Abstract
Three kinds of carbon hosts, Ketjenblack (KB, high surface area and porosity), black pearls 2000 (BP2000, high surface area and moderate porosity), and ordered mesoporous carbon nanospheres (OMCN, low surface area and porosity), have been used as conductive hosts in the sulfur/carbon (S/C) composite cathodes for lithium–sulfur (Li–S) batteries. To correlate the carbon properties (surface area and pore volume), the electrochemical performances of S/C composite cathodes with the same sulfur content (60 wt%) have been investigated in detail. S/KB and S/BP2000 composites with high surface porosity can provide more reactive sites for sulfur, which can result in increasing the utilization rate of sulfur, reducing the polarization, and improving the high-rate capability. Large pore volume can effectively capture the polysulfide species and improve easy passages for ion transport, which can promote long-term cycling stability and reduce the resistance of Li–S batteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium-ion battery (LIB) is one of the most popular power sources for zero-emission electric vehicles and energy storage systems because of its high energy density, high operating voltage, and long cycle life (Liang et al. 2011a). However, its limited capacity and low power density cannot satisfy the increasing requirements of electric vehicles (Bogart et al. 2013; Erickson et al. 2014). Among various battery systems, lithium–sulfur (Li–S) batteries have attracted increasing attention due to their high theoretical energy density (2567 Wh kg−1), high theoretical capacity (1675 mAh g−1), low cost, and environmental friendliness of sulfur (Bruce et al. 2012). So Li–S battery is a promising candidate for next-generation lithium battery. Currently, low sulfur utilization and severe capacity fading are the major obstacles, which tremendously hinder the commercial development of Li–S batteries. The poor conductivity of sulfur and the “shuttle reaction” of soluble polysulfides Li2S n (n ≥ 4) between sulfur cathode and lithium anode are the main reasons (Tang et al. 2014; Yang et al. 2013).
To improve the electrochemical performance of the Li–S batteries, many efforts have been made and various strategies, including surface coating (Wu et al. 2011; Fu and Manthiram 2012), conductive substrates (Ye et al. 2013; Wang et al. 2011; Yu et al. 2013), and novel electrolytes (Liang et al. 2011b; Yuan et al. 2006) have been proposed. Carbon-based materials, graphene (Zhang et al. 2014; Zhao et al. 2014; Yang et al. 2014), mesoporous carbons (Zhang et al. 2013; Wang et al. 2015; Schuster et al. 2012), and carbon nanotubes (CNTs) (Han et al. 2003; Wang et al. 2014) are frequently used for loading sulfur. Porous carbon hosts could effectively improve the conductivity of cathode materials and reduce soluble polysulfide shuttling between the anode and the cathode. However, most literatures mainly focused on the carbon preparation method, sulfur content, and electrochemical testing conditions. In fact, the structural characteristics of carbon hosts, especially surface area and pore volume, are the most important factors to ensure efficient utilization of sulfur. For instance, Wei et al. found that the sulfur/carbon (S/C) composite using pig bone-derived carbon with large BET specific surface area and pore volume showed excellent cycling stability and high utilization of sulfur in the Li–S batteries (Wei et al. 2011). Schuster et al. reported the synthesis of ordered mesoporous carbon material with high inner pore volumes of 2.32 cm3 g−1 and surface areas of 2445 m2 g−1. The corresponding S/C composite material delivered a high reversible charge capacity of up to 1200 mAhg−1 and good cycling stability (Schuster et al. 2012). Therefore, investigation on the porous structural properties of carbon hosts could give a direct comparison and further understand electrochemical functions of carbon hosts in the S/C cathodes for Li–S batteries.
In this study, three kinds of carbon hosts, Ketjenblack (KB, high surface area and porosity), black pearls 2000 (BP2000, high surface area and moderate porosity), and ordered mesoporous carbon nanospheres (OMCN, low surface area and porosity), were used as conductive hosts for sulfur loading. Other factors were controlled in the same conditions for the accurate evaluation of carbon effects. Herein, the effects of porous structure of carbon hosts on preparation and electrochemical performance of sulfur/carbon composites for Li–S batteries were investigated in detail.
Experimental
Synthesis of materials
KB and BP2000 were received from Akzo Nobel N.V. and Carbot, respectively. OMCN was prepared as previously reported (Fang et al. 2010). In a typical synthesis process, 0.6 g phenol, 2.1 ml formalin aqueous solution (37 wt%), and 15 ml (0.1 M) NaOH aqueous solution were mixed and stirred at 70 °C for 0.5 h to obtain low-molecular-weight phenolic resol. Then pluronic surfactant of polyoxyethylene and polyoxypropylene triblock copolymers F127 (0.96 g) was added and stirred at 66 °C for 17 h to form a resol-F127 micelle. Afterwards, 17.7 ml of the obtained solution diluted with 56 ml H2O was transferred into an autoclave and subject to hydrothermal reaction at 130 °C for 24 h. After filtration and rinse, the precipitates were carbonized at 700 °C for 3 h. The final product was marked as OMCN.
The S/C composites were prepared via the vapor phase infusion process. KB, BP2000, or OMCN was mixed with sublimed sulfur (AR, Aladdin, China) in a 3:2 weight ratio, the mixture was put in a glass bottle and then sealed in a glove box, and the sulfur impregnation of carbon materials was obtained by heating the glass bottle at 155 °C for 12 h in a teflon-lined stainless steel autoclave.
Characterization
Raman spectra of various carbon hosts were obtained on a Raman spectrometer employing a 10 mW helium/neon laser with a wavelength of 632.8 nm. Morphologies of the obtained S/C composites were investigated by high-resolution transmission electron microscopy (HRTEM) (JEM-2100F). Surface area, porosity, and pore size were determined using nitrogen adsorption–desorption isotherms collected with a BECKMANCOULTER SA 3100 gas sorption system at liquid nitrogen temperature.
Electrochemical measurements
The electrochemical properties of the S/C composites were evaluated in CR2032-type cells assembled in an argon-filled glove box (MBraun). In order to prepare the electrode laminate, a slurry containing 70 wt% S/C composite, 20 wt% super P, and 10 wt% poly(vinylidene fluoride) (PVDF) dispersed in n-methyl-2-pyrrolidinone (NMP) was cast onto an aluminum current collector. After vacuum drying at 60 °C for 12 h, the laminate was punched into disks (Φ 14 mm) for assembling the CR2032-type coin cells. The active material loading in each electrode (disk) is controlled at about 1.0 mg cm−2. High-purity lithium foil was used as both the counter electrode and the reference electrode. Celgard 2400 polypropylene microporous membrane was used as the separator. The electrolyte was a solution of 1 M lithium bis(trifluoromethanesulfonyl) imide (LiTFSI) in a mixture of 1,3-dioxolane (DOL) and dimethyl ether (DME) (1:1, v/v) with 1 wt% LiNO3. The cell performance was evaluated on a multichannel battery cycler (Arbin BT2000). All the cells were cycled between 1.8 and 3.0 V at a current rate of 0.2 C (1 C = 1675 mA g−1). Electrochemical impedance spectra (EIS) of the Li–S cells were performed on a CHI660D electrochemical workstation, which was carried out in a frequency range between 100 kHz and 10 mHz with a potentiostatic amplitude of 5 mV. All the electrochemical tests were conducted at room temperature.
Results and discussion
Before the preparation of the S/C composites, the structures of three carbon hosts were firstly investigated by Raman spectroscopy (Fig. 1). From Raman spectra of carbon materials, the graphitic carbon (G-band) and disorder carbon (D-band) can be detected. The G-band is a typical character of graphitic carbon corresponding to sp2 bonding, and the D-band is usually related to defects, edge sites, and pores. Herein, all three carbon materials contain the D-band at 1335 cm−1 and the G-band at 1590 cm−1. The strong D-bands of all samples indicate the typical character of porous carbon materials. Raman intensity ratio, I D/I G, provides an important clue to the disordered and/or ordered crystal structure of carbon materials. The I D/I G ratios of KB, BP2000, and OMCN are 1.29, 1.16, and 0.98, respectively. The higher I D/I G ratios of KB and BP2000 are possibly derived from their high porosity. Herein, OMCN has the lowest I D/I G ratio mainly because of its low porosity and high ordered structure.
Porous structures of three carbon materials were further investigated by nitrogen adsorption–desorption isotherms. Figure 2 shows the nitrogen adsorption–desorption isotherms and the corresponding pore size distribution curves of different carbon materials as well as their S/C composites. Figure 2a, b shows the adsorption–desorption isotherms and pore size distribution curves of KB and S/KB. KB exhibits the typical type IV isotherm with a large hysteresis (Fig. 2a), corresponding to a mesoporous structure (Li et al. 2014). The main pore sizes of KB are concentrated on 3–6 nm in Fig. 2b. Similar with KB, BP2000 and OMCN also show the distinct hysteresis and the typical mesoporous carbon materials with type IV isotherms (Fig. 2c, e). But they both have a wide pore size distribution as shown in Fig. 2d, f. Furthermore, all three S/C composites exhibit the depressed hysteresis with the surface area and pore volume significantly decreasing after sulfur loading. All the results above give direct evidence of the encapsulation of sulfur inside the mesoporous structure of the three carbon materials.
Surface area and pore volume of different carbon and S/C composites are summarized in Table 1. The surface area and pore volume of the S/C composites decreased significantly after the impregnation of sulfur in comparison with the corresponding carbon host. For example, KB presents high surface area and pore volume of 1412.38 m2 g−1 and 2.69 cm3 g−1, but they reduce to 94.35 m2 g−1 and 0.61 cm3 g−1 after sulfur loading, respectively. It indicates that a majority of nanopores of carbon frameworks are filled by sulfur particles. Additionally, the filling degree, i.e., the ratio of the sulfur occupied volume and carbon pore volume in the S/C composites, could provide an insight into the electrochemical performance of the S/C composites. All the S/C composites are composed of 40 wt% carbon and 60wt% sulfur. In 1 g S/C composites, the pore volumes of the pristine carbons (0.4 g) should be 1.07 (KB), 0.53 (BP2000), and 0.23 cm3 (OMCN). After loading 0.6 g sulfur, the occupied volumes of sulfur in the S/C composites are 0.46 (S/KB), 0.05 (S/BP2000), and 0.18 cm3 (S/OMCN) deduced by the pore volume subtracting method (subtracting the pore volume of the S/C composite from the pristine carbon). Afterwards, the filling degree in S/C composites is 43 % (S/KB), 9 % (S/BP2000) and 78 % (S/OMCN). Too high filling degree of S/OMCN may not be favorable for the diffusion of the electrolyte and the transportation of Li+ ions into the pores of the carbon host, while the low filling degree of S/BP2000 suggested a large deal of sulfur out of the carbon host. S/KB with moderate volume ratio may show good electrochemical performances by accommodating volumetric expansion during lithiation of sulfur and promoting the infiltration of electrolyte.
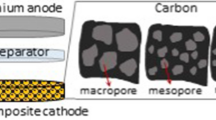
The morphologies and pore structures of various S/C composites were identified as shown in Fig. 3. It can be seen that there are no large bulk sulfur particles on the surface of S/C composites, which also can be certified from the elemental X-ray mappings. These observations demonstrate that sulfur uniformly diffuses into the carbon hosts after the vapor phase infusion process. From the TEM images, three S/C composites have quite different morphologies. S/KB and S/BP2000 have smaller particle size of around 30 nm, but S/OMCN is typical ordered mesoporous nanospheres with an average particle size of around 100 nm. Basically, the porous structure of S/C composites can be observed and there are redundant spaces to accommodate the volumetric expansion during lithiation of sulfur. The element distributions of the prepared S/C composites are shown in Fig. 3 a3–c3, a4–c4. Elemental X-ray mappings clearly show homogeneous distribution of sulfur within the carbon matrix in all three composites. Few sulfur particles were peeled off from carbon hosts even though ultrasonication in ethanol was performed for all the S/C composites before TEM test.
Figure 4 shows the charge–discharge performance of different S/C composites at 0.2 C. Typical voltage profiles of sulfur cathode with two-staged discharge plateaus were observed for all samples in Fig. 4a. The first plateau at 2.3 V is assigned to a rapid kinetic process involving the conversion of elemental sulfur (S8) to long-chain lithium polysulfides (Li2S x , 4 < x < 8). The second plateau at 2.1 V suggests a strong reduction of soluble polysulfide anions to an insoluble low-order Li2S2/Li2S, which is a slower kinetic process than the first and contributes to a large proportion of the capacity (Elazari et al. 2011; Cao et al. 2011; Li et al. 2011). In the first cycle, S/KB, S/BP2000, and S/OMCN deliver the specific charge capacity of 1283, 1020, and 930 mAh g−1, respectively. Moreover, the voltage gap between the charge and discharge plateaus of the cell with S/KB is much smaller than the others. It suggests that electrochemical kinetics have been improved and high surface area could reduce polarization because of the decreased real current density (Zheng et al. 2013). Figure 4b shows the cycling performance of all S/C samples. The discharge capacity of S/C composites decreased sharply in the first few cycles due to the irreversible dissolution of polysulfide species. After that, a relatively stable capacity was achieved after 10 cycles. After 100 cycles, the discharge capacity of the S/KB remains at 789 mAh g−1 with a capacity retention of 78.1 %, which was calculated from the tenth cycle. It is higher than those of S/BP2000 (75.9 %) and S/OMCN (66.1 %). The result suggests that high pore volume and moderate filling degree of sulfur in KB are beneficial to cycling stability of the corresponding S/C composite (S/KB), which could be attributed to the porous structures that effectively mitigate shuttle effect caused by dissolved polysulfide species. Additionally, even though BP2000 has much higher surface area and pore volume than OMCN, still S/BP2000 showed the comparable capacities as S/OMCN before 30 cycles, which could be related to its low filling degree of sulfur in carbon host (9 % for S/BP2000) as mentioned above.
The C-rate behavior of the S/C composites was evaluated via galvanostatic charge–discharge at different C-rates ranging from 0.1 to 2 C. As shown in Fig. 5, the reversible capacity decreased quickly in the first five cycles, while the capacity fading becomes slow with the increase of current rate. At a relatively higher rate of 2 C, S/OMCN shows larger recession of reversible capacity than S/KB and S/BP2000 which possess higher surface area. At such a high current rate, active materials might not react completely and the polarization of the cathode is increased, but the carbon hosts with high surface area could retard the rise of polarization (Yan et al. 2015). As shown in Table 1, KB and BP2000 have comparable surface area, even though the pore volume of KB is twice that of BP2000. While their reversible capacities at 2C are quite close. It indicates that the high surface area of carbon hosts is very important with increasing current density. We reach to a conclusion that high surface area of carbon hosts is beneficial for the high-rate capability of the S/C composites. After higher current density charge–discharges cycles, the S/C cathodes at 0.2 C show small capacity recession, which indicates good capacity recovery after high charge–discharge rate processes.
In order to further explain the mechanism of the electrochemical behaviors of different S/C composites, EIS analysis was carried out after 3 and 100 cycles (Fig. 6). The Nyquist plots of the cathodes after 3 and 100 cycles are composed of two depressed semicircles at high frequency and middle frequency, respectively, and an inclined line at low frequency, which is in accordance with previous reports (Xu et al. 2015; Zhang et al. 2014). The semicircle in high-frequency region corresponds to the resistance of the SEI film which is mainly related to the solid-state layer of accumulated Li sulfides formed on the surface of electrodes, the semicircle at middle frequency is considered as the charge transfer resistance, and the inclined line at low frequency reflects the Li-ion diffusion into the electrode (Zhang et al. 2014). As shown in Fig. 6a, it is clear that the resistance of accumulated Li sulfides with different S/C composite cathodes shows small divergence after 3 cycles, while the resistance of S/KB is lower than those of other carton materials after 100 cycles (Fig. 6b). This result suggests that KB with high surface area and pore volume is most beneficial for capturing the polysulfide species and suppressing the accumulation of Li sulfides.
Conclusions
In this work, the electrochemical performances of S/C composite cathodes have been systematically investigated to analyze the effect of carbon surface area and pore volume for Li–S batteries. The S/KB cathode with higher surface area and pore volume exhibits enhanced electrochemical performance, including capacity, voltage plateau, reversibility, and rate capability in comparison with the other S/C cathodes, which is attributed to the common effects of carbon surface area and pore volume. High surface area provides more reactive sites for sulfur, leading to the increase of the utilization rate of sulfur, reduction of polarization, and an improvement in the high-rate capability. Large pore volume can effectively capture the polysulfide species and improve easy passages for ion transport, which promotes the long-term cycling stability and reduces the resistance of Li–S batteries.
References
Bogart TD, Oka D, Lu X, Gu M, Wang C, Korgel BA (2013) Lithium ion battery performance of silicon nanowires with carbon skin. ACS Nano 8:915–922
Bruce PG, Freunberger SA, Hardwick LJ, Tarascon JM (2012) Li–O2 and Li–S batteries with high energy storage. Nat Mater 11:19–29
Cao YL, Li XL, Aksay IA, Lemmon J, Nie ZM, Yang ZG, Liu J (2011) Sandwich-type functionalized graphene sheet-sulfur nanocomposite for rechargeable lithium batteries. Phys Chem Chem Phys 13:7660–7665
Elazari R, Salitra G, Garsuch A, Panchenko A, Aurbach D (2011) Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li–S batteries. Adv Mater 23:5641–5644
Erickson EM, Ghanty C, Aurbach D (2014) New horizons for conventional lithium ion battery technology. J Phys Chem Lett 5:3313–3324
Fang Y, Gu D, Zou Y, Wu Z, Li F, Che R, Zhao D (2010) A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size. Angew Chem Int Ed 49:7987–7991
Fu Y, Manthiram A (2012) Core-shell structured sulfur-polypyrrole composite cathodes for lithium-sulfur batteries. RSC Adv 2:5927–5929
Han SC, Song MS, Lee H, Kim HS, Ahn HJ, Lee JY (2003) Effect of multiwalled carbon nanotubes on electrochemical properties of lithium/sulfur rechargeable batteries. J Electrochem Soc 150:889–893
Li X, Cao Y, Qi W, Saraf LV, Xiao J, Nie Z, Liu J (2011) Optimization of mesoporous carbon structures for lithium-sulfur battery applications. J Mater Chem 21:16603–16610
Li Q, Zhang Z, Guo Z, Lai Y, Zhang K, Li J (2014) Improved cyclability of lithium-sulfur battery cathode using encapsulated sulfur in hollow carbon nanofiber@nitrogen-doped porous carbon core-shell composite. Carbon 78:1–9
Liang X, Wen Z, Liu Y, Zhang H, Huang L, Jin J (2011a) Highly dispersed sulfur in ordered mesoporous carbon sphere as a composite cathode for rechargeable polymer Li/S battery. J Power Sources 196:3655–3658
Liang X, Wen Z, Liu Y, Wu M, Jin J, Zhang H, Wu X (2011b) Improved cycling performances of lithium sulfur batteries with LiNO3-modified electrolyte. J Power Sources 196:9839–9843
Schuster J, He G, Mandlmeier B, Yim T, Lee KT, Bein T, Nazar LF (2012) Spherical ordered mesoporous carbon nanoparticles with high porosity for lithium-sulfur batteries. Angew Chem Int Ed 51:3591–3595
Tang C, Zhang Q, Zhao MQ, Huang JQ, Cheng XB, Tian GL, Wei F (2014) Nitrogen-doped aligned carbon nanotube/graphene sandwiches: facile catalytic growth on bifunctional natural catalysts and their applications as scaffolds for high-rate lithium-sulfur batteries. Adv Mater 26:6100–6105
Wang H, Yang Y, Liang Y, Robinson JT, Li Y, Jackson A, Dai H (2011) Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett 11:2644–2647
Wang L, Zhao Y, Thomas ML, Byon HR (2014) In situ synthesis of bipyramidal sulfur with 3D carbon nanotube framework for lithium-sulfur batteries. Adv Funct Mater 24:2248–2252
Wang D, Fu A, Li H, Wang Y, Guo P, Liu J, Zhao XS (2015) Mesoporous carbon spheres with controlled porosity for high performance lithium-sulfur batteries. J Power Sources 285:469–477
Wei SC, Zhang H, Huang YQ, Wang WK, Xia YZ, Yu ZB (2011) Pig bone derived hierarchical porous carbon and its enhanced cycling performance of lithium-sulfur batteries. Energy Environ Sci 4:736–740
Wu F, Chen J, Chen R, Wu S, Li L, Chen S, Zhao T (2011) Sulfur/polythiophene with a core/shell structure: synthesis and electrochemical properties of the cathode for rechargeable lithium batteries. J Phys Chem C 115:6057–6063
Xu Q, Hu GC, Bi HL, Xiang HF (2015) A trilayer carbon nanotube/Al2O3/polypropylene separator for lithium-sulfur batteries. Ionics 21:981–986
Yan J, Liu X, Wang X, Li B (2015) Long-life, high-efficiency lithium/sulfur batteries from sulfurized carbon nanotube cathodes. J Mater Chem A 3:10127–10133
Yang Y, Zheng G, Cui Y (2013) Nanostructured sulfur cathodes. Chem Soc Rev 42:3018–3032
Yang X, Zhang L, Zhang F, Huang Y, Chen Y (2014) Sulfur-infiltrated graphene-based layered porous carbon cathodes for high-performance lithium-sulfur batteries. ACS Nano 8:5208–5215
Ye H, Yin YX, Xin S, Guo YG (2013) Tuning the porous structure of carbon hosts for loading sulfur toward long lifespan cathode materials for Li–S batteries. J Mater Chem A 1:6602–6608
Yu L, Brun N, Sakaushi K, Eckert J, Titirici MM (2013) Hydrothermal nanocasting: synthesis of hierarchically porous carbon monoliths and their application in lithium-sulfur batteries. Carbon 61:245–253
Yuan LX, Feng JK, Ai XP, Cao YL, Chen SL, Yang HX (2006) Improved dischargeability and reversibility of sulfur cathode in a novel ionic liquid electrolyte. Electrochem Commun 8:610–614
Zhang K, Li J, Li Q, Fang J, Zhang Z, Lai Y, Tian Y (2013) Synthesis of spherical porous carbon by spray pyrolysis and its application in Li–S batteries. J Solid State Electrochem 17:3169–3175
Zhang J, Dong Z, Wang X, Zhao X, Tu J, Su Q, Du G (2014a) Sulfur nanocrystals anchored graphene composite with highly improved electrochemical performance for lithium-sulfur batteries. J Power Sources 270:1–8
Zhang Z, Lai Y, Zhang Z, Zhang K, Li J (2014b) Al2O3-coated porous separator for enhanced electrochemical performance of lithium sulfur batteries. Electrochimi Acta 129:55–61
Zhao MQ, Zhang Q, Huang JQ, Tian GL, Nie JQ, Peng HJ, Wei F (2014) Unstacked double-layer templated graphene for high-rate lithium-sulphur batteries. Nat Commun 5:3410
Zheng J, Gu M, Wagner MJ, Hays KA, Li X, Zuo P, Xiao J (2013) Revisit carbon/sulfur composite for LI–S batteries. J Electrochem Soc 160:1624–1628
Acknowledgments
This study was supported by the National Science Foundation of China (Grant Nos. 21006033 and 51372060) and the Fundamental Research Funds for the Central Universities (2013HGCH0002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, T., Shi, P., Chen, J. et al. Effects of porous structure of carbon hosts on preparation and electrochemical performance of sulfur/carbon composites for lithium–sulfur batteries. J Nanopart Res 18, 19 (2016). https://doi.org/10.1007/s11051-016-3331-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3331-3