Abstract
A new Cu(II) complex supported on magnetic reduced graphene oxide was prepared and characterized by various techniques, such as FT-IR, XRD, SEM, EDX, TEM, TGA, BET, ICP, and VSM. The synthesized nanocomposite, which has size distribution of 25–30 nm, was employed as catalyst in one-pot synthesis of 1-amidoalkyl-2-naphthols via three-component condensation reaction of amides, aromatic aldehydes, and 2-naphthol, under solvent-free conditions. The introduced catalysis procedure for the synthesis of 1-amidoalkyl-2-naphthol derivatives offers several advantages namely, short reaction times, high yields, facile recyclability, and cost effectiveness.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Substantial research attention has been drawn to the newly emerged multi-component reactions (MCRs) in recent years (Safari et al. 2014; Rakhtshah and Salehzadeh 2016; Tayebee et al. 2014). This is because MCRs can offer the possibility of obtaining divers and complex organic molecules from simple building blocks in a single step (Nasr Esfahani et al. 2016; Gupta et al. 2016). Using MCRs in organic synthesis, especially when they are performed under solvent-free conditions sometimes also called as zipper reactions, can practically minimize the reaction times, unwanted by-products, energy, and environmental pollutions (Zhang et al. 2010). The newly developed MCRs, therefore, have been frequently used for the synthesis of some valuable compounds such as 1-amidoalkyl-2-naphthols. There are many reasons for the importance of 1-amidoalkyl-2-naphthol derivatives, including their potentially useful biological and pharmacological properties (Maleki et al. 2016; Moghanian et al. 2014; Taghrir et al. 2016: Kiasat et al. 2013). 1-Amidoalkyl-2-naphthols are commonly prepared via condensation reaction of aliphatic and/or aromatic aldehydes, 2-naphthol, and acetonitrile or amides in the presence of heterogeneous acid catalyst. Several catalysts, either Lewis or Brønsted acids, have been used to initiate this condensation reaction, such as graphene oxide (Gupta et al. 2016), sulfamic acid (Shaterian et al. 2008a, b), FeCl3@SiO2 (Shaterian et al. 2008a, b), heteropoly acids (Khabazzadeh et al. 2009), Fe(HSO4)3 (Shaterian et al. 2008a, b), K5CoW12O40·3H2O (Nagarapu et al. 2007), and Cu(II) acetylacetonate (Khairnar et al. 2016).
A desirable catalyst in any organic reaction has to be environmentally benign, easily recoverable, cost effective, and highly efficient at mild conditions. Most of the so far used catalysts for the synthesis of 1-amidoalkyl-2-naphthols do not meet all these clear-cut criteria. They suffer from some drawbacks, such as long reaction times, low product yields, the use of strongly acidic media, toxic or corrosive reagents, and complex equipment or experimental conditions. Therefore, designing of new catalysis system for the preparation of medicinally important amidoalkyl-2-naphthols, which has the least disadvantages, still remains a challenge (Nandi et al. 2009; Nagarapu et al. 2007; Kantevari et al. 2007; Singha et al. 2015; Zhu et al. 2012; Chen et al. 2013).
On the other hand, graphene, one of the thinnest materials with two-dimensional carbon sheet structure, has inspired enormous interest in diverse fields including catalytic chemistry. The extraordinary and unique properties of graphene make it one of the most favorable material for wide interesting applications (Sheshmani and Amini 2013; Georgakilas et al. 2012; Su et al. 2013; Allen et al. 2009; Guo et al. 2012; Gemeay et al. 2017; Rayati et al. 2017; Li et al. 2013; Rondinone et al. 1999; Chandekar and Kant 2017; Kumar et al. 2013). Moreover, combining of graphene with a magnetic material, such as CoFe2O4, will provide an excellent means for separation of graphene composites via magnetic decantation as well as reducing the agglomeration tendency of graphene sheets (Yan et al. 2010; Xiong et al. 2014; Rakjumar and Rao 2008; Sofia et al. 2009; Zhang et al. 2009). Magnetic nanoparticles (MNPs) deposited on the surface of graphene sheets can provide a suitable inorganic support for designing and constructing novel nanocatalysts. The fascinating feature of nanocatalysts containing MNPs is their ease of separation from the reaction media by applying an external magnet, which eliminates the need for tedious filtration or centrifugation processes. Another benefit of the MNPs presence in a solid catalyst is providing the required high surface area for desired functionalization (Dupont et al. 2002; Virtanen et al. 2010; Hosseini and Asadnia 2012; Jiang et al. 2009; Zheng et al. 2009; Polshettiwar et al. 2011; Lee et al. 2007; Majidi et al. 2006).
We herein report the synthesis of a new composite consisted of Cu(II) complex immobilized on magnetized reduced graphene oxide (RGO) and its application as catalyst for the synthesis of 1-amidoalkyl-2-naphthols. Three-component one-pot condensations of aldehydes, 2-naphthol, and amides were performed in the presence of this prepared nanocatalyst, under solvent-free conditions to give 1-amidoalkyl-2-naphthol derivatives. The synthesized composite, in which the Cu(II) center acts as Lewis acid, exhibited high catalytic activity in these condensation reactions. To the best of our knowledge, this is the first Cu(II) complex supported on magnetic RGO which is used as catalyst for the preparation of 1-amidoalkyl-2-naphthol derivatives.
Experimental
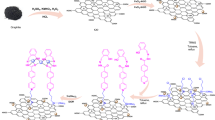
Graphene oxide (GO) was prepared and purified by the Hummers’ method (Rakjumar and Rao 2008) and converted to RGO using sodium borohydride (NaBH4) as reducing agent (Sofia et al. 2009). The RGO/CoFe2O4 composite was synthesized by dispersing of 0.4 g of RGO into 100 mL of deionized water with sonication for 2 h, and to this suspension, 4.04 g of Fe(NO3)3·9H2O and 1.45 g of Co(NO3)2·6H2O, dissolved in 25 mL of water, was added. After adjusting the pH of the mixture at 11–12 with 2 M NaOH and addition of 1.0 g of polyvinylpyrrolidone (PVP), as surfactant, it was heated at 80 °C for 3 h under continuous magnetic stirring. The obtained nanocomposite was collected by applying a permanent magnet and washed with hot water-ethanol and finely powdered after being dried in an oven at 60 °C for 24 h. In the next step, 1.0 g of RGO/CoFe2O4 composite was dispersed in 50 mL of dry toluene using an ultrasonic bath to produce a uniform suspension, and to this solution, 1.0 mL (5.5 mmol) of 3-chloropropyltrimethoxysilane (CPTMS) was added. The mixture was then stirred for 48 h at 80 °C. Finally, the obtained solid product was washed with toluene and dried in an oven to yield CPTMS functionalized RGO/CoFe2O4 (designated as RGO/CoFe2O4/Si-Cl). The Cu(II) complex was immobilized on the surface of RGO/CoFe2O4/Si-Cl as follows: 0.75 g (5.5 mmol) of 4-pyridinecarboxylic acid hydrazide, as ligand (Patel et al. 2013), was added to 1.0 g of well-dispersed RGO/CoFe2O4/Si-Cl in 40 mL of ethanol and the mixture was stirred for 24 h at 60 °C. The resulting solid was mixed with CuCl2·2H2O (0.93 g, 5.5 mmol) in 40 mL of ethanol and stirred for 24 h at 60 °C. The obtained product which is Cu(II) complex anchored on the surface of RGO/CoFe2O4 (designated as RGO/CoFe2O4@Cu(II)) was washed with 60 mL of ethanol (three times each time with 20 mL) and dried in an oven at 60 °C for 24 h.
Synthesis of 1-amidoalkyl naphthols catalyzed by RGO/CoFe2O4@Cu(II)
Of RGO/CoFe2O4@Cu(II), as nanocatalyst, 0.03 g was added to a mixture of 2-naphthol (1 mmol), aldehyde (1 mmol), and amide/urea (1.2 mmol). The mixture was magnetically stirred under solvent-free conditions in an oil bath at 120 °C for a certain period of time. The reaction was stopped when the TLC (hexane/ethyl acetate, 2:5) indicated complete consumption of the starting substrates. After completion, the resultant solid product was washed with hot ethanol and the nanocatalyst was separated by an external magnet and reused as such in new experiment. The obtained solid product of 1-amidoalkyl-2-naphthol was purified by recrystallization in aqueous ethanol solution to give pure product which was identified by comparison of their spectroscopic and physical data with those reported for known samples. The sequential procedure for the synthesis of RGO/CoFe2O4@Cu(II) nanocatalyst is presented in Fig. 1.
Results and discussion
The Cu(II) complex supported on RGO/CoFe2O4, as a heterogeneous nanocatalyst, was characterized with FT-IR, XRD, SEM, EDX, TEM, BET, TGA, and VSM techniques.
In order to confirm the presence of the copper complex on the surface of RGO/CoFe2O4, FT-IR spectra of the as-synthesized materials were obtained and are shown in Fig. 2. The broad band at about 3420–3480 cm−1 was assigned to O-H stretching vibration in all the synthesized compounds. Comparison the spectra of RGO/CoFe2O4 and RGO/CoFe2O4@Si-Cl in Fig. 2A and B reveals an additional strong band at 1000–1200 cm−1 corresponding to the characteristic absorption of Si-O bonds in the latter composite. In the FT-IR spectrum of the RGO/CoFe2O4@Cu(II), the C=O stretching vibration has shifted to lower frequency (1721 cm−l) after coordination of the ligand to copper metal (Fig. 2D). In all these spectra, peaks at about 595 and 421 cm−l were observed which are attributed to the Fe3+-O stretching vibrations of cobalt ferrite.
Figure 3 displays the PXRD patterns of CoFe2O4, RGO/CoFe2O4@Si-Cl, and RGO/CoFe2O4@Cu(II) nano materials. The PXRD pattern of CoFe2O4 (Fig. 3A) shows peaks at 2θ = 30°, 35°, 43°, 54°, 57°, and 63° expected for spinel cubic structure and all these peaks are seen in the PXRD patterns of other synthesized samples (Fig. 3B, C). This indicates that the crystallinity and morphology of CoFe2O4 are preserved during the grafting process. In the PXRD pattern of RGO/CoFe2O4@Si-Cl, peaks at 2θ = 14° and 2θ = 17° are also observed which can be assigned to RGO and silica components, respectively (Sahoo et al. 2013; Tang et al. 2012; Chang et al. 2013). The slight shift observed for these two peaks is probably due to their interactions with magnetic nanoparticles core. The crystallite size of pure CoFe2O4 was 25 nm as estimated by using the well-known Debye-Scherrer formula but increased to 45 nm in the final nanocatalyst.
The SEM and TEM images of RGO/CoFe2O4@Cu(II) are depicted in Fig. 4. The CoFe2O4 nanoparticles, which are well-resolved with almost spherical shapes, anchored with the copper complex can be clearly seen on the surface of RGO (Fig. 4a). Also the transmission electron microscopy (TEM) image of the as-prepared RGO/CoFe2O4@Cu(II) (Fig. 4b) clearly shows that CoFe2O4@Cu(II) on the surface of RGO has almost spherical-shaped morphology. The image indicates that CoFe2O4@Cu(II) (dark spots) was grafted on RGO, and the particles exhibit size distribution of 25–30 nm.
The composition of the as-fabricated RGO/CoFe2O4@Cu(II) nanocomposite was further affirmed by EDX analysis. The EDX spectrum of this composite is shown in Fig. 5 which indicates the presence of copper, as well as other elements such as C, O, N, Cl, and Si existed in the composite. The copper content of the complex supported on RGO/CoFe2O4 was also measured by ICP analysis and was found to be about 1.7%. The chloride content of RGO/CoFe2O4@Cu(II) was determined by potentiometric titration with silver nitrate. The amount of Cl in this composite was found to be about 2.06% and the potentiometric titration curve is shown in Fig. S2. According to the results obtained from ICP measurement and potentiometric titration, the Cu/Cl mole ratio of the composite is approximately 1:2.
The magnetic properties of CoFe2O4 and RGO/CoFe2O4@Cu(II) were studied by a vibrating sample magnetometer (VSM), and the hysteresis loops are shown in Fig. 6. The VSM curve for RGO/CoFe2O4@Cu(II) nanocomposite shows that the value of saturation magnetization (Ms) is 28.5 emu g−1 which is much less than the Ms value of pure CoFe2O4 (61 emu g−1). The decrease of Ms may be due to the entrapment of CoFe2O4 NPs with the nonmagnetic components including silica and the copper complex. The magnetization of the RGO/CoFe2O4@Cu(II)composite, however, is still sufficiently high to ensure its separation and manipulation under an external magnetic field.
The as-fabricated RGO/CoFe2O4@Cu(II) was also studied by thermogravimetric analysis and its TGA curve is given in Fig. 7. A total weight loss of 47% for the nanocatalyst was observed on heating to 600 °C. This study has been performed at temperature range of 60–1000 °C in air atmosphere. Degassing and solvent evaporation at low temperatures accounts for approximately 4% of the weight loss. The second stage is from 120 to 280 °C with a weight loss of 6%, is due to the release of the organic ligand in the Cu(II) complex. The main weight loss which occurs at temperatures from 280 to 450 °C corresponds to the combustion of residue organic ligands as well as decomposition of the composite (37%). DTA analysis of RGO/CoFe2O4@Cu(II) is shown in Fig. S1.
Nitrogen adsorption/desorption is a common method for characterization of mesoporous materials which provides information about the specific surface area, average pore diameter, and pore volume. N2 adsorption-desorption isotherm and BJH pore plot of the as-prepared nanocatalyst are shown in Fig. 8. The BET surface area, pore volume, and pore diameter of RGO/CoFe2O4@Cu(II) are found to be 60.011 m2 g−1, 0.25 cm3, and 16.16 nm, respectively. The observed isotherm for RGO/CoFe2O4@Cu(II) corresponds to type(III)adsorption isotherm, which can also explain the formation of multilayer structure for the nanocomposite.
Evaluation of catalytic activity of RGO/CoFe2O4@Cu(II)
The capability of RGO/CoFe2O4@Cu(II) nanocomposite as a heterogeneous catalyst was evaluated in the synthesis of 1-amidoalkyl-2-naphthol derivatives. To find optimized conditions for the reaction, various parameters, including catalyst dose, type of solvent, and different temperatures, were probed. The model reaction, condensation of benzaldehyde with 2-naphthol and acetamide, was checked with two different amounts of nanocatalyst (0.015 and 0.03 g) and better result was obtained with 0.03 g dose of catalyst, under solvent-free conditions. This reaction proceeds only in the presence of the catalyst because no new product was detected in the absence of this catalyst (see Table 1). The reaction was also studied in different solvents, such as EtOH, CH3CN, CH2Cl2, CHCl3, and n-hexane to compare with the solvent-free conditions. It was found that solvent-free conditions give better results in respect of reaction times and yields (Table 2). The effect of temperature was also checked and based on the obtained results 120 °C was selected for all the examined reactions (see Table 1). The condensation of various amides and aldehydes with 2-naphthol under the optimum conditions was then carried out in the presence of RGO/CoFe2O4@Cu(II) as catalyst. In all the examined reactions, aminoalkylnaphthols were found to be the only products and no other by-products were observed.
In this study, it was also found that aromatic aldehydes containing either electron-donating or electron-withdrawing groups give the target product with high yield. However, aromatic aldehydes with electron-donating groups (OH, N(CH3)2, CH3) required longer reaction times compared to those aldehydes bearing electron-withdrawing groups (Cl, NO2, Br). These observations are in agreement with the previously reported findings [1–7]. Some of these condensation reactions were performed using urea or benzamide instead of acetamide and a set can be seen in Table 3, longer reaction times were needed for these substrates to give substantial yields.
In Table 4, the catalytic activity of the as-prepared nanocatalyst was compared with other recently studied catalysts for the synthesis of amidoalkylnaphthol derivatives. The data show that the catalyst of the present work is superior to the other reported catalysts with respect to reaction times and reaction conditions. The superiority of our introduced nanocatalyst will become more evident when its magnetic property is taken into account which provides a facile and efficient separation for the catalyst in the recycling process.
Recycling and reusability of the catalyst
Facile recovery is considered as an important advantage of the heterogeneous catalysts in organic chemistry and industry. In order to test the as-made catalyst reusability, the synthesis of amidoalkylnaphthol derivatives was achieved in the presence of catalytic amount of RGO/CoFe2O4@Cu(II) under optimized reaction conditions. After completion of the reaction, the catalyst was isolated by applying an external magnetic force, followed by washing it for three times with ethanol. After drying the isolated catalyst at 100 °C for 2 h, it was reused in a new reaction with fresh substrates. The results of reusability tests of this catalyst are presented in Table 5, which indicates the recovered catalyst could be reused for at least five successive times with almost no change in its catalytic performance. The amount of copper was also measured in the recycled nanocatalyst after five runs by ICP-AES analysis. It was found that the copper content of the catalyst remained almost unchanged during the catalytic reactions indicating no detectable leaching of the catalyst into the solution. SEM and TEM images of RGO/CoFe2O4@Cu(II), after reusing it for five runs, are presented in Fig. S3, and the PXRD pattern of the same sample is depicted in Fig. S4. These analyses clearly revealed that the morphology and the structure of the used catalyst, after five successive catalytic reactions, are almost the same as fresh catalyst.
Mechanism for the catalysis reactions
Based on the previously reported research (Nasr esfahani et al. 2016; Zhang et al. 2010) and our observation, a plausible mechanism for the formation of amidoalkylnaphthols from 2-naphthol, acetamide and aldehyde was proposed which is presented in Fig. 9. According to this mechanism, the copper complex can act as a Lewis acid which activates the carbonyl group of the aldehyde to form intermediate (I). A subsequent nucleophilic attack by 2-naphthol on the latter intermediate will result in the formation of intermediate (II). Finally, a Michael reaction occurs through the addition of amide or urea to the last intermediate will lead to the production of 1-amidoalkyl-2-naphthol derivative product.
Conclusion
In brief, a straightforward and facile procedure was used to immobilize a Cu(II) complex onto magnetized reduced graphene to construct a novel nanocatalyst. This composite was prepared in order to be employed as catalyst for the synthesis of 1-amidoalkyl-2-naphthols using multicomponent condensation reactions. It was shown that the as-synthesized nanocatalyst can efficiently catalyze the condensation of 2-naphthol, aldehydes, and amides to give excellent yields of 1-amidoalkyl-2-naphthol derivatives. According to the obtained results and the reaction conditions, the current catalysis system can be considered as an efficient, rapid, and green procedure for the synthesis of the biologically and pharmaceutically important substituted amidoalkylnaphthols.
References
Allen MJ, Tung VC, Kaner RB (2009) Chem Rev 110:132–145
Cai Z, Shu C, Peng Y (2014) Magnetically recoverable nano-sized mesoporous solid acid: effective catalysts for the synthesis of 1-amidoalkyl-2-naphthols. Monatsh Chem 145(10):1681–1687. https://doi.org/10.1007/s00706-014-1246-1
Chandekar KV, Kant KM (2017) Superlattices Microstruct.In press. https://doi.org/10.1016/j.spmi.2017.07.023
Chang CF, Truong QD, Chen JR (2013) RETRACTED: Graphene as excellent support for rapid and efficient near infrared-assisted triptic proteolysis. Colloid Surf B 104:221–228. https://doi.org/10.1016/j.colsurfb.2012.11.040
Chen J, Yao B, Li C, Shi G (2013) An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64:225–229. https://doi.org/10.1016/j.carbon.2013.07.055
Dupont J, de Souza RF, PAZ S (2002) Ionic liquid (molten salt) phase organometallic catalysis. Chem Rev 102(10):3667–3692. https://doi.org/10.1021/cr010338r
Gemeay AH, El-Halwagy ME, El-Sharkawy RG, Zaki AB (2017) Chelation mode impact of copper(II)-aminosilane complexes immobilized onto graphene oxide as an oxidative catalyst. J Environ Chem Eng 5(3):2761–2772. https://doi.org/10.1016/j.jece.2017.05.020
Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, Hobza P, Zboril R, Kim KS (2012) Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev 112(11):6156–6214. https://doi.org/10.1021/cr3000412
Guo J, Li Y, Zhu S, Chen Z, Liu Q, Zhang D, Moon WJ, Song DM (2012) Synthesis of WO3@Graphene composite for enhanced photocatalytic oxygen evolution from water. RSC Adv 2(4):1356–1363. https://doi.org/10.1039/C1RA00621E
Gupta A, Kour D, Gupta VK, Kapoor KK (2016) Graphene oxide mediated solvent-free three component reaction for the synthesis of 1-amidoalkyl-2-naphthols and 1,2-dihydro-1-arylnaphth[1,2-e][1,3]oxazin-3-ones. Tetrahedron Lett 57(43):4869–4872. https://doi.org/10.1016/j.tetlet.2016.09.067
Hosseini SH, Asadnia A (2012) Synthesis, characterization, and microwave-absorbing properties of polypyrrole/MnFe2O4 nanocomposite. J Nanomater 2012:1–6. https://doi.org/10.1155/2012/198973
Jiang WQ, An LT, Zou JP (2008) Molybdophosphoric acid: an efficient Keggin-type heteropoloacid catalyst for the one-pot three-component synthesis of 1-amidoalkyl-2-naphthols. Chin J Chem 26(9):1697–1701. https://doi.org/10.1002/cjoc.200890307
Jiang Y, Guo C, Xia H, Mahmood I, Liu C, Liu H (2009) Magnetic nanoparticles supported ionic liquids for lipase immobilization: enzyme activity in catalyzing esterification. J Mol Catal B 58(1-4):103–109. https://doi.org/10.1016/j.molcatb.2008.12.001
Kantevari S, Vuppalapati SVN, Nagarapu L (2007) Montmorillonite K10 catalyzed efficient synthesis of amidoalkyl naphthols under solvent free conditions. Catal Commun 8(11):1857–1862. https://doi.org/10.1016/j.catcom.2007.02.022
Khabazzadeh H, Saidi K, Seyedi N (2009) Cu-exchanged heteropoly acids as efficient and reusable catalysts for preparation of 1-amidoalkyl-2-naphthols. J Chem Sci 121(4):429–434. https://doi.org/10.1007/s12039-009-0050-7
Khairnar BJ, Girase PS, Mane DV, Chaudhari BR (2016) Der Pharma Chemica 8:137–141
Kiasat AR, Mouradzadegun A, Saghanezhad SJ (2013) Poly(4-vinylpyridinium butane sulfonic acid) hydrogen sulfate: an efficient, heterogeneous poly(ionic liquid), solid acid catalyst for the one-pot preparation of 1-amidoalkyl-2-naphthols and substituted quinolines under solvent-free conditions. Chin J Catal 34(10):1861–1868. https://doi.org/10.1016/S1872-2067(12)60659-7
Kumar ER, Jayaprakash R, Kumar TA, Kumar S (2013) Effect of reaction time on particle size and dielectric properties of manganese substituted CoFe2O4 nanoparticles. J Phys Chem Solids 74(1):110–114. https://doi.org/10.1016/j.jpcs.2012.08.008
Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG (2007) Suh JS, Cheon. J Nat Med 13(1):95–98. https://doi.org/10.1038/nm1467
Li Z, Wu S, Ding H, Zheng D, Hu J, Wang X, Huo Q, Guan J, Kan Q (2013) Immobilized Cu(II) and Co(II) salen complexes on graphene oxide and their catalytic activity for aerobic epoxidation of styrene. New J Chem 37(5):1561–1568. https://doi.org/10.1039/c3nj00099k
Majidi RF, Sanjani NS, Agend F (2006) Encapsulation of magnetic nanoparticles with polystyrene via emulsifier-free miniemulsion polymerization. Thin Solid Films 515(1):368–374. https://doi.org/10.1016/j.tsf.2005.12.102
Maleki B, Sheikh E, Seresht ER, Eshghi H, Ashrafi SS, Khojastehnezhad A, Veisi H (2016) One-pot synthesis of 1-amidoalkyl-2-naphthols catalyzed by polyphosphoric acid supported on silica-coated NiFe2O4 nanoparticles. Org Prep Proc Int 48(1):37–44. https://doi.org/10.1080/00304948.2016.1127098
Moghanian H, Mobinikhaledi A, Blackman AG, Sarough-Farahani E (2014) Sulfanilic acid-functionalized silica-coated magnetite nanoparticles as an efficient, reusable and magnetically separable catalyst for the solvent-free synthesis of 1-amido- and 1-aminoalkyl-2-naphthols. RSC Adv 4(54):28176–28185. https://doi.org/10.1039/C4RA03676J
Nagarapu WL, Baseeruddin M, Apuri S, Kantevari S (2007) Potassium dodecatungstocobaltate trihydrate (K5CoW12O40·3H2O): A mild and efficient reusable catalyst for the synthesis of amidoalkyl naphthols in solution and under solvent-free conditions. Catal Commun 8(11):1729–1731. https://doi.org/10.1016/j.catcom.2007.02.008
Nagawade RR, Shinde DB (2007) Sulphamic acid (H2NSO3H)-catalyzed multicomponent reaction of β-naphthol: an expeditious synthesis of amidoalkyl naphthols. Chin J Chem 25(11):1710–1714. https://doi.org/10.1002/cjoc.200790316
Nandi GC, Samai S, Kumar R, Singh MS (2009) Atom-efficient and environment-friendly multicomponent synthesis of amidoalkyl naphthols catalyzed by P2O5. Tetrahedron Lett 50(51):7220–7222. https://doi.org/10.1016/j.tetlet.2009.10.055
Nasr esfahani Z, Kassaee MZ, Eidi E (2016) Homopiperazine sulfamic acid functionalized mesoporous silica nanoparticles (MSNs-HPZ-SO3H) as an efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphthols. New J Chem 40(5):4720–4726. https://doi.org/10.1039/C5NJ02974K
Patel RN, Sondhiya VP, Shukla KK, Patel DK, Singh Y (2013) Synthesis, crystal structure, electrochemical and bioactivities of pyridine-2-carboxylato bridged copper(II) complexes. Polyhedron 50(1):139–145. https://doi.org/10.1016/j.poly.2012.10.027
Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset JM (2011) Magnetically recoverable nanocatalysts. Chem Rev 111(5):3036–3075. https://doi.org/10.1021/cr100230z
Rakhtshah J, Salehzadeh S (2016) β-cyclodextrin-monosulphonic acid catalyzed efficient synthesis of 1-amidoalkyl-2-naphthols. Appl Organomet Chem 31(2):1–9. https://doi.org/10.1002/aoc.3690
Rakjumar T, Rao GR (2008) Investigation of hybrid molecular material prepared by ionic liquid and polyoxometalate anion. J Chem Sci 120(6):587–594. https://doi.org/10.1007/s12039-008-0089-x
Rayati S, Khodaei E, Shokoohi S, Jafarian M, Elmi B, Wojtczak A (2017) Cu-Schiff base complex grafted onto graphene oxide nanocomposite: synthesis, crystal structure, electrochemical properties and catalytic activity in oxidation of olefins. Inorg Chim Acta 466:520–528. https://doi.org/10.1016/j.ica.2017.07.013
Rondinone AJ, Samia ACS, Zhang ZJ (1999) Superparamagnetic relaxation and magnetic anisotropy energy distribution in CoFe2O4 spinel ferrite nanocrystallites. J Phys Chem B 103(33):6876–6880. https://doi.org/10.1021/jp9912307
Safari J, Zarnegar Z (2014) Synthesis of amidoalkyl naphthols by nano-Fe3O4 modified carbon nanotubes via a multicomponent strategy in the presence of microwaves. Ind Eng Chem Res 20(4):2292–2297. https://doi.org/10.1016/j.jiec.2013.10.004
Sahoo B, Devi KSP, Kumar Sahu S, Nayak S, Maiti TK, Dhara D, Pramanik P (2013) Facile preparation of multifunctional hollow silica nanoparticles and their cancer specific targeting effect. Biomater Sci 1(6):647–657. https://doi.org/10.1039/c3bm00007a
Selvam NP, Perumal PT (2006) A new synthesis of acetamido phenols promoted by Ce(SO4)2. Tetrahedron Lett 47(42):7481–7484. https://doi.org/10.1016/j.tetlet.2006.08.038
Shaterian HR, Yarahmadi H (2008) A modified reaction for the preparation of amidoalkyl naphthols. Tetrahedron Lett 49(8):1297–1300. https://doi.org/10.1016/j.tetlet.2007.12.093
Shaterian HR, Hosseinian A, Yarahmadi H, Ghashang M (2008a) Alumina sulfuric acid: an efficient heterogeneous catalyst for the synthesis of amidoalkyl naphthols. Lett Org Chem 5(4):290–295. https://doi.org/10.2174/157017808784049524
Shaterian HR, Yarahmadi H, Ghashang M (2008b) An efficient, simple and expedition synthesis of 1-amidoalkyl-2-naphthols as ‘drug like’ molecules for biological screening. Bioorg Med Chem Lett 18(2):788–792. https://doi.org/10.1016/j.bmcl.2007.11.035
Sheshmani S, Amini R (2013) Carbohydr Polym 95:348–359
Singha RK, Balaa R, Duvedia R, Kumar S (2015) Iran J Catal 5:187–206
Sofia LTA, Krishnan A, Sankar M, Kala Raj NK, Manikandan P, Rajamohanan PR, Ajithkumar TG (2009) Immobilization of phosphotungstic acid (PTA) on imidazole functionalized silica: evidence for the nature of PTA binding by solid state NMR and reaction studies. J Phys Chem C 113(50):21114–21122. https://doi.org/10.1021/jp906108e
Su DS, Perathoner S, Centi G (2013) Nanocarbons for the development of advanced catalysts. Chem Rev 113(8):5782–5816. https://doi.org/10.1021/cr300367d
Taghrir H, Ghashang M, Bireghan MN (2016) Preparation of 1-amidoalkyl-2-naphthol derivatives using barium phosphate nano-powders. Chin Chem Lett 27(1):119–126. https://doi.org/10.1016/j.cclet.2015.08.011
Tang Y, Huang F, Zhao W, Liu Z, Wan D (2012) Synthesis of graphene-supported Li4Ti5O12 nanosheets for high rate battery application. J Mater Chem 22(22):11257–11260. https://doi.org/10.1039/c2jm30624g
Tayebee R, Amini MM, Rostamian H, Aliakbari A (2014) Preparation and characterization of a novel Wells–Dawson heteropolyacid-based magnetic inorganic–organic nanohybrid catalyst H6P2W18O62/pyridino-Fe3O4 for the efficient synthesis of 1-amidoalkyl-2-naphthols under solvent-free conditions. Dalton Trans 43(4):1550–1563. https://doi.org/10.1039/C3DT51594J
Virtanen P, Salmi TO, Mikkola JP (2010) Supported ionic liquid catalysts (SILCA) for preparation of organic chemicals. Top Catal 53(15-18):1096–1103. https://doi.org/10.1007/s11244-010-9540-6
Xiong P, Hu G, Fan Y, Zhang W, Zhu J, Wang X (2014) Ternary manganese ferrite/graphene/polyaniline nanostructure with enhanced electrochemical capacitance performance. J Power Sources 266:384–392. https://doi.org/10.1016/j.jpowsour.2014.05.048
Yan X, Chen J, Xue Q, Miele P (2010) Synthesis and magnetic properties of CoFe2O4 nanoparticles confined within mesoporous silica. Microporous Mesoporous Mater 135(1-3):137–142. https://doi.org/10.1016/j.micromeso.2010.07.001
Zhang Y, Zhao Y, Xia C (2009) Basic ionic liquids supported on hydroxyapatite-encapsulated γ-Fe2O3 nanocrystallites: an efficient magnetic and recyclable heterogeneous catalyst for aqueous Knoevenagel condensation. J Mol Catal A 306(1-2):107–112. https://doi.org/10.1016/j.molcata.2009.02.032
Zhang Q, Luo J, Wei Y (2010) A silica gel supported dual acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Green Chem 12(12):2246–2254. https://doi.org/10.1039/c0gc00472c
Zheng X, Luo S, Zhang L, Cheng JP (2009) Green Chem 1:455–458
Zhu X, Lee YR, Kim SH (2012) Facile one-pot synthesis of 1-amidoalkyl-2-naphthols by RuCl2(PPh3)3-catalyzed multi-component reactions. Bull Kor Chem Soc 33(8):2799–2802. https://doi.org/10.5012/bkcs.2012.33.8.2799
Funding
The authors wish to acknowledge the support of this work (grant no. 1396) provided by the Research Council of Shahid Chamran University of Ahvaz, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 990 kb)
Rights and permissions
About this article
Cite this article
Kooti, M., Karimi, M. & Nasiri, E. A novel copper complex supported on magnetic reduced graphene oxide: an efficient and green nanocatalyst for the synthesis of 1-amidoalkyl-2-naphthol derivatives. J Nanopart Res 20, 16 (2018). https://doi.org/10.1007/s11051-017-4107-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-4107-0