence of supercritical carbon dioxide. Solid State Sci 11:976–981
Keleş M, Ylmaz MK (2014) Synthesis, characterization and catalytic activity of new aminomethyldiphosphine-Pd(II) complexes for Suzuki cross-coupling reaction. Appl Organomet Chem 28:91–94
Koli VB, Dhodamani AG, Delekar SD, Pawar SH (2017) In situ sol-gel synthesis of anatase TiO2-MWCNTs nanocomposites and their photocatalytic applications. J Photochem Photobiol A Chem 333:40–48
Krisyuk VV, Shubin YV, Senocq F, Turgambaeva AE, Duguet T, Igumenov IK, Vahlas C (2015) Chemical vapor deposition of Pd/Cu alloy films from a new single source precursor. J Cryst Growth 414:130–134
Li X-H, Baar M, Blechert S, Antonietti M (2013) Facilitating room-temperature Suzuki coupling reaction with light: Mott-Schottky photocatalyst for C-C-coupling. Sci Rep 3:1743
Liew KH, Samad WZ, Nordin N, Loh PL, Juan JC, Yarmo MA, Yahaya BH, Yusop RM (2015) Preparation and characterization of HypoGel-supported Pd nanocatalysts for Suzuki reaction under mild conditions. Chin J Catal 36:771–777
Magano J, Dunetz JR (2011) Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem Rev 111:2177–2250
Molnar A (2011) Efficient, selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem Rev 111:2251–2320
Narayanan R (2010) Recent advances in noble metal nanocatalysts for Suzuki and Heck cross-coupling reactions. Molecules 15:2124–2138
Narayanan R, El-Sayed MA (2005) Effect of colloidal nanocatalysis on the metallic nanoparticle shape: the Suzuki reaction. Langmuir 21:2027–2033
Pan H-B, Yen CH, Yoon B, Sato M, Wai CM (2006) Recyclable and ligandless Suzuki coupling catalyzed by carbon nanotube-supported palladium nanoparticles synthesized in supercritical fluid. Synth Commun 36:3473–3478
Perez-Coronado AM, Calvo L, Alonso-Morales N, Heras F, Rodriguez JJ, Gilarranz MA (2016) Multiple approaches to control and assess the size of Pd nanoparticles synthesized via water-in-oil microemulsion. Colloids Surf A Physicochem Eng Asp 497:28–34
Pérez-Lorenzo M (2012) Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. The Journal of Physical Chemistry Letters 3:167–174
Rather S-u, Zacharia R, Hwang SW, Naik M-u-d, Nahm KS (2007) Hydrogen uptake of palladium-embedded MWCNTs produced by impregnation and condensed phase reduction method. Chem Phys Lett 441:261–267
Skaf M, Aouad S, Hany S, Cousin R, Abi-Aad E, Aboukaïs A (2014) Physicochemical characterization and catalytic performance of 10% Ag/CeO 2 catalysts prepared by impregnation and deposition–precipitation. J Catal 320:137–146
Škerget M, Knez Ž, Knez-Hrnčič MŠ (2011) Solubility of solids in sub- and supercritical fluids: a review. J Chem Eng Data 56:694–719
Sobhani S, Zarifi F (2015) Pd-isatin Schiff base complex immobilized on γ-Fe2O3 as a magnetically recyclable catalyst for the Heck and Suzuki cross-coupling reactions. Chin J Catal 36:555–563
Taş E, Çukurovali A, Kaya M (1999) The synthesis and characterization of 10, 11-bis (hydroxyimino)-4, 8, 12, 17-tetraaza 1, 2, 19, 20-o-dicyclo-hexylideneoctacosene and some transition metal complexes. J Coord Chem 48:411–423
Teoh WH, Mammucari R, Foster NR (2013) Solubility of organometallic complexes in supercritical carbon dioxide: a review. J Organomet Chem 724:102–116
Torborg C, Beller M (2009) Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv Synth Catal 351:3027–3043
Ulusal F, Güzel B (2018) Deposition of palladium by the hydrogen assisted on SBA-15 with a new precursor using supercritical carbon dioxide. J Supercrit Fluids 133:233–238
Ulusal F, Darendeli B, Erünal E, Egitmen A, Guzel B (2017) Supercritical carbondioxide deposition of γ-alumina supported Pd nanocatalysts with new precursors and using on Suzuki-Miyaura coupling reactions. J Supercrit Fluids 127:111–120
Vilana J, Amade R, Hussain S, Bertran E, Gómez E, Vallés E (2014) 3D distribution of magnetic CoNi alloy nanoparticles electrodeposited on vertically aligned MWCNT showing exceptional coercive field. Mater Lett 124:8–11
Wu T, Zhang P, Ma J, Fan H, Wang W, Jiang T, Han B (2013) Catalytic activity of immobilized Ru nanoparticles in a porous metal-organic framework using supercritical fluid. Chin J Catal 34:167–175
Xiong H, Nolan M, Shanks BH, Datye AK (2014) Comparison of impregnation and deposition precipitation for the synthesis of hydrothermally stable niobia/carbon. Appl Catal A Gen 471:165–174
Yaşar S, ÖZdemir I, ÇEtinkaya B (2008) Heck and Suzuki reactions of aryl halides catalyzed by 1,3-dialkylimidazolinium/palladium. Chin J Catal 29:185–190
Zhang M, Gao Y, Li C, Liang C (2015) Chemical vapor deposition of Pd(C3H5)(C5H5) for the synthesis of reusable Pd@ZIF-8 catalysts for the Suzuki coupling reaction. Chin J Catal 36:588–594
Funding
This study was funded by the Scientific and Technological Research Council of Turkey (TUBITAK, 111T153).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tezcan, B., Ulusal, F., Egitmen, A. et al. Preparation of metallic Pd nanoparticles using supercritical CO2 deposition: An efficient catalyst for Suzuki cross-coupling reaction. J Nanopart Res 20, 145 (2018). https://doi.org/10.1007/s11051-018-4252-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4252-0