Abstract
Spongy graphene adsorbents have attracted great research interest recently, because of the high adsorption capacity, easy handling, and low operating cost. Fabrication of graphene sponge with other high-performance adsorbents might combine the merits of both materials, thus deserves more investigations. In this study, TiO2–graphene sponge (TiO2–GS) was prepared by the deposition of amorphous TiO2 on graphene oxide (GO) sheets for the adsorption of tetracycline antibiotics, where lyophilization was adopted to obtain the porous structure. TiO2–GS adsorbed tetracycline with a large adsorption capacity of 1,805 mg/g, larger than that of GO (313 mg/g) and GO-chitosan aerogel (1,470 mg/g). The adsorption kinetics, which finally reached the equilibrium at 48 h, was clearly controlled by the diffusion of tetracycline to TiO2–GS in the initial stage according to intraparticle diffusion model. Thermodynamics investigation indicated that the adsorption process was endothermic and promoted at higher temperature, with a positive ΔH of 35.8 kJ/mol. Generally, higher pH facilitated the adsorption of tetracycline on TiO2–GS, except that the adsorption was also very effective at pH 1. In contrast, ionic strength had insignificant influence. The adsorbed tetracycline could be washed out with acidic ice-cold water to regenerate TiO2–GS. The implication to the applications of TiO2–GS in water treatment is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceutical antibiotics, which are extensively used worldwide in human therapy and farming industries, have attracted increasing concerns in recent years, because they have been proven as a class of potent pollutants (Aristilde et al. 2010; Li et al. 2011; Sun et al. 2010). After the discharge into the environment, antibiotics are poorly metabolized and absorbed by plants and animals (Ji et al. 2009, 2010; Wang et al. 2008), which create serious environmental problems. Therefore, efficient, sustainable, and cost-effective wastewater treatment techniques for antibiotics are urgently needed.
Among various water treatment strategies, adsorption is regarded as a facile and effective one (Crini 2006; Forgacs et al. 2004; Rafatullah et al. 2010; Robinson et al. 2001). There are a large number of materials that are employed as adsorbents for water remediation (Crini 2006; Forgacs et al. 2004; Rafatullah et al. 2010; Robinson et al. 2001). Recent findings demonstrate that graphene-based materials have become a new class of promising adsorbents, which show great potential in adsorbing multiple pollutants, including antibiotics, metal ions, dyes, and so on (Xu et al. 2013). For instance, we have shown that graphene oxide (GO) and its composites are good adsorbents for copper ions and methylene blue (Yang et al. 2010, 2011, 2013; Yu et al. 2013; Zhao et al. 2014a). However, the well-dispersed graphene sheets are prone to stack and agglomerate during the processing and using. Thus, the major challenge in this area is to design porous graphene materials with proper functionalities (Xu et al. 2013).
Spongy graphene materials retain the porous structure of graphene and could be processed without stacking or agglomeration. Several studies have shown that graphene sponge (GS) has large surface area and low density (Bi et al. 2012; Wang et al. 2012; Zhao et al. 2012). Many preparation technologies have been developed to produce GS, such as lyophilization and chemical vapor deposition (Bi et al. 2012; Wang et al. 2014). Herein, we reported that porous TiO2–graphene sponge (TiO2–GS) could be used as a recyclable adsorbent for the decontamination of tetracycline. After the characterizations by transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), infrared spectrometry (IR), and Brunauer–Emmett–Teller (BET) technique, the adsorption capacity of TiO2–GS for tetracycline was determined. The adsorption kinetics and thermodynamics were investigated. The influences of pH and ionic strength were also concerned. The regeneration of TiO2–GS was achieved by washing with ice-cold acidic water. The implication to the applications of TiO2–GS in antibiotics adsorption is discussed.
Materials and methods
Preparation of TiO2–GS
Graphite was purchased from Sinopharm Chemical Reagent Co., Ltd., China. Tetracycline was obtained from Alfa Aesar Chemical Reagent Co., Ltd., China. Tetrabutyl titanate was purchased from Riqi Rubber Additives Co., Ltd., China. Other chemicals were all of analytical grade.
GO dispersion was prepared by modified Hummers’ method following our previous reports (Yang et al. 2010, 2011). TiO2–GS was prepared by the following procedures. Briefly, 750 µL of tetrabutyl titanate was dissolved in ethanol (8 mL). The solution was added dropwise into 200 mL of GO dispersion (1 mg/mL) under vigorous stirring. The mixture was stirred for another 20 min before the pH adjustment to 9.0 by ammonia. After the stirring for another 2 h, the product was repeatedly washed with ethanol and deionized water each for three times. The final product was lyophilized to obtain the porous TiO2–GS. TiO2–GS was characterized by TEM (JEM-200CX, JEOL, Japan), XPS (Kratos, UK), IR (Magna-IR 750, Nicolet, USA), and BET technique (ASAP2010, Micromeritics, USA).
Isothermal adsorption
Tetracycline (8.0 mL, pH 9.0, 0.45–2.0 g/L) was added to 5 mg of TiO2–GS. The mixture was shaken on a thermostat under 308 K (CHA-S, Jintan Hankang Electronic Co., China) at 100 rpm for 48 h to reach the apparent equilibrium. After the incubation, the suspension was centrifuged at 12,000 rpm for 5 min (TG16W, Pingfan Instrument and Menter Co., China) to collect the supernatant for the determination of tetracycline. The equilibrium concentration (C e) was obtained with reference to the standard curve of tetracycline at 364 nm. The equilibrium adsorption capacity (q e) was generally considered as one of the most critical parameters for an adsorbent, which could be calculated by \(\left( {C_{0} - C_{\text{e}} } \right)/C_{{{\text{TiO}}_{ 2} - {\text{GS}}}}\). To analyze the isothermal data, three models were applied to fit the data, namely the Temkin model, the Langmuir model, and the Freundlich model (Zhao et al. 2014a).
Adsorption kinetics and thermodynamics
To investigate the kinetics, tetracycline (8.0 mL, pH 9.0, 1.75 g/L) was added to 5 mg of TiO2–GS. The mixture was shaken for certain time. After the incubation at 308 K, the suspension was centrifuged at 12,000 rpm for 5 min to collect the supernatant for the determination of tetracycline concentration. The adsorption capacity (q t) was obtained accordingly. The kinetics data were analyzed by intraparticle diffusion model (Yang et al. 2012).
To investigate the thermodynamics, 5 mg of TiO2–GS was mixed with 8.0 mL of tetracycline (1.75 g/L, pH 9.0) and the mixture was incubated at different temperatures (298–328 K) for 48 h. The equilibrium concentration (C e) and adsorption capacity (q e) were obtained for the calculations of thermodynamics parameters (Wu et al. 2014).
Influence of pH and ionic strength
The original pH of tetracycline was adjusted with HCl or NaOH aqueous solution (PB-10, Sartorius, Germany). At each designed pH levels, 5 mg of TiO2–GS was mixed with 8.0 mL of tetracycline (1.75 g/L) for q e determination at 308 K.
To investigate the influence of ionic strength, NaCl with different original concentrations were mixed with tetracycline (1.75 g/L, pH 9.0). Then, 5 mg of TiO2–GS was mixed with 8.0 mL of tetracycline (1.75 g/L, pH 9.0, Na+ concentration: 0–100 mM) for the measurement of q e at 308 K.
Recycling
To investigate the desorption of tetracycline from TiO2–GS, the used TiO2–GS was washed with ice-cold water solution (pH 3.0) twice. Then, the dried sample was subjected to the q e determination at 308 K. The initial adsorption capacity was regarded as q e,0, and the adsorption capacity of regenerated TiO2–GS was expressed as q e,n , where n was the cycle number. The adsorption/desorption process was conducted up to 5 consecutive cycles. The relative capacity was expressed by q e,n /q e,0 × 100 %.
Results and discussion
Characterization of TiO2–GS
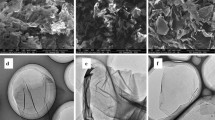
As shown in Fig. 1a, small TiO2 nanoparticles (NPs) were decorated on the GO sheets randomly. The particle sizes are in the range of 10–100 nm and some of the particles were connected with others. The average diameter was 40.1 nm and the particle size distribution was presented in Fig. 1b. Since the sample was not reduced, there were plenty of oxygen atoms detected by XPS. There were about 37 wt% of Ti, 37 wt% of O, and 20 wt% of C in TiO2–GS. The abundance of oxygen-containing groups was confirmed by the IR spectrum (Fig. 1c). The presence of –OH/–COOH groups was indicated by the broad peak at 3,260 cm−1. The small peak at 1,720 cm−1 was attributed to –C=O groups. The porous structure of TiO2–GS was also reflected by the large specific area of 67.2 m2/g. Overall, the characterization results indicated that TiO2–GS had high specific surface area and was full of oxygen-containing groups. TiO2–GS might interact with various pollutants, in particular for those with aromatic rings and/or positively charged (Xu et al. 2013).
Adsorption isotherms
Adsorption isotherm was employed to clarify the distribution of tetracycline between the solid sorbent (TiO2–GS) and the solution in equilibrium over a range of concentrations at a constant temperature. The q e values increased along with the growth of C e values (Fig. 2a). After the C e values were higher than 670 mg/L, the q e values reached a nearly constant value of 1,727 mg/g. The adsorption capacity of TiO2–GS (1,805 mg/g) was very impressive, which was nearly sixfolds of that of GO (313 mg/g) (Gao et al. 2012) and 46-folds of that of GO-Fe3O4 (39.1 mg/g) (Lin et al. 2013). TiO2–GS also performed better than GO-chitosan aerogel (1,470 mg/g) (Zhao et al. 2014a). The adsorption capacity of TiO2–GS was competitive to those high-performance adsorbents for tetracycline, including active carbon, TiO2 nanotubes, and magnetic microspheres (Su et al. 2012). Therefore, we concluded that TiO2–GS was a high-performance adsorbent for antibiotics.
To further analyze the adsorption data, three adsorption isothermal models were adopted to fit the data, namely Temkin model, Langmuir model, and Freundlich model. Langmuir model could not describe the data well, because of the negative intercept value, which was related to the maximum adsorption capacity. The relative small R values of Langmuir model (R = 0.90) and Freundlich model (R = 0.90) indicated that the two models were not suitable. In contrast, Temkin model described the data slightly better (R = 0.98), after excluding the last point. The b value was 1.57 J/mol, which indicated that the adsorption of tetracycline onto TiO2–GS involved both physisorption and chemisorption. Comparing to that of GO-chitosan aerogel (b = 2.83 J/mol) (Zhao et al. 2014a), the b value of TiO2–GS was smaller, implying that the interaction between tetracycline and TiO2–GS was even weaker.
Adsorption kinetics
The adsorption kinetics of tetracycline on TiO2–GS was distinctly different to other graphene adsorbents. As shown in Fig. 3a, at the initial 10 h, no meaningful adsorption occurred. After 10-h incubation, the adsorption capacity increased quickly and reached the equilibrium at about 48 h. In other graphene adsorbents, the adsorption occurred upon the addition of pollutant solution immediately. As we discussed previously, the dispersing stage of graphene adsorbents determined the adsorption kinetics (Xu et al. 2013). Highly dispersed GO sheets could adsorb pollutants within 20 min (Yang et al. 2010, 2011). When graphene sheets were aggregated, the adsorption kinetics was slowed down. The low adsorption speed of tetracycline on TiO2–GS implied that the surface of graphene sheets was not easily accessed by tetracycline.
The adsorption process usually involves multistep process, including bulk diffusion, external mass transfer of adsorbate molecules across the liquid film around the adsorbent, binding of adsorbate molecules on the active sites distributed on the outer surface of the adsorbent, intraparticle diffusion of adsorbate molecules into macro-, meso- and micropores, and adsorption of adsorbate molecules onto active sites distributed within the adsorbent (Maksin et al. 2012). Generally, binding of adsorbate molecules on the active sites in and/or out the adsorbent is very fast, thus, not affects the kinetics much. Intraparticle diffusion and surface diffusion are widely acknowledged as the main controlling factors of the adsorption kinetics (Weber and Morris 1963). Therefore, we fitted the adsorption kinetics data of tetracycline on TiO2–GS with intraparticle diffusion model. The adsorption could be distinguished in two stages. In the first stage (0–10 h), the boundary effect was dominating, with a C value of 23.0 mg/g. The rate constant of adsorption (k i ) was 6.43 mg/g min−0.5. In the second stage, the C value was negative, indicating the vanish of boundary effect. Accordingly, the k i value increased to 62.9 mg/g min−0.5. For the first stage, the C value was very large, comparing to that for the adsorption of tetracycline on TiO2-chitosan (5.89 mg/g) (Zhao et al. 2014a). The boundary effect hindered the diffusion of tetracycline, resulting in the slow adsorption kinetics. Beyond the diffusion, we also found that pre-wetting of TiO2–GS accelerated the adsorption in our preliminary exploration, which implied that the wetting process on TiO2–GS dominated the initial adsorption speed. The underneath mechanism still requires further investigations.
Adsorption thermodynamics
The thermodynamics is a key component of adsorption behaviors. Thermodynamics data benefit the optimization of adsorption condition and provide in-depth understanding of the adsorption process. Herein, four temperatures were designed to explore the thermodynamics of the adsorption of tetracycline on TiO2–GS. As demonstrated in Fig. 4a, the adsorption capacity increased with the growth of temperature, demonstrating that the adsorption was endothermic in nature. Higher adsorption capacity could be obtained at higher temperature. This might be due to the reduced viscosity of the solution and the increased diffusion rate of tetracycline molecules (Salam and Burk 2009). To further analyze the data, we calculated the adsorption thermodynamics parameters (Table 1).
All ΔG values of the adsorption of tetracycline on TiO2–GS were negative in the test temperature range, indicating that the adsorption was spontaneous. More negative ΔG values were found at higher temperature, suggesting that higher temperature was thermodynamically favorable. The absolute values of ΔG were very small, implying that the adsorption was mainly physisorption. The positive ΔH value indicated the endothermic nature of adsorption process, consisting with the fact that larger adsorption capacity was obtained at higher temperature. The positive ΔS reflected an increase of the randomness at the solid/solution interface during the adsorption process Yang et al. 2013, which was the driven force of the adsorption of tetracycline on TiO2–GS. The thermodynamics parameters suggested that the adsorption should be performed at higher temperature if possible. In the contrast, the desorption should be carried out at lower temperature to inhibit the adsorption.
Influence of pH and ionic strength
The pH of the solution plays an important role in the adsorption process since it converts charge species as well as the surface properties of the adsorbent (Zhao et al. 2011). Our results suggested that pH influenced the adsorption of tetracycline on TiO2–GS significantly. As shown in Fig. 5a, the adsorption was inhibited at pH 2 and 3, while the adsorption capacities were very high at the rest of the pH values. A slight inhibition at near-neutral condition was also observed. Previous reports have invoked the mechanism of cation/anion exchange, cation bridging, and/or surface complexation (e.g., H-bonding) to explain the relative strong sorption of tetracycline by soils, iron/aluminum hydroxides, clay minerals, and humic substances (Figueroa et al. 2004; Gu and Karthikeyan 2005). Multiple interactions contributing to the adsorption made the situation very complex. As we discussed previously, electrostatic interaction was a major contributor of the total interaction (Xu et al. 2013). The pH-regulated adsorption should be attributed to the protonation stages of both tetracycline and TiO2–GS. The phenolic hydroxyl groups and amino groups of tetracycline would be protonated at low pH. So were the hydroxyl groups and carboxyl groups on GO sheets. When N atoms were protonated at lower pH and showed positive charges, the electrostatic interaction between tetracycline and TiO2–GS would be strengthened. And the protonation of oxygen-containing groups on both tetracycline and TiO2–GS reduced the electrostatic expulsion, which benefited the adsorption too. On the other hand, the protonation of hydroxyl groups and carboxyl groups on GO sheets at lower pH would reduce electrostatic interaction between amino of tetracycline- and oxygen-containing groups on GO. The total influence of pH depended on the dominating effect among the aforementioned influences.
In contrast to the significant influence of pH, ionic strength had a tiny influence on the adsorption of tetracycline on TiO2–GS (Fig. 5b). At the initial tetracycline concentration of 1.75 g/L, the adsorption capacity ranged from 1,803 mg/g at 0 mM Na+ to 1,726 mg/g at 100 mM Na+. Similar inhabitation trends were also observed by Ji et al. (2010) and Zhao et al. (2014a). The electrostatic protection might be emerged after adding NaCl, which could inhibit the immediate electrostatic interaction between adsorbent and adsorbate. The inhabitation finally led to the lower adsorption capacity. Moreover, the increase of ionic strength would promote the competitive adsorption between tetracycline molecules and Na+ on the adsorptive sites of TiO2–GS. Similar effects were reported by previous studies of GO and carbon NPs (Gu et al. 2007; Yang et al. 2013; Zhao et al. 2014b).
Recycling
For the practical applications, it was highly desirable that the adsorbents could be regenerated through a feasible way after use. The recycling of graphene adsorbents has been widely investigated. Different strategies have been applied to achieve the goal, including elusion with water and other solvents, pH adjustment, adding chelator, burning, and so on. As aforementioned, the adsorption of tetracycline on TiO2–GS was inhibited at low temperatures and specific pH values. Thus, we achieved the recycling of TiO2–GS by washing with ice-cold acidic water (pH 3.0). As demonstrated in Fig. 6, the accumulative adsorption capacity of TiO2–GS retained as high as 90 % for tetracycline even after five consecutive cycles. The facile regeneration might be due to the weak interaction between tetracycline and TiO2–GS. There was about 10 % capacity loss during the regeneration. It might not be surprising, because such capacity loss has been widely reported in the studies of graphene adsorbents (Xu et al. 2013). A possible explanation might be that some active sites were blocked during the wash and lyophilization in the regeneration process, due to the chemisorption and/or stacking of graphene sheets (Xu et al. 2013). The easy regeneration of TiO2–GS definitely would facilitate the practical applications in water treatment, because it reduces the operating cost significantly and avoids generating nano-wastes.
Conclusion
In summary, TiO2–GS adsorbent was fabricated for the removal of tetracycline from aqueous solution, where the composition of TiO2 significantly promoted the adsorption performance. TiO2–GS was found to be a recyclable adsorbent with the adsorption capacity of 1,805 mg/g for tetracycline. The adsorption isotherm was well described by the Temkin isothermal model, giving a b value of 1.57 J/mol. Diffusion limited the adsorption at the initial stage, which was accelerated thereafter. Relative high pH and temperature benefited the adsorption, while ionic strength had insignificant influence on the adsorption. We believe that TiO2–GS might be regarded as promising adsorbent for the remediation of tetracycline.
References
Aristilde L, Marichal C, Miehe-Brendle J, Lanson B, Charlet L (2010) Interactions of oxytetracycline with a smectite clay: a spectroscopic study with molecular simulations. Environ Sci Technol 44:7839–7845
Bi H, Xie X, Yin K, Zhou Y, Wan S, He L, Xu F, Banhart F, Sun L, Ruoff R (2012) Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv Funct Mater 22:4421–4425
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Figueroa R, Leonard A, Mackay A (2004) Modeling tetracycline antibiotic sorption to clays. Environ Sci Technol 38:476–483
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Gao Y, Li Y, Zhang L, Huang H, Hu J, Shah SM, Su X (2012) Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J Colloid Interface Sci 368:540–546
Gu C, Karthikeyan K (2005) Interaction of tetracycline with aluminum and iron hydrous oxides. Environ Sci Technol 39:2660–2667
Gu C, Karthikeyan K, Sibley S, Pedersen J (2007) Complexation of the antibiotic tetracycline with humic acid. Chemosphere 66:1494–1501
Ji L, Chen W, Duan L, Zhu D (2009) Mechanisms for strong adsorption of tetracycline to carbon nanotubes: a comparative study using activated carbon and graphite as adsorbents. Environ Sci Technol 43:2322–2327
Ji L, Chen W, Bi J, Zheng S, Xu Z, Zhu D, Alvarez P (2010) Adsorption of tetracycline on single-walled and multi-walled carbon nanotubes as affected by aqueous solution chemistry. Environ Toxicol Chem 29:2713–2719
Li R, Yuan Q, Zhang Y, Ling J, Han T (2011) Hydrophilic interaction chromatographic determination of oxytetracycline in the environmental water using silica column. J Liq Chromatogr Relat Technol 34:511–520
Lin Y, Xu S, Li J (2013) Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem Eng J 225:679–685
Maksin DD, Kljajevic SO, Dolic MB, Markovic JP, Ekmescic BM, Onjia AE, Nastasovic AB (2012) Kinetic modeling of heavy metal sorption by vinyl pyridine based copolymer. Hem Ind 66:795–804
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Robinson T, McMullanG G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Salam M, Burk R (2009) Thermodynamics and kinetics studies of pentachlorophenol adsorption from aqueous solutions by multi-walled carbon nanotubes. Water Air Soil Pollut 210:101–111
Su S, Lei J, Zhang Z (2012) Preparation of magnetic yeast microspheres and research on the adsorpion of tetracycline. Appl Chem Ind 41:2099–2102
Sun H, Shi X, Mao J, Zhu D (2010) Tetracycline sorption to coal and soil humic acids: an examination of humic structural heterogeneity. Environ Toxicol Chem 29:1934–1942
Wang Y, Jia D, Sun R, Zhu H, Zhou D (2008) Adsorption and cosorption of tetracycline and copper(II) on montmorillonite as affected by solution pH. Environ Sci Technol 42:3254–3259
Wang J, Shi Z, Fan J, Ge Y, Yin J, Hu G (2012) Self-assembly of graphene into three-dimensional structures promoted by natural phenolic acids. J Mater Chem 22:22459–22466
Wang L, Li X, Guo T, Yan X, Tay BK (2014) Three-dimensional Ni(OH)2 nanoflakes/graphene/nickel foam electrode with high rate capability for supercapacitor applications. Int J Hydrogen Energ 39:7876–7884
Weber W, Morris J (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–60
Wu R, Liu J-H, Zhao L, Zhang X, Xie J, Yu B, Ma X, Yang S-T, Wang H, Liu Y (2014) Hydrothermal preparation of magnetic Fe3O4@C nanoparticles for dye adsorption. J Environ Chem Eng 2:907–913
Xu J, Lv H, Yang S-T, Luo J (2013) Preparation of graphene adsorbents and their applications in water purification. Rev Inorg Chem 33:139–160
Yang S-T, Chang Y, Wang H, Liu G, Chen S, Wang Y, Liu Y, Cao A (2010) Folding/aggregation of graphene oxide and its application in Cu2+ removal. J Colloid Interface Sci 351:122–127
Yang S-T, Chen S, Chang Y, Cao A, Liu Y, Wang H (2011) Removal of methylene blue from aqueous solution by graphene oxide. J Colloid Interface Sci 359:24–29
Yang S-T, Luo J, Zhou Q, Wan J, Ma C, Liao R (2012) Adsorption behaviour of methylene blue on carbon nanoparticles. Micro Nano Lett 7:1060–1063
Yang S-T, Luo J, Liu J, Zhou Q, Wan J, Ma C, Liao R, Wang H, Liu Y (2013) Graphene oxide/chitosan composite for methylene blue adsorption. Nanosci Nanotechnol Lett 5:372–376
Yu B, Xu J, Liu J-H, Yang S-T, Luo J, Zhou Q, Wan J, Liao R, Wang H, Liu Y (2013) Adsorption behavior of copper ions on graphene oxide-chitosan aerogel. J Environ Chem Eng 1:1044–1050
Zhao G, Song S, Wang C (2011) Determination of triazine herbicides in environmental water samples by high-performance liquid chromatography using graphene-coated magnetic nanoparticles as adsorbent. Anal Chim Acta 708:155–159
Zhao J, Ren W, Cheng H (2012) Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J Mater Chem 22:20197–20202
Zhao L, Dong P, Xie J, Li J, Wu L, Yang S-T, Luo J (2014a) Porous graphene oxide-chitosan aerogel for tetracycline removal. Mater Res Express 1:015601
Zhao L, Yu B, Li J, Yang S-T, Luo J (2014b) Carbon nanoparticles as recyclable adsorbent for the removal of copper ions. Nanosci Nanotechnol Lett 6:87–93
Acknowledgments
We acknowledge the financial support from Science & Technology Department of Sichuan Province (Pillar Program No. 2013FZ0060), the China Natural Science Foundation (No. 21307101), the Scientific Research Foundation of Shandong Province of Outstanding Young Scientist Award (No. BS2011SW031), and the Project of Postgraduate Degree Construction, Southwest University for Nationalities (No. 2014XWD-S0703).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, L., Xue, F., Yu, B. et al. TiO2–graphene sponge for the removal of tetracycline. J Nanopart Res 17, 16 (2015). https://doi.org/10.1007/s11051-014-2825-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2825-0