Abstract
The eco-toxicological effects of unconventionally prepared nanostructured TiO2 and ZnO were evaluated in this study, since both oxides are keenly investigated semiconductor photocatalysts in the last three decades. Unconventional processing by pressurized hot water was applied in order to crystallize oxide materials as an alternative to standard calcination. Acute biological toxicity of the synthesized oxides was evaluated using germination of Sinapis alba seed (ISO 11269-1) and growth of Lemna minor fronds (ISO 20079) and was compared to commercially available TiO2 Degussa P25. Toxicity results revealed that synthesized ZnO as well as TiO2 is toxic contrary to commercial TiO2 Degussa P25 which showled stimulation effect to L. minor and no toxicity to S. alba. ZnO was significantly more toxic than TiO2. The effect of crystallite size was considered, and it was revealed that small crystallite size and large surface area are not the toxicity-determining factors. Factors such as the rate of nanosized crystallites aggregation and concentration, shape and surface properties of TiO2 nanoparticles affect TiO2 toxicity to both plant species. Seriously, the dissolution of Ti4+ ions from TiO2 was also observed which may contribute to its toxicity. In case of ZnO, the dissolution of Zn2+ ions stays the main cause of its toxicity.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2) and zinc oxide (ZnO) nanoparticles have general importance as absorbers of ultraviolet (UV) light and broad scale use as pigments in plastics, paints, paper coatings and sunscreen lotions. Both materials are keenly investigated photocatalysts and have been considerably studied for the removal of organic compounds from contaminated air and water and for microbial disinfection (Adams et al. 2006; Coronado et al. 2013; Hoffmann et al. 1995).
The technology and research progress gave rise to manufactured nanomaterials (Laborda et al. 2016). The environmental level of manufactured nanomaterials is expected to increase continually which is given by their current widespread application. It was reported that they may enter natural ecosystems through direct application, biosolid application, accidental release, contaminated soil/sediments or atmospheric fallout (Rico et al. 2011). However, properties of manufactured nanomaterials differ from their bulk counterparts. Materials that are safe in a bulk form can become harmful in nanoscale. It is well known that the type of preparation and used precursors for synthesis can affect the micro(structural) and surface properties of manufactured nanomaterials significantly (Matějová et al. 2017). Toxicity of nanomaterials was addressed by many authors (Hofmann-Amtenbrink et al. 2015; Hougaard et al. 2015; Wang et al. 2016), but the unified methodology and procedures of toxicity measurement do not exist yet. First steps in this field have been done recently (Arts et al. 2015; Rasmussen et al. 2016).
Concerning eco-toxicological effects of ZnO nanoparticles, they have been very limited across all taxa (Kahru and Dubourguier 2010). The studies have identified toxic effects of ZnO nanoparticles in both aquatic and terrestrial species; toxicity can occur at concentration around 1 mg/l. This suggests that ZnO nanoparticles, when reaching a certain level in natural environments, can cause significant risk to the environmental biota (Ma et al. 2013). ZnO nanoparticles cause phytotoxicity in a limited number of crops such as Raphanus sativus, Brassica napus, Lactuca sativa, Zea mays and Cucumis sativus (Lin and Xing 2007), Allium cepa (Kumari et al. 2011) and Vicia faba (Manzo et al. 2011). High doses of ZnO nanoparticles have negative impacts on agricultural ecosystem such as declines in soil quality (Priester et al. 2012), reduces growth and biomass (Yoon et al. 2014) and excessive Zn accumulation in plant tissues and seeds (Mukherjee et al. 2014; Priester et al. 2012). However, there are still several knowledge gaps which need to be filled to gain a thorough understanding on ZnO nanoparticles eco-toxicity for risk assessment and management. First, there is a significant lack of characterizations for ZnO nanoparticles and the exposure system in eco-toxicity studies conducted thus far. Since ZnO nanoparticles can elicit toxicity by different modes of action (i.e. particle dissolution, photo-activation etc.) and these modes of action are highly dependent on exposure conditions such as water chemistry of exposure media, irradiation conditions, a thorough characterization of these exposure conditions is essential for proper interpretation of toxicity data as well as valid comparison between different studies. Second, tools and techniques are needed in order to differentiate between the particle-induced toxicity and dissolved Zn2+ ions effects. Third, there are no sufficient data on chronic effects from long-term and low-concentration exposure, which may be more representative for real environmental exposure (Ma et al. 2013).
Concerning eco-toxicological effects of TiO2 nanoparticles, the fate and the long-term effects of this nanomaterial remain unrevealed as well as its impact and risk assessment are also challenging. TiO2 nanoparticles can interact with both biotic and abiotic components of the environment. These interactions rely mostly on their agglomeration or aggregation state. This determines the size in which they are present in the environment and consequently their potential for transport and sedimentation and for uptake by organisms (Adam et al. 2015). It was reported that TiO2 nanoparticles cause toxicity to organisms by producing reactive oxygen species upon interaction with UV light, leading to cell membrane damage (Manke et al. 2013; Sadiq et al. 2011). Jacob et al. (2013) observed that TiO2 nanoparticles play a key role in the modification of activities of enzymatic antioxidants at concentration of 10 and 30 ppm. Moreover, in spinach seedlings, 0.25% of TiO2 nanoparticles resulted in the generation of oxidative stress in chloroplasts, and TiO2 nanoparticles caused elimination of microtubules in Arabidopsis thaliana (Tripathi et al. 2017).
As it was indicated above, the problem of manufactured nanomaterials’ toxicity is even more complex, since the tests being done on pristine nanoparticles under controlled laboratory conditions do not account for their interaction with the real environment. As Bour et al. (Bour et al. 2015) and Judy et al. (Judy and Bertsch 2014) suggest, the studies of nanomaterial toxicity should be carried out in more realistic conditions, and as Menard et al. (Menard et al. 2011) stresses out, the manufactured nanomaterials’ physicochemical properties should be thoroughly characterized and known.
According to Sun et al. (Sun et al. 2014), one of the real release pathways for manufactured nanomaterials to the environment is their collection within wastewater and their concentration in waste sludge. Waste sludge is then deposited on landfill or used as a fertilizer on agricultural land. The testing subjects therefore have to encompass the whole cycle of the manufactured nanomaterials in the environment and range from microorganisms like bacteria (Barnes et al. 2013; Bellanger et al. 2015; Farkas et al. 2015; Mallevre et al. 2014) and algae (Fu et al. 2015; Schiavo et al. 2016) to cells (Hsiao and Huang 2011), plants (Andersen et al. 2016; Clement et al. 2013; Cox et al. 2016), but also fish (Xiong et al. 2011) or mice (Warheit et al. 2015) and others.
This work focuses on the preparation of nanostructured TiO2 and ZnO by unconventional preparation method using pressurized hot (subcritical) water and evaluation of their acute biological toxicity using germination of Sinapis alba seed (ISO 11269-1, 1993) and growth of L. minor fronds (ISO 20079, 2005). Since for the preparation of both nanomaterials pressurized hot water crystallization was used, the effect of this post-treatment step on nanomaterial toxicity can be excluded, as water is a non-toxic solvent. The advantage of performed acute biological toxicity tests compared to often used acute aquatic toxicity tests according to the OECD 201 methodology using freshwater green algae (Desmodesmus subspicatus, Chlorella vulgaris) is the fact that in the tests with S. alba seed, the nanoparticulated samples do not have to be dissolved in water, thus, the sedimentation of nanoparticulated samples, which can affect the toxicity results, is eliminated. The indicator organism (S. alba seed) and the nanoparticulated sample are left in contact on the filter paper, without the possibility of sedimentation. In tests with L. minor fronds, the concentration series of nanoparticles in suspensions are prepared, but the sedimentation is removed by continuous mixing throughout the exposure. Thus, two various experimental arrangements were used for the determination of toxicity of nanoparticulated samples in our study. In both tests with D. subspicatus and C. vulgaris, the nanoparticulated samples form suspensions after mixing with water and settle down, which can affect the toxicity results. Another fact is the evaluation method. In our tests on S. alba and L. minor, the determination of growth inhibition is feasible, excluding the turbidity of the tested nanoparticulated samples. In the tests, e.g. on D. subspicatus, the results are determined by counting the algal cultures under the microscope, using an automatic cell counting, and this process is influenced highly by the turbidity of the sample.
Experimental
Chemicals
All aqueous solutions for chemical experiments were prepared using deionized water (electrical conductivity ∼0.06–0.08 μS/cm). The reference commercial TiO2 anatase-rutile mixture (TiO2 Degussa P25) for eco-toxicity studies was obtained from Degussa (Germany). Chemical for preparation of nanostructured materials such as titanyl sulphate (TiOSO4) was purchased from Precheza a.s. (Czech Republic). Sulphuric acid (H2SO4, p.a.), sodium hydroxide (NaOH, p.a.), sodium carbonate (Na2CO3, p.a.) and zinc chloride (ZnCl2, p.a.) were purchased from Penta a.s. (Czech Republic).
Preparation of precursors for TiO2 and ZnO nanoparticles preparation
The TiO(OH)2 precursor was prepared via thermal hydrolysis. The stock solution of titanyl sulphate (1.25 mol/dm3) was diluted to 0.2 mol/dm3 solution by addition of 0.1 mol/dm3 sulphuric acid. The 0.2 mol/dm3 solution of titanyl sulphate was heated, the spontaneous precipitation of TiO(OH)2 occurred at 80 °C. The temperature of 80 °C was kept for 1 h. The pH was adjusted with 5 mol/dm3 NaOH at pH = 7. After that, the created suspension was left to cool down to ambient temperature. The suspension was filtered, and the filter cake/precipitate was washed to remove the sulphate anions with 4–5 l of deionized water. The precipitate was then dried in Petri dishes at 40 °C overnight. The obtained precipitate was subsequently processed by pressurized hot water to prepare nanoparticulated TiO2.
The Zn(OH)2 precursor was prepared by neutralization. Na2CO3 was dissolved in water and mixed on an electromagnetic stirrer until the solution was clear. ZnCl2 was added to the Na2CO3 solution and white Zn(OH)2 precipitate started to appear. The Na2CO3 was in 50% surplus to ZnCl2. The addition of ZnCl2 was gradual because of CO2 production. The mixture was stirred for 3 h, filtered and washed with deionized water until the pH was 7. The precipitate was dried overnight at 50 °C and powdered in a mortar. The obtained precipitate was subsequently processed by pressurized hot water to prepare nanoparticulated ZnO.
Both precursors were sieved for high-pressure processing to particle-size fraction of 0.160–0.315 mm.
Processing of precursors by pressurized hot (subcritical) water
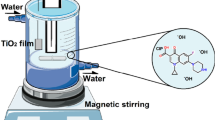
Deionized water (electrical conductivity ∼0.06–0.08 μS/cm) was used as a solvent for pressurized hot water processing. Processing by pressurized hot water was carried out in a laboratory-made unit equipped with a HPLC BETA10 Plus gradient pump (Ecom s.r.o., Czech Republic), a chromatographic oven operating in the temperature range of 25–400 °C, a capillary cooling and a restrictor operating at ambient temperature. A scheme of the experimental setup is shown in Fig. 1a. The TiO2 precursor was placed in a 24-ml high-temperature stainless-steel cell and was processed in a flow regime at pressure of 10 MPa and temperature of 100 °C using 1.05 l of deionized water. The ZnO precursor was placed in a 10-ml high-temperature stainless-steel cell and was processed in a flow regime at pressure of 30 MPa and temperature of 250 °C using 1.5 l of deionized water. The flow rate of water during the high-pressure processing was kept at 3.5–4.5 ml/min. Since both precursors were powders, their special arrangement in the high-temperature stainless-steel cell was used (Fig. 1b) to prevent the plugging of frits inside the cell. The different experimental conditions of processing for both precursors by pressurized hot water were selected based on previous photocatalytic investigations in AO7 photodegradation, when both oxides at selected processing conditions were the most photoactive.
Acute biological toxicity tests
The acute biological toxicity of prepared nanomaterials (TiO2, ZnO, TiO2 Degussa P25) was determined using the L. minor plant growth test and the S. alba seed germination test. For toxicity tests, the nanoparticulated materials were crashed and sieved to particle-size fraction of <0.160 mm.
The L. minor plant test (ISO 20079, 2005) measured the growth inhibition of fronds in the presence of nanoparticulated material compared to the control samples containing only the culture medium. The tests were performed in the growth chamber with the light luminosity of 10,000 lx. L. minor fronds grow as monocultures in different concentrations of the tested substance over a period of 7 days. The objective of the test is to quantify the substance-related effects on vegetative growth over this period based on assessments of frond number and also on assessments of biomass. To quantify the substance-related effects, the growth in the tested solutions is compared with that of the controls and the concentration bringing the specified 50% inhibition of growth is determined and expressed as the EC50 value (Quality 2005).
In the seed germination test, the S. alba (ISO 11269-1, 1993) was used. Inhibition of root growth after 3 days exposure was measured. The tests with S. alba were performed in the darkness in the tempered bath at constant temperature of 21 °C. The test was considered to be valid if the germination of the control sample was ≥90%, and the standard deviation (SD) value was inferior to the double SD value measured in the control (containing only the culture medium). The inhibition concentration value IC50 was derived after plotting the percentage of inhibition of root growth against the concentration.
The nanoparticulated materials were tested in a concentration range 0.01–10 mg/ml for L. minor and 1–180 mg/ml for S. alba.
The positive controls using toxicants recommended in the corresponding ISO standard: 3.5-dichlorophenol and potassium dichromate were also measured to check the sensitivity of the individual tests. The acute biological toxicity tests were performed three times in parallel repetitions.
Characterization of investigated nanoparticulated materials
Nitrogen physisorption at 77 K was performed on a 3Flex automated volumetric apparatus (Micromeritics Instruments, USA) after degassing of materials at 150 °C for more than 18 h under vacuum below 1 Torr. Degassing at low temperature was applied to remove physisorbed water but having no influence on the porous morphology of the developed materials. The specific surface area, S BET, was calculated according to the classical Brunauer–Emmett–Teller (BET) theory for the p/p0 range of 0.05–0.30 (Gregg and Sing 1982). As the specific surface area, S BET, is not a proper parameter in the case of mesoporous solids containing micropores (Schneider 1995), the mesopore surface area, S meso, and the micropore volume, V micro, were also evaluated based on the t-plot method (de Boer et al. 1966) with the C modif constant (Lecloux and Pirard 1979; Schneider 1995). The net pore volume, V net, was determined from the nitrogen adsorption isotherm at maximum p/p0 (∼0.99). The pore-size distribution was evaluated from the adsorption branch of the nitrogen adsorption-desorption isotherm by the Barrett–Joyner–Halenda (BJH) method (Barrett et al. 1951) using the de Boer standard isotherm and assuming cylindrical pore geometry.
X-ray diffraction (XRD) patterns were recorded using a Bruker D8 Advance diffractometer (Bruker AXS) equipped with a fast position-sensitive detector VÅNTEC 1. CoKα irradiation (λ = 0.178897 nm) was used. Measurements of all samples were carried out in reflection mode in symmetrical Bragg–Brentano arrangement.
Fourier-transform infrared (FTIR) spectra were recorded in the range of 400–4000/cm. Samples were measured by ATR technique with diamond crystal on Nicolet 6700 FTIR (Thermo Scientific, USA).
Raman spectra were collected on a XploRA™ Smart System composed of microscope and Raman spectrometer (Horiba Jobin Yvon, France) using 532 nm laser source. The Olympus microscope BX 41/51 with an objective magnification of 50 was used to focus the laser beam on the sample placed on an X–Y motorized sample stage. The filter to reduce laser beam to 25% of initial laser beam and grating 1200 grooves/mm were used.
Scanning electron microscopy with chemical analysis (SEM-EDX) was performed using a Philips XL30 scanning electron microscope (SEM) with energy dispersive X-ray microanalysis (EDX). The SEM images were obtained using back-scattered electrons at an operating voltage of 25 kV.
Transmission electron microscopy (TEM) analysis was done on a JEOL 2100 at 200 kV of accelerating voltage. Prior to analysis, purified and ultra-sonified water for 3 min was added to powder sample placed in small Eppendorf tube. Suspensions were dropped on a copper grid with holey carbon film and dried on air.
The samples of aqueous growing media from L. minor tests containing specified weight to volume ratios of ZnO and TiO2 were measured on a ContrAA 700 atomic absorption spectrometer (AAS) (Analytik Jena, Germany) by electrothermal atomization technique in with L’vov platform tube.
Results and discussion
Characterization of prepared nanoparticulated materials
Evaluated textural and (micro)structural properties of both prepared materials from nitrogen physisorption and XRD measurements, respectively, are summarized in Table 1 (Matejova et al. 2013) and Fig. 2a, b. While prepared TiO2 shows mesoporous structure with 165 m2/g mesopore surface area and minor contribution of micropores, ZnO shows mesoporous-macroporous structure possessing low surface area of 17 m2/g. The structural properties correspond well with evaluated textural properties; TiO2 of anatase crystal structure is nanocystalline with ∼7 nm anatase crystallites (Fig. 2a), while ZnO of wurtzite crystal structure possesses ∼114 nm crystallites. Moreover, beside ZnO wurtzite also Zn2SiO4 willemite of ∼88 nm crystallite size was clearly identified in prepared ZnO (Table 1, Fig. 2b). The presence of Zn2SiO4 willemite (RRUFF 2016) can be explained by preparation procedure; during high-pressure processing using pressurized hot water, Zn2+ ions were released to hot water from the Zn(OH)2 precursor and these Zn2+ ions reacted with SiO2 present in the glass balls which were used as filling substrate in the extraction cell (glass balls were mainly composed of SiO2, Na2O and CaO).
Measured FTIR spectra of TiO2 precursor and nanoparticulated TiO2 are shown in Fig. 3. From the spectra (Fig. 3), it is evident that there are no significant differences between these materials. The most intensive bands at 3201 cm-1 and 1629 cm-1 correspond to the O-H stretching and deformation vibrations, respectively. Broad band under 1000 cm-1 has similar progress as ordinary TiO2 anatase spectrum. The Raman spectra in Fig. 4 prove the similarity of TiO2 precursor and nanoparticulated TiO2. Both spectra show all characteristic bands of anatase modification of TiO2. All these facts indicate that in case of preparation of nanoparticulated TiO2 by thermal hydrolysis using titanyl sulphate, the TiO2 anatase crystallization occurs already during the thermal hydrolysis preparation of the precursor.
In Fig. 3, the FTIR spectra of ZnO precursor and nanoparticulated ZnO are shown as well. A broad and intensive band at 3374 cm-1 and shoulder at 1648 cm-1 belong to the O-H vibrations. According to all presented bands in the spectrum of ZnO precursor (Fig. 3c), it can be estimated that the ZnO precursor is composed of hydrozincite (Zn5(OH)6(CO3)2), which being often used precursor. FTIR spectrum of ZnO (Fig. 3d) shows two characteristic bands at 420 and 486 cm-1 which are not clearly visible in the spectrum. On the other hand, a strong structured band around 900 cm-1 is presented in the spectrum and corresponds to the willemite (Zn2SiO4), which presence was proved also by XRD. Similar conclusions were obtained from Raman measurements (Fig. 4), where the most intensive band at 1080 cm-1 of ZnO precursor belongs to the presence of carbonate (Fig. 4c). The Raman spectrum of ZnO (Fig. 4d) corresponds to the typical ZnO spectrum. No bands corresponding to Zn2SiO4 willemite were observed, but this feature may be caused by strong Raman signal of ZnO as well as by used 532 nm laser.
The surface composition and morphology of prepared TiO2 and ZnO clusters were studied using SEM-EDX technique. EDX spectra of both materials are shown in Fig. 5a, b and reveal that TiO2 nanoparticles contain only Ti and O elements, contrary to ZnO nanoparticles which contain besides Zn and O element also Si element. Results from EDX analysis prove the XRD results, where Zn2SiO4 willemite was identified beside ZnO wurtzite in prepared ZnO. SEM images at ×1000 magnification shown in Fig. 5c, d reveal a significantly different morphology of TiO2 and ZnO clusters. While conjunction of very fine nanoparticles of TiO2 anatase forms spherical TiO2 clusters, the nanoparticles of major ZnO wurtzite and minor Zn2SiO4 willemite form needle-like clusters and single needles. The surface energy of both materials was increased due to their small crystallite size and this feature led to agglomeration of crystallites.
TEM images and the evaluated TiO2 anatase crystallite size distribution (Fig. 6a–c) correspond nicely to XRD results, proving the highest population of TiO2 anatase crystallites of ∼7 ± 2 nm size.
Acute biological toxicity
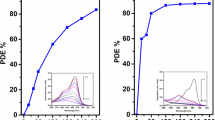
Acute biological toxicity tests on plants L. minor and S. alba showed toxic effects of both prepared nanostructured materials, TiO2 as well as ZnO. However, ZnO demonstrated a significantly higher toxicity than TiO2 (Fig. 7a, b).
Concerning the tests with L. minor, the inhibition of growth rate of L. minor was between 8.76 and 81.68% for TiO2 and between 46.79 and 63.54% for ZnO. The inhibition of the weight of the final biomass of L. minor ranged from 31.90 to 91.48% for TiO2 and from 21.73 to 86.63% for ZnO. Thus, the resulting EC50 toxicity values calculated from the inhibition growth rate of L. minor are following: 5.215 ± 0.138 mg/ml for TiO2 and 1.839 ± 0.161 mg/ml for ZnO.
Concerning the tests with S. alba, the inhibition of S. alba root growth for TiO2 was observed in the range of 12.01–60.48% and for ZnO in the range of 30.04–76.07%. The resulting IC toxicity values calculated from the inhibition of root growth are following: 172.853 ± 22.160 mg/ml for TiO2 and 1.532 ± 0.930 mg/ml for ZnO. TiO2 showed significantly lower toxic effect on seeds of S. alba compared to proven toxicity on L. minor.
The commercially available TiO2 Degussa P25 did not show any toxic effects in both bioassays used, conversely to that the stimulation of L. minor frond growth was observed.
For comparison of the intensity of toxic effect, toxicological indexes EC50 and IC50 of nanoparticulated materials were compared with the positive controls of reference substances (3,5-dichlorophenol and potassium dichromate) and all results are summarized in Table 2.
Discussion of the obtained results and aspects related to toxicity of nanoparticulated ZnO, TiO2 and commercially available TiO2 Degussa P25 to L. minor and S. alba
Arruda et al. reported in their review (Arruda et al. 2015) that the mechanism of nanotoxicity is not still revealed. On the other hand, they reported that the nanotoxicity may be related to the chemical composition, chemical structure, particle-size and surface area of nanoparticles. The nanoparticles’ toxicity may be explained by the following phenomena: (1) the chemical toxicity caused by the chemical composition, e.g. the release of (toxic) ions and (2) the stress or stimuli caused by the nanoparticles surface, size and/or shape. It was proved that the solubility of oxide nanoparticles markedly influences the response of cell culture, and it was revealed that the nanoparticles-mediated toxicity cannot be exclusively attributed to the release of dissolved components of nanoparticles. They emphasized the fact that in many of studies both was not evaluated, the impacts of ions released from the nanoparticles as well as the impacts of nanoparticles on plants, thus, the gained results about nanoparticles toxicity may be confusing.
With respect to toxicity of nanoparticulated ZnO to L. minor, Chen et al. (Chen et al. 2016) investigated the toxicity of nanosized ZnO to L. minor via modulation of nanosized ZnO dissolution by either modification of the pH of the growth medium and/or surface coating of nanosized ZnO and evaluated the impacts on the growth and physiology of L. minor. Chen et al. revealed that nanosized ZnO was dissoluted quickly and completely in the medium at pH 4.5. Moreover, quantitatively similar toxic impacts were determined when L. minor was exhibited to nanosized ZnO as well as to the dissolved Zn equivalent of dissolved nanosized ZnO. Their conclusions that the toxicity of nanosized ZnO can be attributed to dissolved Zn2+ ions was further supported by the results that the phytotoxicity was missing in medium of higher pH values (>7). In those media, the dissolution of nanosized ZnO practically stopped. The decreased toxicity of coated nanosized ZnO, where the slower dissolution of Zn2+ ions took place, corresponded to the main role of dissolved Zn2+ ions in nanotoxicity of ZnO. Their results about the main role of released Zn2+ ions causing ZnO nanotoxicity support our obtained results. In our case, the synthesized ZnO is a mixture of larger nanoparticles of ∼114 nm ZnO wurtzite and ∼84 nm Zn2SiO4 willemite, having the surface area of 17 m2/g. In spite of these larger crystallites of both crystalline phases, the toxicity of synthesized ZnO was significantly higher compared to “true” nanoparticulated TiO2 anatase (∼7 nm crystallite size, 240 m2/g) especially to S. alba. Concerning the tests to L. minor, the toxicity of ZnO was even three times higher than of nanoparticulated TiO2 (Table 2). Within the tests with L. minor, it was proved by AAS analysis that all nutrient aqueous solutions analysed after toxicity tests with ZnO contained the dissolved Zn2+ ions (Fig. 7b). The pH of the solutions moved between 6.5 and 7. Thus, our results support the fact that concerning the ZnO toxicity, the nanosize of crystallites and their surface area does not play the key role and the dissolution of Zn2+ ions to the nutrient medium contribute significantly to ZnO toxicity.
Li et al. (2013) examined the toxicity of nanoparticulated TiO2-P25 (from Evonik industries AG, Essen, Germany), being the equivalent to investigated TiO2 Degussa P25 in our study, to L. minor. In tests in order to exclude the nanoparticles aggregation and to achieve the L. minor exhibition to TiO2 nanoparticles, they applied diluted growth medium. TiO2 nanoparticles did not demonstrated any unfavourable impact on the growth rate of L. minor, even at a high exposure concentration of 5 mg/l and extended exposure time of 14 days. Despite of TiO2 nanoparticles stuck to L. minor cell walls, no cellular uptake was observed. They concluded that albeit TiO2 nanoparticles were not toxic to L. minor, there still exists the possibility of the transfer of TiO2 nanoparticles in aquatic food chains and thus it can contribute to environmental negative impact of nanoparticles. In our study, without doing any additional dilution of growth medium and tests being done exactly according to ISO 20079 (2005) with L. minor, the stimulation of growth medium was observed in the presence of TiO2 Degussa P25 nanoparticles at concentrations of nanoparticles above 6 mg/ml. Based on Li et al. observations (Li et al. 2013), it can be said in our case the aggregation of TiO2 Degussa P25 nanoparticles definitively carried out in growth medium during the toxicity tests. Moreover, AAS analysis did not prove any dissolved Ti4+ ions in all nutrient solutions analysed after toxicity tests with TiO2 Degussa P25. Thus, our results support and broaden the conclusions of Li et al. (2013) that TiO2 Degussa P25 nanoparticles show no toxic effect to L. minor; even in aggregated form, they stimulate the growth of L. minor fronds.
Concerning the toxicity of synthesized nanoparticulated TiO2 anatase, which toxicity was even about two orders lower to S. alba and three times lower to L. minor compared to ZnO (Table 2), it can be said that the exclusive effect of nanosize of TiO2 crystallites or its high surface area on the toxicity to both plant species cannot be proved. In our case, the part of ∼7-nm anatase crystallites was aggregated and the part stayed separately as nanoparticles, but these conditions do not affect the nanotoxicity of TiO2 anatase crystallites in such a way to make it more toxic than ZnO to investigated plant species. On the other hand, with respect to TiO2 toxicity, synthesized nanoparticulated TiO2 anatase (∼7 nm size) was toxic to both tested plant species contrary to TiO2 Degussa P25 (anatase of ∼25 nm size, rutile of ∼54 nm size) which contrarily stimulated the growth of L. minor fronds and showed no toxic effect to S. alba seed. Thus, it can be concluded that in case of TiO2 nanotoxicity, the complex effect of the rate of aggregation of nanosized TiO2 crystallites, size, shape and surface properties will play the role in the stress and stimuli caused to both plant species. Moreover, from our results (Fig. 7a, b), it is evident that the toxicity of nanoparticulated TiO2 depends significantly on the concentration of nanoparticles, being in contact with plant species. In case of TiO2 anatase, its toxicity increased markedly from the nanoparticles concentration of 45 mg/ml for S. alba (Fig. 7a). Our observations about the effect of nanoparticles concentration on germination of S. alba are only in partial agreement with observations from Hatami et al. (2014). They reported contrarily on the stimulatory effect of nanosized TiO2 anatase (of 10–15 nm crystallite size) on germination of S. alba in concentration range of 20–40 mgNPs/ml. In tests with L. minor, the TiO2 anatase toxicity was increasing gradually with increasing nanoparticles concentration in the range of 0.01–10 mg/ml (Fig. 7b). Within tests with L. minor, AAS analysis of nutrient aqueous solutions analysed after toxicity tests, surprisingly, revealed that solutions with TiO2 nanoparticle concentrations >6 mg/ml contained high concentrations of dissolved Ti4+ ions (Fig. 7b). Namely, Ti4+ ions concentrations were the following: 6.5 ± 0.2 mg/l for 6 mgTiO2NPs/ml and 17.1 ± 0.8 mg/l for 10 mgTiO2NPs/ml. These results indicate that even dissolution of Ti4+ ions from TiO2 can take place at certain nanoparticle concentrations and this phenomenon may also contribute to TiO2 toxicity. Whether the dissolution of Ti4+ ions from nanoparticulated TiO2 will occur or not will be dependent on the type of chemical preparation and used titanium precursor.
Conclusions
Nanostructured TiO2 and ZnO were prepared by unconventional processing using pressurized hot water in a flow regime, allowing the preparation of pure nanostructured materials at significantly lower temperature than during standard calcination. In case of TiO2, the subsequent processing by pressurized hot water did not cause any significant changes within TiO2 (micro)structure which was prepared by thermal hydrolysis. In case of ZnO, the processing by pressurized hot water affected crystallization of nanoparticles significantly. Detailed characterization of both prepared nanoparticulated materials revealed that TiO2 anatase of ∼7 nm crystallite size beside the mixture of major ZnO wurtzite (of ∼114 nm crystallite size) and minor Zn2SiO4 willemite (of ∼88 nm crystallite size) were formed under pressurized hot water. Tests of acute biological toxicity demonstrated a significant toxicity of ZnO. The eco-toxicity tests over ZnO showed the toxicity values EC50 = 1.839 ± 0.1605 mg/ml for L. minor and IC50 = 1.532 ± 0.930 mg/ml for S. alba. TiO2 showing significantly lower crystallite size than ZnO exhibited markedly lower toxic effect on seeds of S. alba (IC50 = 172.853 ± 22.16 mg/ml) compared to proven, however, lower toxicity on L. minor fronds (EC50 = 5.215 ± 0.1375 mg/ml) than ZnO. The commercial TiO2 Degussa P25 showed the growth stimulation effect to L. minor fronds and no toxic effect to S. alba root. It was shown the (micro)structural parameters such as the small crystallite size and the large surface area are not the acute biological toxicity-determining factors. The toxicity of TiO2 nanoparticles to both plant species is affected by factors such as the rate of nanosized crystallites aggregation, their concentration, shape and surface properties. The dissolution of Ti4+ ions from TiO2 nanoparticles can also occur at certain nanoparticle concentrations, possibly contributing to its toxicity. In case of ZnO nanotoxicity, the dissolution of Zn2+ ions is the main cause. Finally, it should be emphasized that the statement about TiO2 non-toxicity should be taken with caution since it is significantly affected by used preparation method and/or used titanium precursors as seen in our case.
References
Adam V, Loyaux-Lawniczak S, Quaranta G (2015) Characterization of engineered TiO2 nanomaterials in a life cycle and risk assessments perspective. Environ Sci Pollut Res 22:11175–11192
Adams LK, Lyon DY, Alvarez PJJ (2006) Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40:3527–3532
Andersen CP, King G, Plocher M et al (2016) Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ Toxicol Chem 35:2223–2229
Arruda SCC, Silva ALD, Galazzi RM et al (2015) Nanoparticles applied to plant science: a review. Talanta 131:693–705
Arts JHE, Irfan MA, Keene AM et al (2015) Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul Toxicol Pharmacol 76:234–261
Barnes RJ, Molina R, Xu JB et al (2013) Comparison of TiO2 and ZnO nanoparticles for photocatalytic degradation of methylene blue and the correlated inactivation of gram-positive and gram-negative bacteria. J Nanopart Res 15:1432–1443
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380
Bellanger X, Billard P, Schneider R et al (2015) Stability and toxicity of ZnO quantum dots: interplay between nanoparticles and bacteria. J Hazard Mater 283:110–116
Bour A, Mouchet F, Silvestre J et al (2015) Environmentally relevant approaches to assess nanoparticles ecotoxicity: a review. J Hazard Mater 283:764–777
Chen XL, O'Halloran J, Jansen MAK (2016) The toxicity of zinc oxide nanoparticles to Lemna minor (L.) is predominantly caused by dissolved Zn. Aquat Toxicol 174:46–53
Clement L, Hurel C, Marmier N (2013) Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants–effects of size and crystalline structure. Chemosphere 90:1083–1090
Coronado JM, Fresno F, Hernández-Alonso MD et al (2013) Design of advanced photocatalytic materials for energy and environmental applications. Springer, London
Cox A, Venkatachalam P, Sahi S et al (2016) Silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol Biochem 107:147–163
de Boer JH, Lippens BC, Linsen BG et al (1966) Thet-curve of multimolecular N2-adsorption. J Colloid Interface Sci 21:405–414
Farkas J, Peter H, Ciesielski TM et al (2015) Impact of TiO2 nanoparticles on freshwater bacteria from three Swedish lakes. Sci Total Environ 535:85–93
Fu L, Hamzeh M, Dodard S et al (2015) Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation. Environ Toxicol Pharmacol 39:1074–1080
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity. United States, New York
Hatami M, Ghorbanpour M, Salehiarjomand H (2014) Nano-anatase TiO2 modulates the germination behavior and seedling vigority of some commercially important medicinal and aromatic plants. J Biol Eng 8:53–59
Hoffmann MR, Martin ST, Choi WY et al (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Hofmann-Amtenbrink M, Grainger DW, Hofmann H (2015) Nanoparticles in medicine: current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomedicine-Nanotechnology Biology and Medicine 11:1689–1694
Hougaard KS, Campagnolo L, Chavatte-Palmer P et al (2015) A perspective on the developmental toxicity of inhaled nanoparticles. Reprod Toxicol 56:118–140
Hsiao IL, Huang YJ (2011) Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Sci Total Environ 409:1219–1228
Jacob DL, Borchardt JD, Navaratnam L, Otte ML, Bezbaruah AN (2013) Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int J Phytoremediation 15(2):142–153
Judy JD, Bertsch PM (2014) Bioavailability, toxicity, and fate of manufactured nanomaterials in terrestrial ecosystems. Adv Agron 123:1–64
Kahru A, Dubourguier HC (2010) From ecotoxicology to nanoecotoxicology. Toxicology 269:105–119
Kumari M, Khan SS, Pakrashi S et al (2011) Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater 190:613–621
Laborda F, Bolea E, Cepria G et al (2016) Detection, characterization and quantification of inorganic engineered nanomaterials: a review of techniques and methodological approaches for the analysis of complex samples. Anal Chim Acta 904:10–32
Lecloux A, Pirard JP (1979) The importance of standard isotherms in the analysis of adsorption isotherms for determining the porous texture of solids. J Colloid Interface Sci 70:265–281
Li L, Sillanpaa M, Tuominen M et al (2013) Behavior of titanium dioxide nanoparticles in L. minor growth test conditions. Ecotoxicol Environ Saf 88:89–94
Lin DH, Xing BS (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Ma HB, Williams PL, Diamond SA (2013) Ecotoxicity of manufactured ZnO nanoparticles–a review. Environ Pollut 172:76–85
Mallevre F, Fernandes TF, Aspray TJ (2014) Silver, zinc oxide and titanium dioxide nanoparticle ecotoxicity to bioluminescent Pseudomonas putida in laboratory medium and artificial wastewater. Environ Pollut 195:218–225
Manke A, Wang LY, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. doi:10.1155/2013/942916
Manzo S, Rocco A, Carotenuto R et al (2011) Investigation of ZnO nanoparticles’ ecotoxicological effects towards different soil organisms. Environ Sci Pollut Res 18:756–763
Matejova L, Koci K, Reli M et al (2013) On sol-gel derived Au-enriched TiO2 and TiO2-OZrO2 Photocatalysts and their investigation in photocatalytic reduction of carbon dioxide. Appl Surf Sci 285:688–696
Matějová L, Kočí K, Troppová I et al (2017) TiO2 and nitrogen doped TiO2 prepared by different methods; on the (micro)structure and photocatalytic activity in CO2 reduction and N2O decomposition. J Nanosci Nanotechnol. doi:10.1166/jnn.2017.13936
Menard A, Drobne D, Jemec A (2011) Ecotoxicity of nanosized TiO2. Review of in vivo data. Environmental Pollution 159:677–684
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S, et al (2014) Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138
Priester JH, Ge Y, Mielke RE et al (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc Natl Acad Sci U S A 109:E2451–E2456
Quality Water (2005) Determination of the toxic effect of water constituents and waste water to duckweed (Lemna minor)–duckweed growth inhibition test (ISO CD 20079)
Rasmussen K, Gonzalez M, Kearns P et al (2016) Review of achievements of the OECD Working Party on Manufactured Nanomaterials’ Testing and Assessment Programme. From exploratory testing to test guidelines. Regul Toxicol Pharmacol 74:147–160
Rico CM, Majumdar S, Duarte-Gardea M, et al (2011) Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem 59:3485–3498
RRUFF (2016) On-line database, http://rruff.info/Willemite/R100109 (Willemite R100109, accessed 26/5/2016).
Sadiq IM, Dalai S, Chandrasekaran N et al (2011) Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp and Chlorella sp. Ecotoxicol Environ Saf 74:1180–1187
Schiavo S, Oliviero M, Miglietta M et al (2016) Genotoxic and cytotoxic effects of ZnO nanoparticles for Dunaliella tertiolecta and comparison with SiO2 and TiO2 effects at population growth inhibition levels. Sci Total Environ 550:619–627
Schneider P (1995) Adsorption isotherms of microporous-mesoporous solids revisited. Appl Catal A Gen 129:157–165
Sun TY, Gottschalk F, Hungerbuhler K et al (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Pollut 185:69–76
Tripathi DK, Shweta, Sing S et al (2017) An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem 110:2–12
Wang D, Lin Z, Wang T et al (2016) Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? J Hazard Mater 308:328–334
Warheit DB, Boatman R, Brown SC (2015) Developmental toxicity studies with 6 forms of titanium dioxide test materials (3 pigment-different grade & 3 nanoscale) demonstrate an absence of effects in orally-exposed rats. Regul Toxicol Pharmacol 73:887–896
Xiong DW, Fang T, Yu LP et al (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ 409:1444–1452
Yoon SJ, Kwak JI, Lee WM et al (2014) Zinc oxide nanoparticles delay soybean development: a standard soil microcosm study. Ecotoxicol Environ Saf 100:131–137
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
Financial support from the Grant Agency of the Czech Republic (project reg. No.14-23274S) is gratefully acknowledged. This work was also financially supported by EU structural funding Operational Programme Research and Development for Innovation (project No. CZ.1.05/2.1.00/19.0388). Authors’ grateful thanks is also aimed to Dr. Pavel Buček from IET VŠB-Technical University of Ostrava for AAS analyses.
Rights and permissions
About this article
Cite this article
Troppová, I., Matějová, L., Sezimová, H. et al. Nanostructured TiO2 and ZnO prepared by using pressurized hot water and their eco-toxicological evaluation. J Nanopart Res 19, 198 (2017). https://doi.org/10.1007/s11051-017-3877-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3877-8