Abstract
Objective
This study aims to determine the diagnostic utility of galactomannan enzyme immunoassay (GM EIA) in invasive aspergillosis (IA) in children with hematological malignancy (high risk population) in terms of sensitivity, specificity, negative predictive value (NPV) and positive predictive values (PPV) at various cut offs while validating the revised EORTC/MSG 2019 criteria in order to obtain the best cut-off.
Material and Methods
For 100 pediatric patients, serum and respiratory samples were collected. Clinical, mycological workup (potassium-hydroxide mount, fungal culture) and GM EIA was done to classify proven, probable, and possible IA as per EORTC-MSG guidelines,2019. Sensitivity, specificity, PPV and NPV were calculated of GM indices at cut-off 0.5, 0.7 and 1, and validated with revised EORTC -MSG, 2019.
Results
Of 100 patients enrolled, 75 were diagnosed with ALL, 14 with AML, two with Hodgkin's, three had non-Hodgkin lymphoma, and six had undifferentiated leukemia. With routine mycological findings, 51 were classified as probable IA, 11 as possible IA, and 38 as no IA. Aspergillus flavus was the most prevalent on culture (56.9%, 29/51) followed by A. fumigatus (29%, 15/51) A. niger (7.8%, 4/51), A. terreus (3.9%, 2/51) and A. nidulans (2%, 1/51). GM EIA demonstrated sensitivity 82.3%, specificity 97.4%, PPV 98.1%, and NPV 77.1% at cut-off 0.67 when comparing probable/possible IA v/s no IA groups. The GM EIA had the best sensitivity (82.4%), specificity (81.8%), PPV (95.5%), and NPV (50%) at cut off 0.78 when the probable IA group was compared to the possible IA. Seven patients succumbed of whom 5 had GMI ≥ 2.
Conclusion
This study deduces the optimal cut-off for serum GM EIA to be 0.67 obtained by ROC analysis when comparing possible and probable IA versus no IA and reinforces the definition of probable category of EORTC-MSG criteria, 2019. At 0.5 ODI the sensitivity (87.1%) and NPV (80.5%) are high, thus making it the most suitable cut-off for detecting true positive and ruling out IA respectively, in pediatric patients with hematological malignancy. GM EIA when performed adjunctive to clinico-radiological findings can prove to be screening, diagnostic and prognostic test for IA in pediatric hematological malignancy patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive aspergillosis (IA) is a major life-threatening opportunistic infection in hematological malignancy patients, especially in the pediatric age group. IA accounts for more than 80% of invasive fungal diseases (IFD), and along with invasive candidiasis (IC) the burden is more than 95% of all IFD in all risk groups [1,2,3,4] Worldwide estimates reveal that over 1.8 million cases of IFDs occurred in 2017, out of which around 250,000 cases were IA [5, 6].
Although clinical signs are nonspecific, any early radiological finding on high resolution computed tomography (HRCT) scan with culture-negativity raises the suspicion. Galactomannan enzyme immunoassay (GM EIA) has proven to be a cornerstone in diagnosis of IA in children with hematological malignancy where invasive sampling can be life threatening and often contraindicated. GM is a soluble, heat stable polysaccharide in cell wall of Aspergillus species, detected in serum, bronchoalveolar lavage (BAL) fluid and cerebrospinal fluid (CSF), 5–8 days before onset of symptoms and 7–12 days prior to HRCT abnormalities. This provides a window of opportunity to start pre-emptive antifungal therapy [7].
The European Organization for Research and Treatment of Cancer (EORTC) and the Mycoses Study Group (MSG) devised a classified IFD into possible, probable, and proven categories based on host factors, clinical features, histopathology, radiological and mycological investigations and in 2019 updates established pediatric-specific IFD definitions which include GM EIA as a criterion [8].
Definitive diagnosis is made by direct demonstration of fungal elements or a positive culture from the tissue obtained by invasive procedure such as trachea-bronchial biopsy. In the pediatric hematological malignancy patients, it is often not compliable due to grave complications of thrombocytopenia and coagulopathy [9, 10]. Positive microscopy and cultures from nonsterile samples lower respiratory specimen along with GM EIA positivity in high-risk patients are strongly suggestive of probable IA. Expedient diagnosis of IA is of paramount importance for overall patient management and preventing mortality.
Objectives
The objective of this study is to determine the diagnostic utility in terms of sensitivity and specificity of serum GM EIA in IA in pediatric patients with hematological malignancy. To evaluate the GM EIA findings at cut-off ODI 0.5,0.7, 1 with EORTC/MSGcriteria2019 [8].
Materials and Methods
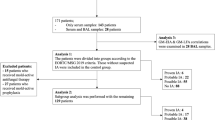
This study was conducted at Safdarjung Hospital, a tertiary care center in New Delhi in the departments of Microbiology, Pediatrics, and Radiodiagnosis over the period of 18 months from December 2020 to June 2022. A total of 100 pediatric patients, aged between 1 to 12 years with diagnosed hematological malignancies were included in the study. The patient selection was done considering the clinical features, host factors etc. given in context to pediatric patients in the revised EORTC/MSG guidelines, 2020. These patients were antifungal naïve with high degree of suspicion for IFI. For all patients informed consent were taken from the parents/guardian. Following patients were excluded; infants and neonates due to high index of GM false positivity, patients on piperacillin tazobactam and patients with other hematological disorders on immunosuppressive therapy. Statistical analysis was done using the statistical software SPSS Version 21.0 (IBM, USA). Ethical clearance was obtained.
For each patient 2 ml of blood was collected under aseptic condition and transported immediately to the laboratory. Patients were pretreated with nebulised salbutamol and then hypertonic saline for sputum induction. High volume induced sputum was collected, quality of sputum accessed by microscopic examination and then processed as per protocol as per protocol mentioned in prior study [11] Briefly for high volume culture (HVC), an aliquot of undiluted specimen upto 1 ml was cultured on two Sabouraud’s dextrose agar (SDA) incubated at 30 °C for upto 14 days and potassium hydroxide mount was done. Lactophenol cotton blue (LPCB) mount was made from the growth on the SDA to look for septate hyphae, conidiophores, and arrangement of conidia for identification of the fungal species.
Serum GM EIA was performed using Bio-Rad Platelia™ Aspergillus Ag kit as per the manufactures’ instructions. Results of GM EIA were analyzed at optical density (OD) cut-offs 0.5, 0.7, and 1 to determine the best cut-off satisfying the EORTC/MSG criteria 2019 [8]. After classifying these patients into probable, possible and NO IA categories, GM positivity in (probable + possible) v/s NO IA and probable IA v/s possible IA were analysed using ROC analysis and the cut-off with best combination of sensitivity and specificity was determined.
Results
A total of 100 pediatric patients, 63 male 37 female with median age of 6 years were enrolled in the study and taking into consideration the host factors, radiological findings, and mycological findings, they were categorised into proven, probable, possible, and no evidence of IA according to EORTC/MSG 2019 guidelines.
Out of 100, 61 patients were diagnosed cases of B-cell ALL (most common), followed by T-cell ALL 14, AML 14, 3 cases of non-Hodgkin’s lymphoma, 2 of Hodgkin’s lymphoma and 6 as unclassified/undifferentiated leukemia as shown in Table 1. Forty six percent of patients with B-cell ALL (28/61), 57% (8/14) T-cell ALL, 42% (6/14) AML,33% (1/3) cases of NHL, 100% (2/2) cases of Hodgkins lymphoma and 66% (4/6) cases of undifferentiated leukemia patients were categorized as probable IA, satisfying the EORTC/MSG guidelines. There was no significant association (Fisher’s exact test) between the type of hematological malignancy in this study cohort and occurrence of IA (p value = 0.304).
Fever was the most common presenting symptom (82%), followed by cough (40%), pleuritic chest pain (24%), dyspnoea (16%), sore throat (7%), hemoptysis (5%), symptoms of malignancy like weight loss (16%), lymphadenopathy (13%) and other clinical features like loss of appetite, poor growth, diarrhoea, vomiting, epistaxis, gum bleeding, pain abdomen, bone pain, rash, in 36% patients. None of the clinical features were significantly associated with IA cases at the time of presentation.
Chest imaging by X-ray and/or HRCT scan was done for all the patients. For 94 of the patients HRCT was performed. For 6 patients, chest Xray was the sole radiological investigation. 58 (58%) patients had significant findings, most common being reticular alveolar infiltrates (52% of the positive findings). The classical radiological features of IA, i.e., halo sign and cavity on HRCT scan was present in 3 and 4 cases, respectively. Other radiology findings were non-specific consolidations (15), ground glass opacities (12), pleural effusion (2), perihilar infiltrates (2) and mass lesion (1). Of the 58, 49 patients were diagnosed as probable IA and 9 as possible IA. In these probable cases the radiological finding was non-specific. However, there was a statistically significant association of HRCT scan abnormality with true positives for GM (p < 0.001).
In the fungal cultures 51% (51/100) yielded growth positive for Aspergillus and 49% (49/100) of the patients were culture negative. In the aetiological profile Aspergillus flavus was most common 56.8% (29/51) followed by A. fumigatus 29.4% (15/21) A. niger 7.8% (4/51) A. terreus 3.9% (2/51) and A. nidulans 1.9% (1/51). With routine mycological findings, 51 were classified as probable IA, 11 as possible IA, and 38 as no IA.
Serum GM was measured in all (n = 100) patients. As per Platelia™ Aspergillus antigen kit ELISA literature, the significant cut-off ODI ≥ 0.5 was taken. 59 (59%) patients were GM positive and 41 (41%) seronegative at this cut-off.
Out of the 59 positive cases, 49 (83%) were diagnosed as probable IA and 5 (8.4%) as possible IA. False positive GM ≥ 0.5 was found in 5 (8.4%) patients in association with Candida sepsis in 1 patient, grade III/IV mucositis in 2 patients and 2 patient had no identifiable cause.
Performance of GM EIA at different ODI cut-offs ≥ 0.5, ≥ 0.7 and ≥ 1.0 was compared with fungal culture from nonsterile sites and sensitivity, specificity, PPV and NPV for GM EIA were calculated. The findings observed are mentioned in Table 2. The best cut-off observed was ≥ 0.7 at which sensitivity, specificity, PPV and NPV were 88.2%, 91.8%, 91.8 and 88.2, respectively.
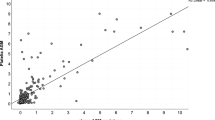
Serum GMEIA results were benchmarked against the revised EORTC/MSG 2019 definitions for IA [8]. Taking these definitions are reference standard [10], sensitivity, specificity, PPV and NPV of GM EIA calculated at cut-off ODI ≥ 0.5 were 87.1%, 86.8%, 91.5 and 80.5 for proven / probable / possible IA. The specificity and PPV increased to100% at cut-off ≥ 0.7 and ≥ 1 shown in Table 3. ROC curve analysis was done to determine the best cut-off and it was found to be 0.67 ODI depicted in Fig. 1.
While comparing probable IA versus possible IA groups sensitivity, specificity, PPV and NPV detected at cut-off ≥ 0.5 were 96.1%, 54.5%, 90.7 and 75 respectively. On increasing the cut-off ODI to ≥ 0.7 and ≥ 1, specificity and PPV increased to 63.6% and 91.8 respectively at ODI ≥ 0.7 and 81.8% and 93.8 respectively at ODI ≥ 1 represented in Table 4. Sensitivity although, decreased drastically. ROC curve analysis was done to determine the best cut-off and it was found to be 0.78 ODI and depicted in Fig. 2.
The outcome of the patients was determined in terms of the number of patients (n) who were discharged after a successful chemotherapy and/or antifungal therapy (87), had in-hospital mortality (7) or developed complications of respiratory failure and required ICU admission (6). Of the 87 patients who were discharged, 43 were diagnosed as probable IA, 9 as possible IA, and 37 with no evidence of IA. GMI ranged from 0.5 to 3.9 in the probable category. Of the patients that required intensive care 3 were diagnosed as probable IA, 2 as possible IA, and 1 with no evidence of IA. In the possible and probable IA category, GMI ranged from 0.4 to 1.5. Out of the 7 patients that succumbed, 5 were diagnosed as probable IA and 2 had no evidence of IA. In probable IA patients who died GM index was ≥ 2. The patients in the no IA category had associated Candida sepsis.
Discussion
IA is one of the most common IFI in children with hematological malignancy, leading to significant morbidity and mortality [12]. The present study was undertaken to assess the diagnostic utility of GM EIA in pediatric patients with hematological malignancy with suspected IA.
In accordance with EORTC/MSG criteria 2019, 51% (51/100) cases were diagnosed as probable IA and 11% (11/100) cases as possible IA. There were no cases of proven IA because invasive sampling for tissue or biopsy specimens could not be done [9, 10].
In this study, 45.0% of the patients were in the induction phase of chemotherapy whereas 33.0% and 22.0% were in the consolidation and maintenance phase, respectively. Similarly in the recent retrospective study from Turkey, 77% of the patients in the probable IA category had received induction chemotherapy [13], Although dissimilar findings in the study cohort were observed in another study from North India, where 23% of patients were in the delayed intensification followed by maintenance phase [14].
The most common associated risk factor for IA in this study was febrile neutropenia, presented in27% of the children, followed by pancytopenia due to bone marrow suppression in 6% and in 2 out 3patients who received a corticosteroid therapy > 20 days prior to admission. GM was also positive and they were eventually categorized as probable IA. Of the 27 patients with febrile neutropenia, 16 were categorized as probable IA, 2 as possible IA and 9 patients as no IA due to lack of corroborative mycological and radiological evidence. In a prior study from North India, similar findings were obtained and febrile neutropenia, induced by intensive chemotherapy, was the most common associated risk factor for IA [14].
As far as radiological features are concerned; HRCT evidence of reticular alveolar infiltrates (non-specific) was the commonest finding and was associated with higher GM positivity (24/59). The classical features of IA like halo sign and cavity were present in only 3 and 4 cases respectively. There was a statistically significant association of CT scan finding with true positives for GM (p < 0.001). Similar observations were made in EORTC/MSG 2019 guidelines where in the authors stated that HRCT findings are less specific in children than those reported in adults [8]. and in a prior study from North India, where the most common CT-scan finding was multiple pulmonary nodules [14]. In contrast to this, in another study from Turkey classical radiological findings on HRCT were obtained that had a strong statistically association with GM positivity and thus the clinicians advised CT scan in all febrile neutropenic patients without waiting for GM results [15]. In a recent study from North India on adult patients with blood malignancy and disorders enrolled for a hematopoietic stem cell transplantation (HSCT), the authors concluded that positive serum GM antigenemia, a history of invasive pulmonary aspergillosis (IPA) and EORTC clinical criteria of chest HRCT in pre-HSCT strongly indicated post-HSCT IPA [16].
In the fungal cultures, 51% of all induced sputum collected yielded growth positive for Aspergillus in this study. The reported rates of sputum culture positivity for Aspergillus spp. varies widely, which probably reflects the variability in inclusion criteria and the small number of participants included in some studies. Our patient population was already at high risk for invasive aspergillosis and this selection bias may explain the high positivity. Some studies in different patient population have reported sputum positivity up to 56% [17] In another study similar technique for fungal culture using high volume undiluted sputum was adopted which resulted in better yield of Aspergillus growth [11].
The most common species isolated on culture was A. flavus, followed by A. fumigatus. In the study from a private tertiary care centre, New Delhi, relatable results were observed [18]. In a prior study from this institute done in adult population similar results were observed, where A. flavus again was the most common species isolated [19]. In a study from a reference centre in North India, air sampling was done in North India which revealed the presence of increased concentration spores of A. flavus, owing to the hot and humid tropical climate, resulting in greater incidence of A. flavus related infections [20].
GM presence in the circulation correlates with invasive growth of Aspergillus through the pulmonary capillaries, and angio-invasion has been correlated with fungal burden and GM production. This explains the variable performance of serum GM in different patient populations. Serum GM is detectable in patients with hematological malignancies and allogeneic hematopoietic stem cell transplant recipients who have a higher burden of disease [21] The patients included in this study are children with diagnosed hematological malignancy – particular patient cohort. The patient selection is done considering the clinical features, host factors, etc. given in context to pediatric patients in the revised EORTC/MSG guidelines in 2019 and GM is used as an adjunctive tool to diagnose IA. The numbers in our study reflect the risk group (hematological malignancy), high burden of aspergillosis in this patient group. Furthermore, the EORTC/MSG revised guidelines in 2019 [8] state GM assay performs similarly in children and adults when used as an adjunctive tool to diagnose IA, as was seen in this study and prior studies [22, 23] In this study the best optimal cut-off for GM antigenemia obtained by ROC analysis was determined to be 0.67. Different authors in various studies have found wide range of sensitivity, specificity, NPV, and PPV while testing serum GM EIA at different ODI cut-offs. A comparison of performance of GMEIA in diagnosis of IA in pedriatric population in different national and international studies is depicted in Table 5.
Antifungal prophylaxis is given in these patients with hematological malignancy, but for this study, the first serum sample for GM and respiratory samples for fungal culture were collected before initiation of antifungal prophylaxis. GM testing has shown decreased sensitivity from 80 to 30% and reduced isolation of molds in respiratory samples in patients on mold active antifugal prophylaxis [24, 25].
The study population being children 1 to 12 years of age with hematological malignancy and suspected IFI, performance of bronchoscopy to get tissue sample or lower respiratory specimen was not feasible. These children were at risk of life-threatening complications of thrombocytopenia and coagulopathy, so weighing the risk and benefit associated, invasive sampling could not be performed. This is the major limitation of the study.
Existing diagnostic tools for IA largely rely on biomarkers like GM detection in serum or BAL, both of which have their limitations. The use of sputum sample is non-invasive and Aspergillus detection is feasible [26]. There is on-going trend of using non-invasive methods in these settings and have been found to be equally effective for another organism like tuberculosis [27].
A high index of suspicion is mandatory, particularly because of prolonged immunosuppression in these patients receiving intensive chemotherapy and corticosteroid therapy and presence of febrile neutropenia. While a significant radiological finding on chest HRCT scan highly suggests IA, a normal chest radiograph does not rule out IA. Conventional mycological investigations essential for deriving a diagnosis of IA; however, the sensitivity and specificity of these parameters are variable. Although isolation and evidence of Aspergillus from a sterile specimen like tissue or biopsy is the gold standard, it is not usually feasible owing to complications of coagulopathy and thrombocytopenia. GMEIA, when performed adjunctive to clinicoradiological findings and other mycological investigations, can prove as an effective diagnostic marker for IA. Although the EORTC/MSG 2019 criteria state a single serum GM positivity at an ODI of 1 as significant [28], this study suggests that an ideal serum GM EIA cut-off of 0.67 can define the probable IA category in pediatric patients with hematological malignancy. However, at 0.5 ODI the sensitivity (87.1%) and NPV (80.5%) are better, thus making it the most suitable cut-off for detecting true positive and ruling out IA respectively. A negative GM rules out practically the diagnosis of IA but does not exclude diagnosis of other IFI. Furthermore, prompt return to negative GM may be a good indicator of clinical response to antifungal therapy and predict favourable outcome. Although, GM value of ≥ 2 is associated with higher risk of fatal outcome in high-risk population with hematological malignancy and prolonged immunosuppression [21, 29]. Thus, this study concludes that serum GM can be used as an effective screening [30], diagnostic and prognostic marker in the pediatric patients with hematological malignancy with suspected IA.
References
Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67:1567–601.
Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63.
Sezgin M, Tüfekçi Ö, Baytan B, Ören H, Çelebi S, Ener B, et al. Invasive fungal infections in children with leukemia: the clinical features and Prognosis. Turk J Haematol 2021,;94–100.
Otto WR, Green AM. Fungal infections in children with haematologic malignancies and stem cell transplant recipients. Br J Haematol. 2020;189(4):607–24.
Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 2017;3:57–68.
Global Action Fund for Fungal Infections (GAFFI). Priority fungal infections. Available online: http://www.gaffi.org/media/fact-sheets/ (accessed on 27 Jan 2023).
Rozaliyani A, Sedono R, Jusuf A, Rumende C, Aniwidyaningsih W, Burhan E, et al. A novel diagnosis scoring model to predict invasive pulmonary aspergillosis in the intensive care unit. Saudi Med J. 2019;40:140–6.
Donnelly J, Chen S, Kauffman C, Steinbach W, Baddley J, Verweij P, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses study group education and research consortium. Clin Inf Dis. 2019;71:1367–76.
Reiss E, Shadomy H, Lyon G. Aspergillosis fundamental medical mycology. NJ: Hoboken Wiley Blackwell; 2012; 357–90.
Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110:3532–9.
Vergidis P, et al. High-volume culture and quantitative real-time PCR for the detection of aspergillus in sputum’. Clin Microbiol Inf. 2020;26(7):935–40.
Tragiannidis A, et al. Invasive aspergillosis in children with acquired immunodeficiencies. Clin Inf Dis. 2011;54(2):258–67.
Çağlar İ, Özkerim D, Tahta N, Düzgöl M, Bayram N, Demirağ B, et al. Assessment of serum galactomannan test results of pediatric patients with hematologic malignancies according to consecutive positivity and threshold level in terms of invasive aspergillosis diagnosis: cross-sectional research in a tertiary care hospital. J Pediatr Hematol Oncol. 2019;42:e271–6.
Jha A, Bansal D, Chakrabarti A, Shivaprakash M, Trehan A, Marwaha R. Serum galactomannan assay for the diagnosis of invasive aspergillosis in children with hematological malignancies. Mycoses. 2013;56:442–8.
Avcu G, Karapinar D, Akinci A, Sivis Z, Sahin A, Bal Z, et al. Utility of the serum galactomannan assay for the diagnosis of invasive aspergillosis in children with acute lymphoblastic leukemia. Intern J Infect Dis. 2017;54:8–12.
Sharma R, Singh C, Khadwal A, Prakash G, Malhotra P, Jain A, et al. Role of pre-transplant chest high-resolution computed tomography and serum galactomannan index in predicting post-transplant invasive pulmonary aspergillosis in allogeneic hematopoietic cell transplant recipients. Transpl Infect Dis. 2021;23(4):e13632.
Fraczek MG, et al. Volume dependency for culture of fungi from respiratory secretions and increased sensitivity of Aspergillus quantitative PCR. Mycoses. 2013;57(2):69–78.
Dinand V, Anjan M, Oberoi JK, Khanna S, Yadav SP, Wattal C, et al. Threshold of galactomannan antigenemia positivity for diagnosis of invasive aspergillosis in neutropenic children. J Microbiol Immunol Inf. 2016;49:66–73.
Mohindra R, Capoor MR, Puri S, Raheja H, Gupta DK, Gupta B, et al. Evaluation of serum galactomannan enzyme immunoassay at two different cut-offs for the diagnosis of invasive aspergillosis in patients with febrile neutropenia. Indian J Med Microbiol. 2017;35(2):237–9.
Rdramurthy S, Paul R, Chakrabarti A, Mouton J, Meis J. Invasive aspergillosis by Aspergillus flavus: epidemiology, diagnosis, antifungal resistance, and management. J fungi. 2019;5:55–78.
Maertens J, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in Neutropenic Hematology patients. Cancer. 2008;115(2):355–62.
Huppler AR, Fisher BT, Lehrnbecher T, Walsh TJ, Steinbach WJ. Role of molecular biomarkers in the diagnosis of invasive fungal diseases in children. J Pediatric Infect Dis Soc. 2017;6:32–44.
Fisher BT, Zaoutis TE, Park JR, et al. Galactomannan antigen testing for diagnosis of invasive aspergillosis in pediatric hematology patients. J Pediatric Infect Dis Soc. 2012;1:103–11.
Duarte RF, et al. Serum Galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective Antimold Prophylaxis. Clin Inf Dis. 2014;59(12):1696–702.
Khoo AL, et al. Cost-effectiveness of serum galactomannan surveillance during mould-active antifungal prophylaxis. J Fungi. 2021;7(6):417. https://doi.org/10.3390/jof7060417.
Xiao W, et al. Sputum signatures for invasive pulmonary aspergillosis in patients with underlying respiratory diseases (SPARED): study protocol for a prospective diagnostic trial. BMC Inf Dis. 2018;18(1):4369.
Luabeya AK, et al. Noninvasive detection of tuberculosis by oral swab analysis’. J Clin Microbiol. 2019;57(3):10–1128.
Mercier T, Castagnola E, Marr KA, Wheat LJ, Verweij PE, Maertens JA. Defining Galactomannan positivity in the updated EORTC/MSGERC consensus definitions of invasive fungal diseases. Clin Inf Dis. 2021;72:S89-93.
Han SB, Kim SK, Lee JW, Yoon J-S, Chung N-G, Cho B, et al. Serum Galactomannan index for early prediction of mortality in immunocompromised children with invasive pulmonary aspergillosis. BMC Inf Dis. 2015;15(1):15.
Kumar J, Singh A, Seth R, Xess I, Kabra SK. Galactomannan Antigen Test for Early Diagnosis of Invasive Aspergillus Infection in Pediatric Febrile Neutropenia. Indian Pediatr. 2018;55(3):257–8.
Badiee P, Karimi M, Alborzi A, Pourabbas B, Shakiba E, Mardaneh J. Invasive aspergillosis in pediatric hematology oncology ward. Iran J Blood Cancer. 2010;2:67–70.
Choi SH, Kang ES, Eo H, Yoo SY, Kim JH, Yoo KH, et al. Aspergillus galactomannan antigen assay and invasive aspergillosis in pediatric cancer patients and hematopoietic stem cell transplant recipients. Pediatr Blood Cancer. 2013;60:31622.
Gefen A, Zaidman I, Shachor-Meyouhas Y, Avidor I, Hakim F, Weyl Ben-Arush M, et al. Serum galactomannan screening for diagnosis of invasive pulmonary aspergillosis in children after stem cell transplantation or with high-risk leukemia. Pediatr Hematol Oncol. 2015;32(2):146–52.
Özen S, Özdemir H, Evren E, Taskin EC, Arga G, Konca HC, et al. The role of galactomannan test results in the diagnosis of pediatric invasive aspergillosis. Infect Dis. 2022;54:269–76.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Handling Editor: Martin Hoenigl.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, S., Capoor, M.R., Singh, A. et al. Diagnostic Utility of Galactomannan Enzyme Immunoassay in Invasive Aspergillosis in Pediatric patients with Hematological Malignancy. Mycopathologia 188, 1055–1063 (2023). https://doi.org/10.1007/s11046-023-00798-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-023-00798-y