Abstract
Cyanazine residue poses a great threat to human health and its derivatives would remain in soils, natural waters, and other environmental domains for a long time. Herein, a simple, rapid, and ultra-sensitive analytical method for the determination of cyanazine (CZ) based on inner filter effect (IFE) of Au nanoparticles (AuNPs) on the fluorescence of CdTe quantum dots (QDs) is first described in this study. With the presence of citrate-stabilized AuNPs, the fluorescence of GSH-capped CdTe QDs was remarkably quenched by AuNPs via IFE. The fluorescence of the AuNP–CdTe QD system was recovered upon addition of CZ. CZ can adsorb on to the surface of AuNPs due to its cyano group that has good affinity with gold, which could induce the aggregation of AuNPs accompanying color change from red to blue. Thus, the IFE of AuNPs on CdTe QDs was weakened, and the fluorescence intensity of CdTe QDs was recovered accordingly. A good linear correlation for detection of CZ was exhibited from 0.05 to 9 μM, and the detection limit reached 0.1568 μM, which was much lower than the safety limit required by the USA, the UK, and China. In order to probe into the selectivity of AuNPs towards CZ over other pesticides, various frequently used pesticides were mixed with AuNPs. AuNP composite solution shows good selectivity towards CZ among other pesticides. This method was successfully carried out for the assessment of CZ in real samples with satisfactory results, which revealed many advantages such as high sensitivity, low cost, and non-time-consuming compared with traditional methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanazine (CZ) is a synthetic triazine herbicide that has been widely used to control broadleaf weeds and grasses in agricultural crops throughout the world, not only in the developed countries including United States but also in the developing countries including China. In the 1990s, cyanazine ranked as the fifth most commonly used herbicide in the United States, with an estimated 32 million pounds applied annually (U.S. EPA 2004). On account of its worldwide situation, many studies have focused on ecological and health hazards of these compounds (triazines) and their toxic effects. It is a great threat to human health and the environment, and its derivatives would remain in soils, natural waters, and other environmental domains for a long time (Gholivand et al. 2012). There would be a variety of toxic effects upon exposure to the triazine compounds. For instance, they can cause endocrine disruptions, regular hormone function interruptions, birth defects, reproductive tumors, and weight loss in mother and embryos (Gebel et al. 1997). The U.S. EPA classified CZ as a restricted use pesticide, canceled CZ product registrations and prohibited the sale and use of existing stocks of CZ after September 30, 2002. Although CZ is banned in the United States, it is still used in various African nations (e.g., South Africa, Niger, etc.), Asia and the Pacific Region (e.g., Australia, India, New Zealand, the Philippines, etc.), Europe (e.g., Hungary, Portugal, the United Kingdom, etc.), Central Asia, Canada, and South America (Lynch et al. 2006).
Practical methods with high sensitivity and selectivity for simple and rapid detection of CZ residues are crucial for human health and environment pollution monitoring. Many researches described different analytical methods employed for quantitative determination of several triazines, as well as their degradation products at low levels (Sabik et al. 2000; Pacakova et al. 1996; Tadeo et al. 1996). Chromatography, capillary electrophoresis (Carabias-Martinez et al. 2000), enzyme immunoassay, spectrophotometry, HPLC, micellar electrokinetic chromatography (Turiel et al. 2001; Lin et al. 1999), cyclic voltammetry (Zapardiel et al. 2000), and differential-pulse polarography (Ignjatovic et al. 1993) on solid electrodes, photosynthetic electron transport (PET) electrochemical biosensors (Steegborn and Skladal 1997), PET colorimetric detection (Brewster and Lightfield 1993), and adsorptive stripping voltammetry in dispersed media (Pedrero et al. 1995) can be mentioned among the most employed techniques.
In spite of precision for these analytical methods, however, most of them are time consuming, complex sample pretreatment, and skilled personnel always needed, which limit their point-of-care or in-field applications. Thus, development of simple, rapid, and cost-effective sensor system for the detection of CZ is still urgently needed. Recent years, the combination of QDs and AuNPs in one micro matrix is expected to result in ideal sensors. AuNPs have been successfully applied for detecting a large variety of targets owing to their excellent photoelectrical properties (Xiao and Yu 2010; Ma et al. 2011) and the accompanying color change of solution (Li and Rothberg 2004; Zhu et al. 2012; Kanagaraj et al. 2012; Sun et al. 2011; Zheng et al. 2013; Imene et al. 2014; Tan et al. 2014; Li et al. 2014; Zhang et al. 2014). Hence, colorimetric sensor for pesticide and herbicide by controlling the surface condition of AuNPs is a hotspot in trace sensing.
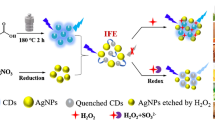
The inner filter effect (IFE) of AuNPs on QDs has been considered as an efficient strategy in developing fluorescent methods. The IFE refers to the absorption of the excitation and/or emission light of fluorophores by absorbers in the detection system (Yuan and Walt 1987; Zhang et al. 2012; Li et al. 2013; Shaikh et al. 2015). Compared to the conventional fluorescence resonance energy transfer-based fluorescent methods, IFE does not require chemical linkage between absorber and fluorophore. And it has been applied for the detection of various analytes (Shang and Dong 2009; Zhai et al. 2011; Cao et al. 2013). Herein, we developed a simple and sensitive CZ sensing system based on the IFE of AuNPs on the fluorescence of CdTe QDs, where AuNPs and CdTe QDs were chosen as the absorber–fluorophore pair to modulate the emission of the fluorophore because of the tremendously larger extinction coefficient of AuNPs as well as the stronger fluorescence and better photostability of QDs. The principle of the proposed IFE-based assay for CZ is illustrated in Scheme 1 by AuNPs and QDs as the partner of absorber/fluorophore. The fluorescence intensity of QDs could be quenched by AuNPs based on IFE (Xu et al. 2011a; Cui et al. 2012). CZ can adsorb on to the surface of AuNPs due to its cyano group that has good affinity with gold (Yan et al. 2014; Xu et al. 2011b; Kuhn et al. 2010). After the adsorption of CZ, citrate ions were removed from the surface of AuNPs, which resulted in the diminishment of the electrostatic repulsion force and caused the aggregation of AuNPs. The aggregation of AuNPs led to the fluorescence intensity of QDs recovery accordingly. The results obtained were useful for the development of CZ detection, and the relevant method could present a potential on-site screening tool for the rapid detection of CZ in different practical samples without special instrumentation. This work is helpful for the construction and application of AuNPs–QDs analytical methods in environmental monitoring. To the best of our knowledge, this is the first dual strategy for the fluorescent and visual sensor for CZ monitoring.
Materials and methods
Materials and apparatus
All the chemicals were commercially available as analytical reagent grade and used as received unless otherwise stated. Chloroauric acid and trisodium citrate were purchased from Sinopharm Chemical Reagent Co., Ltd. cyanophos, cyanofenphos, chlorothalonil, dichlobenil, ioxynil, thiacloprid, phoxim, fenvalerate, cypermethrin, cyfloxylate, fenpropathrin, and cyanazine were supplied by Chongqing Entry Exit Inspection and Quarantine Bureau. Na2TeO3, CdCl2, NaBH4, and GSH were bought from Chengdu Kelong Chemical Reagent Factory. All the solutions were prepared by double distilled water which was generated by a Millipore Direct-Q Water system (Molsheim, France).
The morphology of newly synthesized AuNPs was characterized by transmission electron microscopy (TEM) (zeiss Libra200, Carl Zeiss Jena, Germany). UV–Vis absorption spectra were achieved on a Lamda-900 UV–Vis spectrophotometer (Perkin-Elmer). Digital camera (Nikon D7000) and scanner (EPSON perfection V10) were utilized to acquire digital photos. The fluorescence spectra were obtained using a LS55 fluorescence spectrometer (PerkinEImer, USA).
Preparation of AuNPs
We prepared AuNPs according to the method reported in the literature (Grabar et al. 1995) with minor modification. Briefly, firstly the fresh trisodium citrate solution (6 mL 10 mg/mL) was added into the boiling 200 mL HAuCl4 aqueous solution (0.1 mg/mL) with vigorous stirring to yield wine red gold nanoparticle colloid, and the obtained colloid was kept boiling for another 30 min. The AuNP colloidal solution was obtained and stored at 4 °C refrigerator for further utilization. The diameter of the AuNPs synthesized was about 13 nm according to the result of TEM. The concentration of AuNPs was determined according to the UV–Vis absorption at 520 nm with the extinction coefficient of 2.7 × 108 M−1 cm−1 for 13 nm nanoparticles (Demers et al. 2000).
Syntheses of CdTe QDs
We prepared GSH-capped CdTe nanocrystals according to the method reported in the literature (Bao et al. 2006). CdCl2 (0.08 mol L−1, 8 mL) was diluted to 100 mL in a one-necked flask, and trisodium citrate dihydrate (200 mg), Na2TeO3 (0.02 mol L−1, 8 mL), GSH (100 mg), and sodium borohydride (NaBH4, 100 mg) were added with stirring. When the color of the solution changed to claybank, the flask was attached to a condenser and refluxed at 100 °C under open-air conditions. Notably, the citrate must be added to avoid the deposition of cadmium tellurite (CdTeO3). According to the calculation method mentioned in a previous research (Yu et al. 2004), the molar concentration of the CdTe QD solution was calculated to be 1.3 × 10−6 M, diameter of CdTe QDs as-synthesized calculated to be about 3.26 nm. The as-prepared CdTe QD solution was diluted to 32.5 nm for further use.
Cyanazine detection
The CZ standard substance was dissolved in ethanol solution for preparing the standard solution. Various amounts of 1.0 mL CZ standard solution and 2.0 mL AuNP solution were successively added into a 5.0 mL calibrated test tube; then the solution was shaken thoroughly and equilibrated for 3 min until the solution was fully mixed at room temperature (25 °C). The color change of the mixture solution was also recorded by a digital camera. Absorbance spectra of the mixture solution were recorded with a UV–Vis spectrometer.
Various amounts of 0.3 mL CZ standard solution and 0.6 mL AuNP solution were mixed in a 5.0 ml calibrated test tube, and the mixture was incubated at room temperature (25 °C) for 3 min. Then, 4 mL CdTe QD solution was successively added into the above mixture. The fluorescence spectra were recorded from 200 to 800 nm for CZ detection.
Real samples’ detection
In order to check the applicability of the proposed methodology for analysis of CZ detection in real samples, colorimetric detection experiments were carried out in environmental and agricultural samples, including water and cabbages. The spiked samples were prepared according to former studies with slight modification (Sun et al. 2011; Shi et al. 2013). Water samples (tap water and river water) were collected from our laboratory and Jialing river water. The water samples (15 mL) were filtered with filter paper (9 cm). Fresh cabbage was purchased from a local supermarket, and accurately weighed cabbage of 10.0 ± 0.10 g was homogenized with a household cutter and transferred 5 g of them into beakers, respectively, as real samples. Mixture was dispersed for 20 min with ultrasonication, and insoluble residues were removed via centrifuging. Finally, spiked samples were obtained by adding CZ aqueous solution (1 mL) with certain concentration. The experiment of each sample was conducted in triplicate. The average recoveries were calculated.
Results and discussion
Characterization of AuNPs
AuNPs were characterized by ultraviolet and visible (UV–Vis) spectrophotometer. To probe the morphology and dispersity of AuNPs intuitively, TEM was carried out. It is obviously shown in Fig. 1a that citrate-stabilized AuNP colloid displays good monodispersity. About 100 nanoparticles had been counted, as depicted in Fig. 1b, which showed narrow size distribution with grain diameter about 13 nm. As shown in Fig. 1c, the UV–Vis spectrum of AuNPs was dominated by a single, intense peak at 520 nm, and this suggested that the as-prepared AuNPs were in good monodispersity. The molar concentration of the obtained citrate-stabilized AuNPs was calculated to be 4.3 nM.
Characterization of CdTe QDs
Figure 2 shows the UV–Vis absorption spectrum (a) and the fluorescence emission spectra (b) of CdTe QDs. As shown in Fig. 2, the UV–Vis absorption spectra of CdTe QDs displayed absorption peak at 549 nm. The fluorescence spectrum of CdTe QDs showed a single wavelength excitation at 602 nm with excitation wavelength at 340 nm. The exciting slits are 15 nm, and emission slits are 9 nm. The inset in Fig. 2 shows the photos of CdTe QDs (a) under sunlight and the optical photos of CdTe QDs(b) under UV lamps.
Sensitivity of AuNPs for CZ
In order to investigate the sensitivity of sensor, CZ (1 mL) with a series of concentration ranging from 0 to 50 μM was added into the AuNP solution (2 mL) under the optimal detection condition. As shown in Fig. 3a, it displayed that the color of mixture changed from wine red to blue and deepen gradually as increase of concentration of CZ. These results were further demonstrated by UV–Vis spectrum, as shown in Fig. 3b, absorption peak broaden at 520 nm and increased at 650 nm gradually. In addition, as depicted in Fig. 3c, AuNPs showed a commendable linear relationship (R 2 = 0.9963) with CZ concentration varying from 2 to 9 μM with a low detection limit down to 0.2201 μM (LOD = 3σ/S). These results confirmed that AuNPs displayed satisfactory sensitivity towards CZ at low concentration.
a The photographic images of AuNPs after addition of cyanazine; b absorption spectra of AuNPs incubated with various concentrations of cyanazine (0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 50 μM); c linear plot of the R(A650/A520) for AuNPs versus the concentration of CZ ranging from 2 to 9 μM; d TEM images of AuNPs before addition of CZ; e TEM images of AuNPs after addition of CZ. The concentration of AuNPs is 4.3 nM
According to the previous reports (Zhang et al. 2012; Li et al. 2013; Shang and Dong 2009; Zhai et al. 2011; Cao et al. 2013; Xu et al. 2011a, b; Kuhn et al. 2010), the fluorescence of QDs could be quenched in the presence of AuNPs through the IFE. To verify the existence of the IFE between AuNPs and QDs, 4 mL QDs were mixed with different volumes of AuNPs (from 0 to 0.6 mL), and the fluorescence intensity changes of the QDs were monitored (Fig. 4a). From Fig. 4a, it can be seen that the fluorescence intensity of QDs 602 nm in QDs continuously decreased with the increase of the AuNP volumes (0–0.6 mL). Notably, in the presence of 0.6 mL AuNPs, the fluorescence intensity of CdTe QDs decreased by over 80 %. This could be attributed to the high extinction coefficient of AuNPs, which was one advantage of using metal NPs as the absorber compared with conventional chromophores. Therefore, the fluorescence of CdTe QDs at 602 nm can be modulated by the absorbance of AuNPs via IFE, and 0.6 mL AuNPs was used in the further experiments. As shown in Fig. 4b, upon the addition of CZ and AuNPs, the fluorescence intensity of QDs602 in the QDs is continuously changed with the increasing concentration of CZ. In addition, as depicted in Fig. 3c, AuNPs showed a commendable linear relationship (R 2 = 0.9919) with CZ concentrations varying from 0.5 to 9 μM with a low detection limit down to 0.1578 μM (LOD = 3σ/S). The analytical performances of developed methods (absorbance of AuNPs and fluorescence intensity of CdTe QDs) are compared in Table 1.
a The fluorescence spectra of QDs in the presence of different volumes of AuNPs (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 mL); b the fluorescence spectra of QDs in the presence of CZ with different concentrations (0, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 50 μM); c linear plot of the relative fluorescence intensity (F/F0) at 602 nm of the AuNP–QD system versus the concentration of CZ ranging from 0.5 to 9 μM. The concentration of QDs is 32.5 nM
Selectivity of AuNPs for CZ
In order to probe into the selectivity of AuNPs towards CZ over other pesticides, various frequently used pesticides (1 mL of 50 μM) were mixed with AuNPs (2 mL of 4.3 nm). The chemical structures of these pesticides are shown in Fig. 5. Upon addition of analytes, as can be seen from Fig. 6a, the color of solution containing CZ changed from wine red to blue obviously and AuNPs accumulated. Simultaneously, absorption peak of CZ/AuNP solution was broadened while no apparent change in other mixture (Fig. 6b). The ratio of absorption at 650 to 520 nm (B = A650 nm/A520 nm) quantify the spectra change (Fig. 6c). Mechanism of the selectivity of colorimetric sensor for cyanazine is that the aggregation of AuNPs induced by cyanazine. Cyano group in cyanazine could adsorb on to the surface of AuNPs and the distance between AuNPs reduced, which made the AuNPs aggregated and color of AuNP colloid changed from red to blue. The Cyanophos, Cyanofenphos, Chlorothalonil, Dichlobenil, Ioxynil, Phoxim, Fenvalerate, Cypermethrin, Cyfloxylate, and Fenpropathrin, although all contain cyano group, could not cause the aggregation of AuNPs. This may be caused by the huge steric hindrance around the cyano group which exists in the pesticides structure of the intermediate domain or directly connects to heterocyclic structure. Thiacloprid has little influence on the detection, but the sensitivity was much lower than that for CZ. Therefore, AuNP composite solution shows good selectivity towards CZ among other pesticides. In essence, both of the change of color and fluorescence are resulted by aggregation of AuNPs. That means the mechanism of fluorescent method is also based on the aggregation of AuNPs induced by the affinity between CZ and AuNPs. So we have not evaluated the selectivity of sensing method by fluorescence here.
a The photographic images of AuNP solution (2 mL) with various pesticides (50 μM); b UV–Vis spectra of AuNPs with various pesticides; c B(A650 nm/A520 nm) of AuNPs with various pesticides (from 1 to 12, they are cyanophos, cyanofenphos, chlorothalonil, dichlobenil, Ioxynil, thiacloprid, phoxim, fenvalerate, cypermethrin, cyfloxylate, cyanazine, fenpropathrin)
Detection of real samples
To explore the practical application, colorimetric detection experiments were carried out for environmental and agricultural samples, including water and cabbages. Water sample (tap water and river water) was collected from our laboratory and Jialing river water. Fresh cabbage was purchased from a local supermarket. Real samples were spiked with CZ at various concentrations ranging from 2 to 22 μM. We can see from Table 2 that a satisfactory recovery in these spiked samples was in the range of 96.58–100.7 %, and the RSD was lower than 3 %. These results suggested that the QD/AuNP sensors developed here possess potential application in environmental samples and agricultural products.
Conclusion
In summary, we have successfully developed a simple, rapid, and highly effective QD sensors for CZ detection based on the IFE of AuNPs on QDs for the first time. The assay uses common AuNPs without the need for tedious surface modification steps, making the method practical and cost-effective. Dual-strategy system presented here displayed some advantages compared with only colorimetric or fluorescent method. In this study, QDs are used for the construction of an effective fluorescence sensor, and AuNPs are used as fluorescence quencher and colorimetric reporter. As shown in Table 1, comparing with only colorimetric method, fluorescent sign produced from dual-strategy system shows higher accuracy and lower detection limit. On the other hand, colorimetric sign delivered by dual-strategy system can be obviously seen through naked eyes in certain extent, which is more convenient contrast to only fluorescent method. In general, dual-strategy system demonstrates better flexibility compared with only colorimetric or fluorescent method.
The proposed assay presents satisfactory linear range (0.5 μM to 9 μM), low detection limit (0.1578 µM), good accuracy, and specificity in the detection of CZ. In order to probe into the selectivity of AuNPs towards CZ over other pesticides, various frequently used pesticides were mixed with AuNPs. These pesticides also containing cyano group could not cause the aggregation of AuNPs. This may be caused by the huge steric hindrance around the cyano group which exists in the pesticides structure of the intermediate domain or directly connects to heterocyclic structure. Thiacloprid has little influence on the detection, but the sensitivity was much lower than that for CZ. Therefore, AuNP composite solution shows good selectivity towards CZ among other pesticides. Furthermore, the novel method has provided an effective reliable detection of CZ in real sample. In addition, real samples analysis also showed good ability of anti-disturbance with satisfactory recovery. These all demonstrated that AuNPs would be a promising candidate for fast CZ analysis in real application. We anticipate that this method will facilitate the detection of CZ in the field. Importantly, this is the first study to reveal that QD–AuNP complexes could be used to detect CZ. We propose that the novel method has advantages over several current analytical methods, and that its use in practical applications is feasible.
References
Bao HF, Wang EK, Dong SJ (2006) One-pot synthesis of CdTe nanocrystals and shape control of luminescent CdTe-cystine nanocomposites. Small 2(4):476–480
Brewster JD, Lightfield AR (1993) Rapid biorecognition assay for herbicides in biological matrices. Anal Chem 65(18):2415–2419
Cao XY, Shen F, Zhang MW, Guo JJ, Luo YL, Li X, Liu H, Sun CY, Liu JB (2013) Efficient inner filter effect of gold nanoparticles on the fluorescence of CdS quantum dots for sensitive detection of melamine in raw milk. Food Control 34(1):221–229
Carabias-Martinez R, Rodriguez-Gonzalo E, Dominguez-Alvarez J, Hernandez-Mendez J (2000) Determination of triazine herbicides in natural waters by solid-phase extraction and non-aqueous capillary zone electrophoresis. J Chromatogr A 869(1–2):451–461
Cui X, Liu M, Li BX (2012) Homogeneous fluorescence-based immunoassay via inner filter effect of gold nanoparticles on fluorescence of CdTe quantum dots. Analyst 137(14):3293–3299
Demers LM, Mirkin CA, Mucic RC, Reynolds RA, Letsinger RL (2000) A fluorescence-based method for determining the surface coverage and hybridization efficiency of thiol-capped oligonucleotides bound to gold thin films and nanoparticles. Anal Chem 72(22):5535–5541
Gebel T, Kevekordes S, Pav K, Edenharder R, Dunkelberg H (1997) In vivo genotoxicity of selected herbicides in the mouse bone-marrow micronucleus test. Arch Toxicol 71(3):193–197
Gholivand MB, Torkashyand M, Malekzadeh G (2012) Fabrication of an electrochemical sensor based on computationally designed molecularly imprinted polymers for determination of cyanazine in food samples. Anal Chim Acta 713:36–44
Grabar KC, Freeman RG, Hommer MB, Natan MJ (1995) Preparation and characterization of Au colloid monolayers. Anal Chem 67(4):735–743
Ignjatovic LM, Markovic DA, Veselinovic DS, Besic BR (1993) Polarographic behavior and determination of some S-triazine herbicides. Electroanalysis 5(5–6):529–533
Imene B, Cui ZM, Zhang XY, Gan B, Yin YC, Tian YY, Deng HT, Li HB (2014) 4-Amino-3-mercaptobenzoic acid functionalized gold nanoparticles: synthesis, selective recognition and colorimetric detection of cyhalothrin. Sens Actuators B 199:161–167
Kanagaraj K, Affrose A, Sivakolunthu S, Pitchumani K (2012) Highly selective fluorescent sensing of fenitrothion using per-6-amino-beta-cyclodextrin: Eu(III) complex. Biosens Bioelectron 35(1):452–455
Kuhn S, Baisch B, Jung U, Johannsen T, Kubitschke J, Herges R, Magnussen O (2010) Self-assembly of triazatriangulenium-based functional adlayers on Au(111) surfaces. Phys Chem Chem Phys 12(17):4481–4487
Li HX, Rothberg LJ (2004) Label-free colorimetric detection of specific sequences in genomic DNA amplified by the polymerase chain reaction. J Am Chem Soc 126(35):10958–10961
Li JW, Li XM, Shi XJ, He XW, Wei W, Ma N, Chen H (2013) Highly sensitive detection of caspase-3 activities via a nonconjugated gold nanoparticle-quantum dot pair mediated by an inner-filter effect. ACS Appl Mater Interfaces 5(19):9798–9802
Li Z, Wang Y, Ni YN, Kokot S (2014) Unmodified silver nanoparticles for rapid analysis of the organophosphorus pesticide, dipterex, often found in different waters. Sens Actuators B 193:205–211
Lin CE, Hsueh CC, Wang TZ, Chiu TC, Chen YC (1999) Migration behavior and separation of s-triazines in micellar electrokinetic capillary chromatography using a cationic surfactant. J Chromatogr A 835(1–2):197–207
Lynch SM, Rusiecki JA, Blair A, Dosemeci M, Lubin J, Sandler D, Hoppin JA, Lynch CF, Alavanja MCR (2006) Cancer incidence among pesticide applicators exposed to cyanazine in the agricultural health study. Environ Health Perspect 114(8):1248–1252
Ma YR, Niu HY, Zhang XL, Cai YQ (2011) One-step synthesis of silver/dopamine nanoparticles and visual detection of melamine in raw milk. Analyst 136(20):4192–4196
Pacakova V, Stulik K, Jiskra J (1996) High-performance separations in the determination of triazine herbicides and their residues. J Chromatogr A 754(1–2):17–31
Pedrero M, Alonso MR, Devillena FJM, Pingarron JM (1995) Adsorptive stripping voltammetry in dispersed media—application to the determination of the herbicide terbutryn. Electroanalysis 7(7):644–648
Sabik H, Jeannot R, Rondeau B (2000) Multiresidue methods using solid-phase extraction techniques for monitoring priority pesticides, including triazines and degradation products, in ground and surface waters. J Chromatogr A 885(1–2):217–236
Shaikh AJ, Rabbani F, Sherazi TA, Iqbal Z, Mir S, Shahzad SA (2015) Binding strength of porphyrin-gold nanoparticle hybrids based on number and type of linker moieties and a simple method to calculate inner filter effects of gold nanoparticles using fluorescence spectroscopy. J Phys Chem A 119(7):1108–1116
Shang L, Dong SJ (2009) Design of fluorescent assays for cyanide and hydrogen peroxide based on the inner filter effect of metal nanoparticles. Anal Chem 81(4):1465–1470
Shi HJ, Zhao GH, Liu MC, Fan LF, Cao TC (2013) Aptamer-based colorimetric sensing of acetamiprid in soil samples: sensitivity, selectivity and mechanism. J Hazard Mater 260:754–761
Steegborn C, Skladal P (1997) Construction and characterization of the direct piezoelectric immunosensor for atrazine operating in solution. Biosens Bioelectron 12(1):19–27
Sun JF, Guo L, Bao Y, Xie JW (2011) A simple, label-free AuNPs-based colorimetric ultra sensitive detection of nerve agents and highly toxic organophosphate pesticide. Biosens Bioelectron 28(1):152–157
Tadeo JL, Sanchez-Brunete C, Garcia-Valcarcel AI, Martinez L, Perez RA (1996) Determination of cereal herbicide residues in environmental samples by gas chromatography. J Chromatogr A 754(1–2):347–365
Tan SL, Zhao HY, Tian DM, Wang F, Liu JA, Li HB (2014) Piperidine-calix [4] arene modified gold nanoparticles: imidacloprid colorimetric sensing. Sens Actuators B 204:522–527
Turiel E, Martin-Esteban A, Fernandez P, Perez-Conde C, Camara C (2001) Molecular recognition in a propazine-imprinted polymer and its application to the determination of triazines in environmental samples. Anal Chem 73(21):5133–5141
U.S. EPA (U.S. Environmental Protection Agency) (2004) Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). http://www.epa.gov/opp00001/regulating/fifra.pdf. Accessed 2 Jan 2005
Xiao N, Yu CX (2010) Rapid-response and highly sensitive noncross-linking colorimetric nitrite sensor using 4-aminothiophenol Modified gold nanorods. Anal Chem 82(9):3659–3663
Xu L, Li BX, Jin Y (2011a) Inner filter effect of gold nanoparticles on the fluorescence of quantum dots and its application to biological aminothiols detection. Talanta 84(2):558–564
Xu Q, Du S, Jin GD, Li HB, Hu XY (2011b) Determination of acetamiprid by a colorimetric method based on the aggregation of gold nanoparticles. Microchim Acta 173(3–4):323–329
Yan X, Li HX, Li Y, Su XG (2014) Visual and fluorescent detection of acetamiprid based on the inner filter effect of gold nanoparticles on ratiometric fluorescence quantum dots. Anal Chim Acta 852:189–195
Yu WW, Qu LH, Guo WZ, Peng XG (2004) Experimental determination of the extinction coefficient of CdTe, CdSe and CdS nanocrystals. Chem Mater 16(3):560 (vol 15, pg 2854, 2003)
Yuan P, Walt DR (1987) Calculation for fluorescence modulation by absorbing species and its application to measurements using optical fibers. Anal Chem 59(19):2391–2394
Zapardiel A, Bermejo E, Perez JA, Chicharro M (2000) Determination of s-triazines with copper and glassy carbon electrodes. Flow injection analysis of aziprotryne in water samples. Fresenius J Anal Chem 367(5):461–466
Zhai YM, Jin LH, Wang P, Dong SJ (2011) Dual-functional Au-Fe3O4 dumbbell nanoparticles for sensitive and selective turn-on fluorescent detection of cyanide based on the inner filter effect. Chem Commun 47(29):8268–8270
Zhang MW, Cao XY, Li HK, Guan FR, Guo JJ, Shen F, Luo YL, Sun CY, Zhang LG (2012) Sensitive fluorescent detection of melamine in raw milk based on the inner filter effect of Au nanoparticles on the fluorescence of CdTe quantum dots. Food Chem 135(3):1894–1900
Zhang XY, Sun ZY, Cui ZM, Li HB (2014) Ionic liquid functionalized gold nanoparticles: synthesis, rapid colorimetric detection of imidacloprid. Sens Actuators B 191:313–319
Zheng JM, Zhang HJ, Qu JC, Zhu Q, Chen XG (2013) Visual detection of glyphosate in environmental water samples using cysteamine-stabilized gold nanoparticles as colorimetric probe. Anal Methods 5(4):917–924
Zhu DR, Li XK, Liu X, Wang JN, Wang ZX (2012) Designing bifunctionalized gold nanoparticle for colorimetric detection of Pb2+ under physiological condition. Biosens Bioelectron 31(1):505–509
Acknowledgments
The present study was supported by the Natural Science Foundation of China (31171684), Key Technologies R&D Program of China (2014BAD07B02) Key Technologies R&D Program of Sichuan Province of China (2013FZ0043), Fundamental Research Funds for the Central Universities (CQDXWL-2013-030), Open fund of Liquor-Making Biotech and Application Key Laboratory of Sichuan Province (NJ2014-03), and Graduate Student Research Innovation Project in Chongqing (CYB14013) Sharing fund of Chongqing university’s large equipment.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Liang Dong declares that he has no conflict of interest. Changjun Hou declares that he has no conflict of interest. Huanbao Fa declares that he has no conflict of interest. Mei Yang declares that she has no conflict of interest. Huixiang Wu declares that he has no conflict of interest. Caihong Shen declares that he has no conflict of interest. Danqun Huo declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, L., Hou, C., Yang, M. et al. Highly sensitive colorimetric and fluorescent sensor for cyanazine based on the inner filter effect of gold nanoparticles. J Nanopart Res 18, 164 (2016). https://doi.org/10.1007/s11051-016-3398-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3398-x