Abstract
Certain food additives may contain a sizeable fraction of particles in the nanoscale. However, little is known about the fate, behaviour and toxicological effects of orally-ingested nanoparticles. This study investigated the uptake and biodistribution of nano- and larger-sized titanium dioxide (TiO2) using an in vitro model of gut epithelium and in vivo in rat. The results of the in vivo study showed that oral administration of 5 mg/kg body weight of TiO2 nano- or larger particles did not lead to any significant translocation of TiO2 (measured as titanium) either to blood, urine or to various organs in rat at any of the time intervals studied over a 96 h post-administration period. Different methods used for dispersing particles did not affect the uptake, and orally administered TiO2 was found excreted in the faeces over a period of time. The in vitro study provided further evidence for the lack of translocation of TiO2 across the gut epithelium model. The overall evidence from both in vivo and in vitro studies did not support that oral ingestion of nano- or larger particles of TiO2 via food would result in any significant internal exposure of the consumer to the nanoparticles. The dietary TiO2 nanoparticles are likely to be excreted in the faeces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The agri-food sector has long been an attractive target for new enabling technologies that can offer an innovation or improvement to the existing feedstocks, processes, products or to enhance safety and security of food products. Nanotechnology is the most recent example of a new enabling technology, which is promising to revolutionise the whole of the agri-food chain (Chaudhry et al. 2010; Frewer et al. 2011). Nanotechnology is a broad term which represents a range of processes, materials and applications that span physical, chemical, biological and electronic sciences and engineering fields. A common denominator amongst all these is the manipulation of materials in the nanoscale (between 1 and 100 nanometres). Nanomaterials may exist in different forms—e.g. with one dimension in the nanoscale (layers, coatings, sheets), two dimensions in the nanoscale (rods, tubes) or all three dimensions in the nanoscale (nanoparticles).
The fundamental drivers behind most nanotechnology applications are the potential for development of new or improved functionalities, and a reduction in the use of chemical substances in different consumer products and applications. The current scope of nanotechnology applications in the food sector spans from a possible reduction in the use of agrochemicals during food production, to the development of new food tastes and textures, safer and more nutritious food/feed products, cheaper and rapid diagnostics for pathogens and contaminants, and novel packaging materials that can help keep the food products safe and hygienic in the supply chain (Chaudhry et al. 2008, 2010; Chaudhry and Castle 2011).
Despite the projected benefits, the use of nano-sized ingredients and additives in food products has also raised concerns over their potential risk to consumer health. Emerging evidence from a number of published studies has suggested that some materials, when manufactured at nanoscale, may show significant deviations in relation to physicochemical properties, interaction with biological entities and/or toxicological effects, compared to conventional equivalents. Of particular concern is the likelihood that some nanoparticles, especially in the lower nanometre (nm) range, may penetrate biological membrane barriers (e.g. Jani et al. 1990, Geiser and Kreyling 2010). This may allow some insoluble (or partially-soluble) and biopersistent nanoparticles to reach those parts of the body that are otherwise protected by membrane barriers against the entry of larger-sized particulate substances. If systemically available, such nanoparticles may interact with different biological moieties close to the molecular level and this may lead to harmful effects. Due to high surface energy, nanoparticles may also adsorb or bind other substances (Šimon and Joner 2008), and thus act as a carrier of unwanted and potentially harmful substances to different parts of the body.
The uptake, translocation and biodistribution of engineered nanomaterials (ENMs) is, however, likely to be modulated by a number of factors. For example, orally ingested nanoparticles may degrade, solubilise or bind with other moieties in food, and in the gastrointestinal (GI) tract. They may also agglomerate/aggregate to larger sizes and as a result be excreted without being absorbed in the body. It also needs to be emphasised that any nano-specific properties of a material are intrinsically linked to the physical integrity of the constituting nano-structures. As and when a nanomaterial loses the nano-structure, it will no longer be any different from its conventional (non-nano) equivalent. This aspect helps to narrow down the focus of consumer safety concerns to those nanoparticles that can remain intact in food/feed, survive the harsh digestive environment in the GI tract, and are able to manifest harmful effects in the gut or other parts of the body following translocation. Titanium dioxide (TiO2) is a perfect example of an insoluble, relatively inert and persistent material, the conventional form of which is an approved additive for food use (E171). Although not intended for food use in the nano-form, E171 is now known to contain a significant fraction of the particles (~36 %) in the nanoscale (Weir et al. 2012). Despite a history of E171 use in food products, the extent of systemic uptake and biokinetic behaviour of TiO2 nanoparticles following oral ingestion is not known. The current study was undertaken to address this knowledge gap by investigating the absorption and biodistribution of nano- and submicron- sized particles of TiO2 using in vivo and in vitro models of oral intake. The study was designed around the use of a set of well-characterised TiO2 materials to generate baseline data to inform risk assessment of TiO2 in food products.
Materials and methods
Nanomaterials
Titanium dioxide (E171) was originally permitted in Europe in anatase form as a colour for use in certain foodstuffs. Following an European Food Safety Authority (EFSA) opinion (2004), which considered that also the use of rutile platelets would not pose safety concerns, the purity criteria for E171 were amended to include both anatase and rutile forms (Commission Directive 2006/33/EC). Other international specifications for TiO2, such as those of Joint FAO/WHO Expert Committee on Food Additives (JECFA), also allow the use of both anatase and rutile forms in food products. In view of this, the TiO2 materials selected for this study included both rutile and anatase forms, in nano- and submicron particle size ranges (Table 1).
Characterisation of nanomaterials and nanomaterial suspensions
The suppliers’ description of the TiO2 materials in terms of crystalline forms and nominal particle sizes is provided in Table 1. For use in this study, particle size distribution and agglomeration state of each material was determined using a number of characterisation methods. These included transmission electron microscopy (TEM), scanning electron microscopy (SEM) and CLS disc centrifugation for all the test materials, and additionally by single particle ICP-MS (sp-ICP-MS) and hydrodynamic chromatography for the materials used in the in vitro study. Each of the characterisation methods required a varying degree of dilution. These dilutions were prepared from a working stock dispersion of each material.
Further characterisation for some of the materials used in in vitro study was also carried out using dynamic light scattering (DLS), which confirmed that all the dispersions were composed of polydispersed particles, whereas the 25 nm Nanocomposix material was relatively monodispersed with agglomerates of around 136 nm. The results also showed the complexity of determining the size of a polydispersed particulate suspension using DLS method. In DMEM, all areas under the curves were very small, pointing to weak signals possibly caused by constituents of DMEM. There also seemed to be some overlap in the particle size ranges between the TiO2 suspension and the DMEM blanks. This overlap was, however, of low intensity and was most likely due to DMEM constituents.
Due to the highly agglomerative nature of TiO2 nanoparticles, different methods were attempted to obtain stabilised dispersions. These included applying high-powered sonication (Ultrasonic Processor at level 5, Heat Systems), and/or coating with ovalbumin. Furthermore, to ensure that the nanomaterials used in this study were composed of particles in the nanoscales, the dispersions were ultrasonicated, and nano-fractions were isolated by removing larger particles from the dispersions through centrifugation (detailed in “Physicochemical features of the TiO2 materials used” section).
In vivo studies
All animal studies described here were performed according to the national guidelines for the care and use of laboratory animals under approval of The Home Office license (under the Animals [Scientific Procedures] Act 1986). A specially designed cage system was used to house individual rats to collect the faeces and urine samples during the experiment (McKenzie et al. 2010). Sprague–Dawley 8-week-old male rats (purchased from Harlan Labs) were used in the in vivo study. Each rat was individually weighed before the experiment. The weights ranged between 200 and 429 g (Mean 347.5 +/− 46.6 g). The required amount of TiO2 dose was calculated for each rat and administered through a plastic syringe equipped with a plastic tube. The faecal samples were collected on a nylon mesh held above the tray which collected the urine. The tray collecting urine was held directly below the cage. Each cage housed a single rat.
Preliminary in vivo study
A preliminary in vivo study was carried out to measure the background levels of titanium in rats before the main study. Three (3) rats were used for this study, one of which was treated with the vehicle only to provide samples for determination of background exposure to TiO2 from diet and the environment, and the other two were orally administered with NanoAmor TiO2 (nominal particle size ~15 nm, measured size 40 nm) at 2 and 5 mg/kg bw. Samples (200 μl) of blood were collected at appropriate time intervals after TiO2 administration. Samples of urine and faeces were also collected and analysed for titanium content by ICP-MS.
Main in vivo study
The main in vivo study in rat involved oral administration of nano- or larger-sized TiO2 materials, followed by investigation into the potential translocation of titanium to blood and urine and distribution to various organs over a post-administration period of 96 h. The TiO2 materials described in Table 1 were administered via oral gavage to groups of six adult male rats as a suspension either in deionised water or 5 % ovalbumin solution. For oral dosing, rats were weighed and anesthetised with isoflurane, and the test materials in dispersion were orally administered to each rat. Samples of blood, urine and faeces were collected at appropriate time intervals (Table 2). Samples of blood, urine and faeces were collected immediately after oral gavage, and then at different time intervals over 4 days. Blood samples (0.3–0.4 ml) were collected from the tail vein and stored in lithium heparin tubes. Analysis of the sample showed no detectable titanium above background levels in the urine samples, which ruled out possible leaching of TiO2 from the faecal pellets into urine. After 96 h post-administration of TiO2, rats were killed and tissue samples of liver, brain, heart, kidney, spleen, GI tract (large and small intestine) were collected. The tissue samples were kept frozen at −80 °C and the urine, blood and faecal samples were kept in a cold room at 6–8 °C until analysed. For titanium analysis, the samples were digested in acid/hydrogen peroxide and TiO2 quantified (as Ti ion) by ICP-MS analysis.
In vitro translocation study
The in vitro work was designed to assess the potential of TiO2 nanoparticles to cross the GI-epithelial barrier. For this, a model based on co-culture of human enterocytes (Caco-2 cells) and M-cells was used. The model, described by Des Rieux et al. (2007), incorporates the two specialised cell types making it a more physiologically relevant model compared to monoculture-based models. The model has recently been used in a study of the translocation of silver (Ag) nanoparticles (Bouwmeester et al. 2011). In the current study, translocation of TiO2 nano- and micro-particles through the gut epithelium model was tested in a transwell system used sonicated TiO2 dispersions in ovalbumin solution. For comparison, the smallest particles were also tested after sonication to disperse the agglomerates in aqueous dispersions without ovalbumin. The cell monolayer integrity in the co-cultures was established by measuring trans-epithelial electrical resistance (TEER), as well as by evaluating cell permeability to Fluorescein isothiocyanate (FITC)-dextran (4 and 12 kDa) and Lucifer Yellow. The viability of the cells was assessed by determining mitochondrial activity using a WST-1 assay. The translocation of TiO2 particles was determined by means of hydrodynamic chromatography (HDC) coupled with ICP-MS measurements.
Nanoparticle suspensions
The TiO2 particles used in the in vitro study were Nanocomposix 25 nm, Sigma <25 nm, Sigma <100 nm, and Sigma <5 µm (Table 1). The TiO2 materials were suspended in water at a concentration of 4 mg/ml. After sonication with a probe tip (setting 5 for 60 s. Sonicator XL-2000), the particles were stabilised using 20 mg/ml ovalbumin (Sigma A5503). These stock suspensions (2 mg/ml nanoparticles in 10 mg/ml ovalbumin) were diluted by adding DMEM (supplemented with FCS, NEAA and PEST) and gently vortexed prior to cell exposure. All exposure experiments were performed in the presence of serum.
Cell lines and culture media
Human colon (colorectal adenocarcinoma) Caco-2 cell line (HTB-37TM) and human Burkitt’s (Lymphoma cells) Raji B line (CCL-86TM) were obtained from American Type Culture Collection (ATCC) and used at passages 31–34 and 9–18 respectively. Caco-2 cells were cultured in BioWhittaker’s Dulbecco's modified Eagle’s minimal essential medium (DMEM, 4.5 g/L glucose, with l-Glutamine, Lonza Verviers, Belgium) supplemented with 1 % penstrep (Sigma), 1 % non-essential amino acids (Lonza) and 10 % heat inactivated foetal bovine serum (FBS (Gibco)). Raji B cells were grown in RPMI 1640 medium (Gibco) supplemented with 1 % penicillin–streptomycin and 10 % FCS.
In vitro model of human intestinal epithelium
Caco-2 cells were grown in 75 cm2 flasks (Corning NY) in 12 ml DMEM supplemented with 10 % (v/v) foetal bovine serum (FCS) and 1 % (v/v) each of NEAA and PEST. Cells were incubated at 37 °C in humidified air (95 % RH) and 5 % carbon dioxide (Thermo® Scientific HERAcell 240). Culture medium was refreshed every other day, and at 80 % confluency the adherent cells were passed by sub-cultivation at ratio 1:6 or 1:7.
The inverted co-culture model used was adapted from Des Rieux et al. (2007) (Fig. 4). Briefly, Caco-2 cells were seeded in 12-well Transwell plates (Corning, NY) at a seeding density of 35,700 cells/cm2 (i.e. 40,000 cells/insert; 1.12 cm2 effective membrane growth area) and allowed to attach and grow for 3–5 days with culture media being refreshed every other day. Sterilised silicon tubes (internal diameter, 14 mm; height, 20 mm) were fixed to the basolateral part and the inserts inverted in a sterile glass cell culture dish (diameter, 245 mm; height, 20 mm). The Caco-2 cell monoculture was maintained in this inversed orientation and submerged in culture medium until day 21–23. The silicon (basolateral) chamber was refreshed with DMEM every other day until day 16–18. At day 16–18, Raji cells, cultured in suspension in 10 % w/v FBS-enriched RPMI medium, were centrifuged at 1,200 rpm (Heraeus Labofuge 400 R) for 7 min and resuspended in DMEM. Cells were counted and co-cultured with Caco-2 cells by dispensing 500 µl resuspended Raji cells into the basolateral chamber of the insert (35,700 Raji cells/cm2). The Caco-2/Raji co-culture was maintained until day 21–23 when the exposure and translocations studies were conducted. It is worth highlighting that the transwell system was only inverted during culturing. For exposure to the TiO2 materials, the transwells were oriented in the correct upward position, meaning that the Caco-2/Raji co-culture in the apical chamber faced upwards. Unless stated otherwise, all media and solutions were pre-warmed to 37 °C before use.
Monolayer integrity
The monolayer integrity was determined by measuring TEER and the paracellular permeability of the monolayer to Lucifer Yellow (LY; mw 450) and FITC-Dextran (4 and 10 kDa). TEER measurement of cell monolayer was conducted in freshly added DMEM using Millipore Millicell-ERS for cells grown in inserts. The increase in TEER across cell monolayer was monitored every time culture media were refreshed, until inserts were inverted and it was no longer practicable. Measurements were also taken pre- and post-exposure to compounds to compare effect on tight junction. Percentage change in TEER was calculated using (Teer i −Teerp)/Teer i ) × 100, where Teer i and Teerp represent the pre-exposure and post-exposure TEER respectively. The paracellular permeability of cell monolayer was evaluated using Lucifer Yellow (low molecular weight) and FITC-Dextran 4 & 10 kDa (Sigma, Steinheim, Germany) with and without treatment with 2.5 mM ethylene glycol-bis (beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA; Sigma, Steinheim, Germany). EGTA was employed as a tight junction and adherents junction disruptor via its activation of protein kinase C and Ca2+ chelating effect and as such capable of increasing paracellular permeability (Deli 2009; Tomita et al. 1996). EGTA solutions were prepared in HBSS for the EGTA positive groups. EGTA was added apically and basolaterally to a final concentration of 2.5 mM and incubated for 60 min (2 × 30 min with refreshment of EGTA after 30 min). Inserts were washed with HBSS following this treatment and exposed, for 90 min, to test compounds (DMEM) apically (basolateral fraction 2.5 mM EGTA/DMEM). Samples from both chambers in both groups were collected and the amount of Lucifer Yellow and FITC-Dextran was measured by fluorimetry (BioTek® synergy HT) at excitation/emission wavelength of 485/530 nm.
The results are reported as percentage of compound in the basolateral chambers from the total added compound.
Exposure conditions
A non-cytotoxic concentration of TiO2 nanoparticles was selected for the translocation studies. For this, a WST-1 viability assay was carried out on Caco-2 cells. The WST-1 assay is based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases, which acts as a marker for cell viability. Briefly, 100 µl Caco-2 cells were seeded in 96-well plates at a concentration of 100,000 cells/ml. Forty-eight (48) hours after seeding, an equal volume (100 µl) of nanoparticles (at 10, 50, 100 and 250 µg/ml) was added. After 24 h exposure, cell viability was determined using the WST-1 assay (ROCHE, Mannheim, Germany). For this, 20 µl of the WST-1 reagent was added, and after 2 h incubation at 37 °C optical density (OD) was measured at 450 nm in a BioTek Synergy™ HT microplate Reader. The OD value of the cells with nanoparticles before WST-1 addition was subtracted from the OD value after 2 h WST-1 incubation.
The percentage cell viability was calculated using the following equation:
Tests were carried out to establish a non-cytotoxic but relevant dose for the cell exposures. For WST-1 viability assay, the aim was to set exposures at a dose that decreased viability of Caco-2 cells at round 20 %. For all three sizes of the TiO2 nanoparticles tested, a decrease in cell viability was found with an increase in nanoparticle concentration. However, these effects were more prominent for the larger sizes than the smaller ones. Based on these observations, a concentration of 250 µg/ml was chosen for translocation studies. The translocation of TiO2 particles was therefore tested at a concentration of 250 µg/ml over a 24 h exposure period. 500 µl of each concentration was added to the apical chamber of 21-day-old Caco-2/Raji cells co-culture monolayer in 12-well Transwell plates. Cells were incubated for 24 h. DMEM was used as culture medium in the translocation experiments and samples were collected from both apical and basolateral compartments of the transwell plates, also the cells were collected (after SDS treatment), and cell culture medium was gently removed.
Analysis of titanium in samples
Quantification of titanium was carried out by inductively coupled plasma mass spectrometry (ICP-MS) using medium resolution. For this, the TiO2 particles were solubilised by alkali or acid digestion (see below), appropriately diluted, and measured using a ThermoFisher ‘Axiom’ ICP mass spectrometer tuned within 3,000–5,000 resolution to isolate the titanium signal from potential polyatomic interferences. Alkali digestion was used to solubilise TiO2 particles in aliquots of blood (0.1 ml) and urine (0.5 ml). The samples were transferred to polypropylene autosampler tubes and digested in sodium hydroxide (0.5 ml, 1 M) using a hot block set at 100 °C for 60 min. After cooling, the contents were made up to 10 ml with alkaline solution (0.05 % w/v EDTA, 0.05 % w/v Triton X-100, 1.0 % w/v NH4OH). Acid digestion was used for tissue samples, 0.2 g aliquots of which after grinding the whole sample were transferred to polypropylene autosampler tubes and digested in sulphuric acid (0.5 ml, ~18 M) using a hot block set at 100 °C for 60 min. After cooling, hydrogen peroxide (0.5 ml, 30 % v/v) was added carefully before heating the tubes for a further 120 min. After cooling, the contents were made up to 10 ml with Millipore deionised water (conductivity 18 MΩ). A set of standards (50, 20, 10, 5 and 0 ng/ml) was also prepared in the same matrix and all tubes were fortified with scandium, rhodium and indium (all at 2 ng/ml) for use as internal standards. The digestion of faecal samples posed a particular challenge and the acid digestion was adapted to ensure complete digestion of TiO2 particles. For this, aliquots of faecal samples (ranging from 0.2 to 0.4 g) plus QC material NIST1548a (total diet) were digested in 5 ml nitric acid using quartz high pressure closed vessels and microwave heating. After cooling, the digests were transferred to plastic test tubes and diluted up to 10 ml with Millipore water (18 MΩ). A set of stock standards (500, 200, 100, 50 and 0 ng/ml) was prepared in the same matrix. Diluted digests (0.025 ml) were further diluted with nitric acid (0.5 ml, 50 % v/v) plus internal standard solution (4.5 ml, nitric acid, 1 % v/v; hydrochloric acid 0.5 % v/v; containing scandium, rhodium, indium, all at 10 ng/ml). The stock standards (0.5 ml) were further diluted with internal standard solution (4.5 ml) to produce matrix-matched standards (50, 20, 10, 5 and 0 ng/ml).
The following masses were monitored: 45Sc, 47Ti, 49Ti, 103Rh, 115In. Liquid samples were aspirated into the plasma using a standard setup of a concentric nebuliser/double pass spray chamber. Platinum-tipped sampler and skimmer cones were used throughout the work.
All batches analysed by ICP-MS contained quality-controlled reagent blanks, spiked blanks, spiked samples, a reference material (NIST 1548a, total diet, non-certified value 4,700 ng/g titanium). Reagent blanks were included to monitor background contamination. A selection of reagent blanks and samples were also spiked with a known amount of each form of titanium (aqueous and nanoparticle) to estimate recovery. Alkali digestion could not cope with solid reference material, and therefore a volume of acid digest was used instead. Separate titanium spikes were used from a commercial aqueous standard and titanium dioxide in suspension.
Any TiO2 particles translocating to and contained in the cells, as well as the remaining particle concentration in the apical compartment, was measured using single particle inductively coupled mass spectrophotometry (SP-ICP-MS). The cells were released from the membrane by adding 10 µl 10 % SDS followed by a 15 min incubation at 37 °C. 200 µl of the apical, 500 µl of the basolateral phase, and half of the cells were snap frozen in liquid nitrogen, and transferred to −80 °C. The remainder of the sample, of each fraction, was subjected to SP-ICP-MS analysis. For this, samples were analysed as described in Peters et al. (2014). In brief, samples were analysed with a Thermo X series-2 ICP-MS equipped with an autosampler and conical glass concentric nebulizer and operated at an RF power of 1,400 W. Data acquisition was performed in the time-resolved analysis (TRA) mode. The dwell time was set at 3 ms with a typical acquisition time of 60 s per measurement. Because of the short dwell time, it was not possible to switch between different isotopes (switching needed around 50mn), and therefore only one titanium isotope, m/z ratio 48, was monitored during the measurements. Polyatomic interferences are therefore possible, for example 32C16O and 36Ar12C, and also isobaric interference by 48Ca. However, these interferences would result in a continuous background, whereas TiO2 particles resulted in discontinuous signals (peaks) and could thus be distinguished from the background. Data were transferred to a comma-separated values (CSV) file, which was processed in Microsoft Excel for calculation of particle sizes, particle size distributions and particle concentrations. As no TiO2 standard with known size and concentration of the engineered nanoparticles (ENPs) was available, the system was calibrated using the <100 nm TiO2 ENPs that were used in the translocation experiment. The size measurements derived from these experiments are therefore only relative.
Statistical analysis
The measurement results were analysed in a linear mixed model for main effects only. Fixed Effects: nominal size + pre-treatment + vehicle, with rat, day and analytical run as crossed random effects. This model and method was chosen in response to the way that the experiment had been undertaken, rather than as a reflection of an underlying model for the effects that the various kinds of treatment might have. A value of p < 0.05 was used for determining whether a main effect in the model was statistically significant.
The main questions emanating from the test results were: is titanium present in the treatment organs at higher concentrations than the control organs?; What is the size of the effects of particle size, pre-treatment, and vehicle on the concentration of titanium?; And how large may the addition of titanium of different nominal particle size be?
Results
Physicochemical features of the TiO2 materials used
The characterisation experiments showed that all of the nanomaterials acquired from different sources invariably contained a considerable proportion of larger (non-nano) sized structures. These were present in the material samples both in the form of large- sized objects and agglomerates/aggregates of nanoparticles, with sizes approaching up to micro-metre range. A typical SEM image is shown in Fig. 1, and a typical particle size distribution profile as measured by CLS disc centrifugation is shown in Fig. 2, showing that all of the supplied ‘nanomaterials’ tested contained around ~80 % by weight of larger (typically between 200 and 500 nm) structures. Conversion of mass-based particle size distribution to particle number-based values, however, still showed the majority of the particles to be the nanoscale (Fig. 2).
A typical SEM showing the presence of submicron- and micron-sized objects in a TiO2 material that was purchased as a nanomaterial. Although the image shows Sigma TiO2 material (nominal particle size <25 nm), other nanomaterials used in this study also showed a similar presence of larger-sized objects of up to 1 µm size. These were removed from dispersions used in this study by centrifugation as described in “Physicochemical features of the TiO2 materials used” section
A typical particle size distribution of TiO2 materials measured by CLS Disc Centrifuge. Particle size distribution in terms of relative weight or particle number versus particle diameter measured by CLS disc centrifugation. The dispersion used was prepared as described in Table 1. (Sigma, cat # 637254, TiO2-anatase, 99.7 %, <25 nm)
High-powered sonication, followed by centrifugation, separated the nano-sized fraction by sedimenting larger-sized clusters. The nano-dispersions obtained by this method were stable in aqueous media over a few hours. The nanoscale fractions isolated as a supernatant from this procedure were either used straightaway as such, or freeze-dried and resuspended before use. Average nano-fraction obtained by this method was around 17 % (n = 3) of the original as measured by weighing after freeze-drying. Similar results were obtained by DLS method, although the hydrodynamic sizes measured by DLS are generally larger than those measured by other methods. The results of DLS measurements showed that between 10 and 17 % of the particles had a size in the nanoscale, whilst the remaining particles were in larger size range (600–700 nm). It was found that particle agglomeration had again taken place in freeze-dried nano-fractions on resuspension in aqueous media (a typical example is shown in Fig. 3). The materials and sample preparation methods were therefore adapted appropriately to obtain a uniform nano-fraction for use in in vivo and in vitro experiments (described in Table 1).
Overlay of particle size distribution of not-fractionated (green), fractionated (red) and fractionated & freeze-dried (blue) TiO2 material measured by DLS disc centrifugation. The dispersions used were prepared as described in Table 1. (NanoAmor—5,430 MR, 99.7 %, TiO2-anatase, ~15 nm). (Color figure online)
In vivo studies
Preliminary in vivo study
The results of analysis of blood and urine (not shown) from pilot in vivo study indicated that very little TiO2 was absorbed within 24 h at both 2 and 5 mg/kg bw treatment levels. However, the background levels of titanium were still significantly above the limit of detection. It was thought that diet was the source of high background titanium levels, and therefore a low titanium diet (0.44 ng/g) was established and three other rats were fed on this diet for 15 days. Separately, three rats fed on normal diet were used as control. The levels of titanium detected in blood (4–21 ng/ml) and urine (12–46 ng/ml) from the control rats and those fed on low titanium diet were still significantly greater than the limit of detection (8 ng/g). Also, unexpectedly, large amounts of titanium were detected in the faeces of the control rats (5.33–9.02 µg/g), which were even greater than the amount that could have possibly been ingested through the feed in control rats. This led to further investigations into the other possible sources of titanium intake in the rats. Analysis of water did not show any detectable levels of titanium, but an unexpected source was found to be the cardboard “toys” that were provided in the cages as ‘environmental enrichers’ which contained a considerable amount of titanium (~4.5 µg/g). As the rats with high background titanium levels were not suitable for this study, a fresh breeding nucleus of rats was established by feeding on a ‘titanium-free’ diet, and not providing them with the cardboard toys. The parental generation was maintained on this regime for 3–4 weeks before mating. Titanium levels in the tissues and faeces of the 50–60-day-old offspring of these rats were monitored to establish low background levels before use in the main in vivo study.
Main in vivo study
The main in vivo study was carried out using the materials described in Table 1 and the rats bred on titanium-free diet. For oral gavage, each TiO2 material was suspended at 10 mg/ml in deionised water or 5 % ovalbumin solution, and was orally administered to rats. Subsequent analysis of titanium in aliquots of the test samples by ICP-MS showed that the amount administered was equivalent to 4.6 mg/kg bw TiO2. This was taken into consideration and a correction factor was applied when recoveries of TiO2 were calculated. Five groups of rats (six rats per treatment) were used in the main experiment. Half of the rats were dosed on first day of the week, and the remaining half on first day of the following week. The sequence of the administrations was also randomised between two experiments to avoid operator bias.
Oral administration of nano- or micro-sized fractions of TiO2 was followed up by study of the potential translocation and distribution of the particles (measured as titanium by ICP-MS analysis) to blood, urine, faeces and tissue samples of liver, kidney, spleen, heart, brain, GI tract over a period of 96 h. The results of titanium analysis in rat blood, urine, faeces and tissue samples are summarised in Tables 2, 3, 4, 5. The results are shown as mean values calculated from the averages of 12 duplicate values (n = 6). Initial samples were taken at a time of 0–2 h. The sampling of blood was staggered over a period of 2 h between individual rats. The 0 h represents samples taken immediately from the first rat following oral administration, and 2 h represents a maximum time delay between the administration and collection times to permit the sampling at the appropriate time point for each rat. This same sampling sequence was carried over for all of the sampling times on the subsequent days post-treatment.
With the exception of excretion patterns of Ti in faecal samples, the result of statistical analysis (Table 6) showed that there were no significant effects between TiO2 treatments following oral exposure to TiO2 particles; and the variation in measurement results within each organ was consistent with that caused by analytical measurement variation. Estimates of the mean concentration of titanium in treatments by particle size based on variation driven by analytical measurement uncertainty are given in Table 7. In general, the mean concentrations of titanium in organs from rats that had been treated were not significantly higher than those that had not been treated. In the GI tract samples, however, the mean concentration of titanium taken from rats treated with ‘<100 nm’ particles was significantly higher than the mean concentration of titanium in the samples taken from rats in the control group (Difference = 86.7 ± 82.44 ng/g with 95 % confidence).
Table 8 shows estimates of the variance associated with different sources of experimental variation. Variation associated with the measurement of titanium dominates. None of the “fixed effect” factors (titanium particle size, pre-treatment, vehicle) had a significant effect on the observed level of titanium in organs. The variation observed in the results was dominated by residual variation of individual results and batches into which samples were grouped for analysis. These observations are consistent with the measurement of titanium at levels close to or below the measurement method’s limit of detection (LOD), where the observed variation associated with the analysis of samples can be expected to dominate over the observed variation in the estimated level between samples.
The results (Tables 2, 3, 4, 5) show that oral administration of TiO2 nano- (or larger) particles did not lead to translocation of titanium to blood, urine, or distribution to various organs in rat at any of the time intervals studied during the 96 h post-treatment. With the possible exception of one particle size, the mean concentration of titanium in the GI tract was not significantly affected by treatment.
The orally administered TiO2 materials in all cases were found to be excreted in the faeces (Table 5), although this happened at different elimination rates in different test animals. Trace amounts of titanium were also detected in the GI tract samples. The recoveries of TiO2 in faecal samples for the two Sigma materials (<100 and <5,000 nm) were much lower than the samples from the animals treated with other materials. Since TiO2 was not absorbed/translocated from the GI tract in any of the materials tested (Tables 3, 4, 5), this discrepancy indicates a possible analytical error due to incomplete solubilisation of the Sigma materials in faecal samples. These two materials were composed of rutile form of TiO2, which is much more difficult to solubilise even in strong acid media than anatase. For example, the greater solubility of anatase in hydrofluoric acid (HF) solution has been used in isolating pure rutile particles from a mixture of rutile and anatase TiO2 powder (Ohno et al. 2001).
In all instances, the TiO2 materials were not completely cleared from the faeces at the end of the 96 h sampling period. However, since there was no absorption/translocation of TiO2 detected across the GI tract in any case, it was considered unjustifiable to use more animals to extend the sampling period beyond 96 h to account for all the administered dose of TiO2.
In summary, the findings of the in vivo study indicate that the orally ingested nano- and larger TiO2 particles are not absorbed in any appreciable amount above the detection limits from the GI tract in rat, and are excreted over a period of several days in the faeces (Fig. 4).
Schematic representation of the inverted co-culture model (from des Rieux et al. 2007)
In vitro study
The results of WST-1 assay to test the effects on Caco-2 cell viability (Fig. 5) indicated that all of the TiO2 nanoparticles tested decreased cell viability with increasing nanoparticle concentration. These effects were most prominent for the larger sizes, and thus appear to be indicative of the physical effect of the particle rather than the nano-size. At a concentration of 250 µg/ml, the concentration used for translocation study, cell viability was reduced (relative to control cells) to 90.4, 78.8 and 79.2 % for the <25 nm, <100 nm and <5 µm particles respectively. No significant effects on cell viability were observed using the Nanocomposix (25 nm) TiO2 nanoparticles (data not shown).
Monolayer integrity
TEER measurement of cell monolayers was conducted in freshly added DMEM. Measurements were taken pre- and post-exposure to evaluate the effect of the compounds on monolayer integrity. The TEER values after exposure to Lucifer Yellow, 4 kDa Dextran and 10 kDA Dextran were variable but the percentage change was around 15 %. EGTA treatment resulted in lower TEER readings (−66 to −70 % for Lucifer Yellow, Dextran 4 kDa and Dextran 10 kDA, respectively), indicating the functionality of the monolayer. The cell viability was further confirmed by translocation marker study below.
The cell monolayer permeability was evaluated using Lucifer Yellow (low molecular weight) and FITC-dextran 4 and 10 kDa (medium and high molecular weight indicators) with and without treatment with 2.5 mM EGTA. The tight junctions of the EGTA-treated transwells were open resulting in translocation of Lucifer Yellow and dextrans (Fig. 6).
Translocation of TiO2 nanoparticles
For the translocation experiments, the Caco-2/Raji cell co-culture model was used. To assess the potential cytotoxicity only Caco-2 cells were used. These cells were cultured 48 h before exposure (as described in “Exposure conditions” section). It is well known that proliferating Caco-2 cells are more sensitive to toxicity of NPs than differentiated cells as used in the translocation experiment, this has been shown using silver nanoparticles (Bohmert et al. 2014; Gerloff et al. 2013). Although the results showed a decreased viability at the doses evaluated, but not below 79 %. The TEER measurements in the 21-day-cultured monolayers of Caco-2/Raji were also not reduced indicating that the exposure did not critically affect the integrity of the monolayer.
The translocation of TiO2 nanoparticles was evaluated in two separate experiments. Cell fractions were also collected for analysis in one of the experiments. The exposures were performed in triplicate. TEER values after the exposure to TiO2 particles were generally higher than before the exposure, and it can be concluded from the TEER changes that cellular monolayers remained intact during the exposure (see Fig. 7).
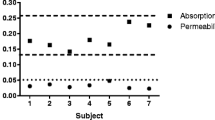
Relative changes in TEER after 24 h exposure to TiO2 compared to changes in TEER in controls (e.g. not exposed to TiO2). *Ova ovalbumin used for nanoparticle stabilisation, # water no ovalbumin added, theoretically less stable suspension, $ Ova/DMEM control sample without nanoparticles, same amount of ovalbumin
To measure possible translocation of the TiO2 nanoparticles; apical, basolateral fractions were subjected to SP-ICP-MS analysis. Because low translocation was observed in the first experiment, cell fractions from the second experiment were subjected to analysis by ICP-MS in single particle mode (sp-ICP-MS). The translocation of TiO2 over the transwell membrane without cells was not studied. However, another study with 50 and 100 nm polystyrene particles had shown that the particles were able to readily pass through the transwell membrane (data not included).
Proper interpretation of sp-ICP-MS data requires a standard with known size and concentration of nanoparticles. Such a standard for TiO2 nanoparticles is currently not available. The system was, therefore, calibrated using the <100 nm TiO2 nanoparticles, that were used in the translocation experiment. The size measurements derived from these experiments are therefore only relative. Comparison of the standards in water, ovalbumin and ovalbumin/DMEM suggested that the presence of ovalbumin stabilised the particles, preventing agglomeration and thus leading to a relatively higher concentration of the free particles. The background value of titanium, measured by SP-ICP-MS, in water was <1 µg/l.
Stock solution (250 µg/ml TiO2 -> 150 µg/ml Ti) of the <100 nm OVA particles, resulted in particles with a median size of 56 nm (calculated based on mass of Ti only) and a concentration of 85 µg/ml, corresponding to a recovery of 57 % (corrected for Ti/TiO2 difference in MW). A 500 µl aliquot of the stock solution of <25 nanoparticles was added to the apical fraction. Due to lack of nanoparticle standards, the particle number in this fraction could not be reliably measured by sp-ICP-MS. The basolateral fraction contained <1 µg/l titanium nanoparticles not significantly different from the blank. Compared to this, 136 ± 31 µg/l titanium (mean of triplicate ± SEM single translocation experiment) was measured in the cellular fraction.
After 24 h of incubation with TiO2 dispersions, the concentration of <100 nm particles was around 1 µg/l apically. In the cell fraction, the concentration was 16 ± 0.7 µg/l (mean of triplicate ± SEM single translocation experiment). The titanium concentration in the basolateral phase was below detection level (<1 µg/l). The <5 µm particles were only recovered in the cell fraction 50 ± 4.7 µg/l (mean of triplicate ± SEM single translocation experiment), both basolateral and apical fractions contained <1 µg/l. For the 25 nm nanocomposix particles, 500 µl of a concentration 250 µg/ml was added to the apical fraction. Again, due to lack of standards, the number of particles in this fraction could not reliably be measured by sp-ICP-MS. In the cell fraction, the concentration was found to be 258 ± 16 µg/l (mean of triplicate ± SEM single translocation experiment). The titanium concentration in the basolateral phase was again below detection level (<1 µg/l).
These findings indicate that the TiO2 materials tested were polydispersed particles and their agglomeration/aggregation state changed over a period of 24 h (both in terms of size and polydispersity).
The study of possible translocation of TiO2 nanoparticles through the co-culture model was followed by analysing the apical, basolateral and cell fractions by sp-ICP-MS. Chemical analysis showed some titanium in the cell fractions but not in the basolateral fraction indicating that nanoparticles had settled between or in the cells. It is possible that there was some retention of titanium in Caco-2 cells or the gut epithelial cells. However, because of the large number of TiO2 particles apparently adhered to the cell surface, it was not possible to detect or quantify any nanoparticles that might have been internalised by the cells.
In summary, despite the difficulties in determining the particle distribution by sp-ICP-MS, the results of chemical analysis clearly showed that neither of the nano- or larger TiO2 particles crossed the in vitro gut epithelium model used in the study.
Discussion
An important consideration in relation to testing a nanomaterial is that they may behave differently from conventional (soluble) chemical substances in biological media. For example, insoluble nanoparticles when dispersed in aqueous media will exist in a suspension rather than solution. They also tend be more agglomerative than their larger-sized counterparts. Knowing the agglomeration/aggregation state of nanomaterials is therefore important for any investigation of the biokinetic behaviour of nanoparticles. In view of these considerations, this study placed a due emphasis on the characterisation of the TiO2 test materials used in the experiments. The findings showed that TiO2 nanoparticles are very agglomerative in nature, and therefore any TiO2 materials acquired from commercial sources as ‘nanomaterials' need to be appropriately characterised to ensure that the particles and/or clusters are within the size range selected for the study of nano parameters. The nano- and micro-sized materials used in this study were dispersed by ultrasonication and fractionated to obtain particle fractions in the nanoscale before use in the experiments.
Despite a large body of research carried out in recent years, the available information on properties, behaviour and effects of orally ingested nanoparticles is still limited. In this regard, a major knowledge gap exists in relation to ADME parameters. Although a few studies have suggested possible uptake and translocation of nanoparticles ingested via oral route, they have not been conducted in a systematic manner. In particular, they lack characterisation of the nanomaterials tested, and it is not clear whether the substances translocating from the GI tract were nanoparticles or solubilised forms of the test materials. For example, Jani et al. (1994) reported that TiO2 particles (rutile, 500 nm) translocated to systemic organs, such as the liver and spleen, following oral gavage of 12.5 mg/kg to female Sprague–Dawley rats for 10 days. Translocation of TiO2 was detected in the lungs and peritoneal tissues, but not in the heart or kidney. A more recent study by Wang et al. (2007) used three particle sizes of TiO2 (25 nm, 80 nm and fine particles) to dose mice orally at 5 g/kg. The TiO2 materials were dosed as a suspension in 0.5 % hydroxypropyl methylcellulose. The study reported that 7 out of 30 mice died within 3 days of oral administration of TiO2 (attributed to injury during oral administration). The analysis of tissue samples showed up to 240 ng/g Ti in tissues. After 2 weeks, 4,000 ng of Ti could be detected in spleen of mice dosed with 80 nm TiO2 nanoparticles, and 500 ng/g of Ti in liver of mice dosed with 25 and 80 nm TiO2 nanoparticles. In total, around 0.05 % of the administered dose was found distributed across the whole body. The study however has several drawbacks. Sufficient characterisation of the nanomaterials used was not carried out. The administered dose (5 g/kg/bw) was very high. Frequent oesophageal ruptures during the dosing procedure were reported that led to the death of some animals. The measurement of translocation was based on chemical analysis of titanium, and as such it is not clear whether the titanium detected in various organs was due to translocation of TiO2 from the GI tract in nanoparticle or solubilised form. It is also not clear whether the observed effects were due to TiO2 toxicity, or due to overloading the gut at such a high dose of the test materials.
Another recent study has suggested that 5 day exposure to 1 or 2 mg/kg bw per day anatase TiO2 resulted in significantly increased Ti levels in ovary and spleen and was indicative of hormonal effects. The primary particles size of these TiO2 materials was <25 nm, which agglomerated to mean diameter up to 1.6 µm with around 13 % of the particles remaining smaller than 100 nm (Tassinari et al. 2013).
Similarly, a study by Gui et al. (2013) on 90 day intragastric exposure to 2.5, 5 and 10 mg/kg anatase TiO2 nanoparticles with a crystallite size of 5.5 nm and a hydrodynamic size around 300 nm (in 0.5 % hydroxypropyl methylcellulose dispersion) has indicated kidney effects, such as renal inflammation, tissue necrosis and renal apoptosis. In addition, evaluation of titanium dioxide by the International Agency for Research on Cancer (IARC) (2010) has noted induction of lung tumours in inhalation and intratracheal instillation studies in rats. However, oral, subcutaneous and intraperitoneal administration of TiO2 did not show any significant increase in the tumour frequency. IARC classified TiO2 as a Group 2B carcinogen (possibly carcinogenic to humans) on the basis that there is inadequate evidence in humans but sufficient evidence in experimental animals for the carcinogenicity of titanium dioxide.
Information from studies on other nanomaterials suggests that the diffusion rate of particulate materials through the gastrointestinal (GI) mucus is likely to be influenced by a number of factors, predominantly size, charge, and surface coating (Lai et al. 2007). In general, translocation from the GI tract has been reported to be greater for nanoparticles than larger particles (Desai et al. 1996, des Rieux et al. 2006). Within the nano-size range (1–100 nm), smaller nanoparticles (1–10 nm) have been found to cross the mucus layer faster than those in the upper nano range (Hillyer and Albrecht 2001; Hoet et al. 2004). As a result, the translocation of smaller nanoparticles has been shown to be comparatively greater than the larger nanoparticles. For example, oral administration of colloidal gold nanoparticles (4, 10, 28 and 58 nm) to mice has been reported to result in an increasing distribution of the smaller nanoparticles to different organs (Hillyer and Albrecht 2001). A study by Kim et al. (2008) has shown accumulation of silver nanoparticles in the GI tract of Sprague–Dawley rats, followed by kidney and liver, lungs, testes, brain and blood following repeated oral administration of nano-silver (60 nm). In an in vitro study on human epithelial cell cultures using fluorescence-labelled silica (SiO2) nanoparticles, Chen and Mikecz (2005) showed that the smaller nanoparticles could enter cell nuclei.
The results of the current in vivo study showed that oral administration of 5 mg/kg of TiO2 in any of the nano- (or larger) particle sizes tested did not lead to translocation of TiO2 (measured as titanium) to blood, urine or distribution to various organs (liver, kidney, spleen, heart, brain) in rat at any of the time intervals studied during the 96 h post-administration period. There was no difference in the absorption/translocation of the TiO2 materials tested that had different size ranges (nano- or micro-metre). Although suspending TiO2 nanoparticles in ovalbumin added another variable compared to the dispersions of larger particles, this also did not lead to any significant difference in the absorption/translocation of the test materials from the GI tract. However, since a few individual measurement values for TiO2 were close to, or just above, the background levels, which also varied between different tissue samples, the possibility for the absorption of a trace amount of the nanoparticles in some animals cannot be completely excluded. In all cases, the orally administered TiO2 materials were found to be excreted in the faeces, although elimination rates varied between the individual animals. The test materials were not completely cleared from the GI tract at the end of the 96 h of experiment, and a significant tail-end of residual TiO2 remained in the faeces and in the GI tract at the end of the 72 h sampling period.
The in vitro study also indicated a lack of TiO2 translocation across the gut epithelium model based on a co-culture of Caco-2 and M-cells. The TiO2 particles seemed to settle between or in the cells, as analytical measurements showed titanium in the cell fractions, but not in the basolateral fraction. All three sizes of the TiO2 nanoparticles tested decreased the viability of Caco-2 cells with increasing nanoparticle concentration. However, these effects were most prominent for the larger sizes rather than smaller ones. In all cases, the results of chemical analysis clearly indicated that neither of the tested TiO2 nano- or larger particles crossed the model gut epithelium.
Thus, the overall conclusion both from the in vivo study in rat, and the in vitro human gut epithelium model, is that the TiO2 nanoparticles (and larger particles for that matter) are not likely to be absorbed in the GI tract following oral intake, and are likely to be excreted in the faeces over a period of time. Due to the absence of detectable blood plasma levels of titanium, the toxicokinetic parameters such as T1/2, Tmax or area under the curve (AUC) could not be determined.
It is also worth highlighting that a recent review by (Powell et al. (2010) has suggested that metal/oxide nanoparticles can be taken up by M -Cells of Peyer’s Patches and passed on to the underlying macrophages where they may accumulate and appear as pigmentation in cells at the base of human intestinal lymphoid aggregates. It is, therefore, possible that during the slow GI passage of TiO2 observed in this study, there is some absorption of TiO2 (as nanoparticles or titanium ion) in the epithelial cells of the GI tract. Attempts were made in this study to clean as much as possible the samples of in vitro membrane and the GI tract (by flushing water through) before analysis for titanium content, but the likelihood of some cellular uptake in the gut epithelium cannot be excluded. The difficulties in distinguishing between the trace amounts of intra- or extra-cellular titanium are evident from the presence of a residual amount of TiO2 in faeces even after 4 days of oral administration, which indicates that it is not easily cleared from biological surfaces. Determining any intracellular TiO2 will need further research as the amounts are most likely to be extremely small and need to be separated from particles adhered to external cell surfaces.
The findings of this study also need to be seen in perspective because TiO2 nanoparticles (due to inert nature and highly agglomerative behaviour) may not be a suitable representative of other nanomaterials that may be used in food/feed applications, and which may have different physicochemical properties, including more dispersed particles in the nanoscale. Further research is therefore needed to obtain such baseline knowledge on other types of nanomaterials that are, or may be, used in food/feed products.
References
Bohmert L, Girod M, Hansen U, Maul R, Knappe P, Niemann B, Weidner SM, Thunemann AF, Lampen A (2014) Analytically monitored digestion of silver nanoparticles and their toxicity on human intestinal cells. Nanotoxicology 8:631–642
Bouwmeester H, Poortman J, Peters RJ, Wijma E, Kramer E, Makama S, Puspitaninganindita K, Marvin HJ, Peijnenburg AA, Hendriksen PJ (2011) Characterization of translocation of silver nanoparticles and effects on whole-genome gene expression using an in vitro intestinal epithelium co-culture model. ACS Nano 5(5):4091–4103
Chaudhry Q, Castle L (2011) Food applications of nanotechnologies—an overview of opportunities and challenges for developing countries. Trends Food Sci Technol 22(2011):595–603
Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, Aitken R, Watkins R (2008) Applications and implications of nanotechnologies for the food sector. Food Addit Contam 25(3):241–258
Chaudhry Q, Castle L, Watkins R (eds) (2010) Nanotechnologies in food. Royal Society of Chemistry Publishers, London. ISBN 978-0-85404-169-5
Chen M, von Mikecz A (2005) Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Exp Cell Res 305(1):51–62
Commission Directive 2006/33/EC of 20 March 2006 amending Directive 95/45/EC as regards sunset yellow FCF (E 110) and titanium dioxide (E 171)
Deli MA (2009) Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta 1788(4):892–910
des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V (2006) Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release 116:1–27
des Rieux A, Fievez V, Théate I, Mast J, Préat V, Schneider Y-J (2007) An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharm Sci 30(5):380–391
Desai MP, Labhasetwar V, Amidon GL, Levy RJ (1996) Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res 13(120):1838–1845
EFSA [European Food and Safety Authority] (2004) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission related to the safety in use of rutile titanium dioxide as an alternative to the presently permitted anatase form, Question No EFSA-Q-2004-103, Adopted on 7 December 2004. EFSA J 163:1–12
Frewer L, Norde N, Fischer A, Kampers F (eds) (2011) Nanotechnology in the agri-food sector. Implications for the future. Wiley, Weinheim. ISBN: 978-3-527-33060-7.
Geiser M, Kreyling WG (2010) Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol 20(7):2
Gerloff K, Pereira DI, Faria N, Boots AW, Kolling J, Forster I, Albrecht C, Powell JJ, Schins RP (2013) Influence of simulated gastrointestinal conditions on particle-induced cytotoxicity and interleukin-8 regulation in differentiated and undifferentiated Caco-2 cells. Nanotoxicology 7:353–366
Gui S, Sang X, Zheng L, Ze Y, Zhao X, Sheng L, Sun Q, Cheng Z, Cheng J, Hu R, Wang L, Hong F, Tang M (2013) Intragastric exposure to titanium dioxide nanoparticles induced nephrotoxicity in mice, assessed by physiological and gene expression modifications. Part Fibre Toxicol 10:4. doi:10.1186/1743-8977-10-4
Hillyer JF, Albrecht RM (2001) Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci 90:1927–1936
Hoet P, Bruske-Hohlfeld I, Salata O (2004) Nanoparticles—known and unknown health risks. J Nanobiotechnol 2:1–15
Jani P, Halbert GW, Langridge J, Florence AT (1990) Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol 42(12):821–826
Jani P, McCarthy D, Florence AT (1994) Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int J Pharmacol 105(2):157–168
Kim YS, Kim JS, Cho HS, Rha, DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon H, Jeong J, Han BS, Yu J (2008) Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Tox 20:575–583
Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang Y-Y, Cone R, Hanes J (2007) Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. PNAS 104:1482–1487
McKenzie J, Charlton A, Donarski J, MacNicoll A, Wilson J (2010) Peak fitting in 2D 1 H–13 C HSQC NMR spectra for metabolomic studies. Metabolomics 6(4):574–582
Ohno T, Sarukawa K, Matsumura M (2001) Photocatalytic activities of pure rutile particles isolated from TiO2 powder by dissolving the anatase component in HF solution. J Phys Chem B 105(12):2417–2420
Peters RJB, van Bemmel G, Herrera-Rivera Z, Helsper HPFG, Marvin HJP, Weigel S, Tromp PC, Oomen AG, Rietveld AG, Bouwmeester H (2014) Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles. J Agric Food Chem 62:6285–6293
Powell JJ, Faria N, Thomas-McKay E, Pele LC (2010) Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun 34(3):J226–J233
Šimon P, Joner E (2008) Conceivable interactions of biopersistent nanoparticles with food matrix and living systems following from their physicochemical properties. J Food Nutr Res 47:51–59
Tassinari R, Cubadda F, Moracci G, Aureli F, D’Amato M, Valeri M, De Berardis B, Raggi A, Mantovani A, Passeri D, Rossi M, Maranghi F (2013) Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology. doi:10.3109/17435390.2013.822114 Early online posted on July 25, 2013
Tomita M, Hayashi M, Awazu S (1996) Absorption-enhancing mechanism of EDTA, caprate, and decanoylcarnitine in Caco-2 cells. J Pharm Sci 85(6):608–611
Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168(2):176–185
Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N (2012) Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46(4):2242–2250
Acknowledgments
The authors gratefully acknowledge funding for this research from the Food Standards Agency. Acknowledgments are also due to Roy McArthur for statistical analysis and Malcolm Baxter and Michael Walls for analysis of samples using ICP-MS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MacNicoll, A., Kelly, M., Aksoy, H. et al. A study of the uptake and biodistribution of nano-titanium dioxide using in vitro and in vivo models of oral intake. J Nanopart Res 17, 66 (2015). https://doi.org/10.1007/s11051-015-2862-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-2862-3