Abstract
The landscape of invasive Candida infections in patients with hematologic malignancy has evolved due to the adoption of anti-fungal prophylaxis, advances in oncological therapies, and developments in antifungal therapies and diagnostics. Despite these scientific gains, the morbidity and mortality caused by these infections remain unchanged, highlighting the importance of an updated understanding of its epidemiology. Non-albicans Candida species are now the predominant cause of invasive candidiasis in patients with hematological malignancy. This epidemiological shift from Candida albicans to non-albicans Candida species is partially a consequence of selective pressure from extensive azole use. Further analysis of this trend suggests other contributing factors including immunocompromise caused by the underlying hematologic malignancy and the intensity of its associated treatments, oncological practices, and regional or institution specific variables. This review characterizes the changing distribution of Candida species in patients with hematologic malignancy, describes the causes driving this change, and discusses clinical considerations to optimize management in this high-risk patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few decades, advances in the treatment of hematologic malignancies have greatly increased the relative survival of this patient population. The CDC estimates that more than half a million people were living more than five years after their diagnosis of myeloma, leukemia, or lymphoma as of 2019 [1]. One cost of advances in allogeneic hematopoietic stem cell transplant (HSCT), chimeric antigen response therapy (CAR-T), and biologic immunotherapies is the growing population of patients at risk of opportunistic and hospital acquired infections such as invasive candidiasis. Invasive candidiasis, defined as Candida infection identified from a sterile site, is most commonly candidemia (positive blood cultures), but can also present with infection involving sites such the eye or the central nervous, musculoskeletal, or hepatosplenic systems. Overall, the rates of invasive candidiasis in patients with hematologic malignancy and HSCT have decreased over the past few decades with the advent of effective antifungal prophylaxis, but these infections remain a significant cause of morbidity and mortality [2,3,4,5].
While pioneers in bone marrow transplantation once feared Candida albicans as a major cause of infection [6, 7], the worldwide incidence of C. albicans infections is declining [8, 9] and most invasive Candida infections in these patients are now caused by non-albicans Candida species [2, 7, 10,11,12]. The predominant non-albicans Candida species causing invasive infections varies among institutions because of differences in regional patterns of antifungal use as well as oncologic practices such as indwelling catheters and conditioning chemotherapy regimens [2, 13, 14]. Similar to antibiotics, antifungal resistance patterns are driven by local antifungal use and selective pressure [15]. In addition to a shift in Candida species distribution, patient level risk factors for invasive Candida infection have evolved over the past several decades of hematologic malignancy treatment [2].
Thus, appreciating the current epidemiology of invasive Candida infections in patients with hematologic malignancies is critical to tailoring effective prevention, detection, and treatment.
Variations in the Distribution of Invasive Candida Infections
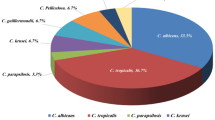
Though more than a dozen Candida species may cause human infection, over 90% of all invasive Candida infections are caused by five major species: Candida albicans, Candida glabrata, Candida parapsilosis, Candida krusei, and Candida tropicalis [8, 12]. The predominant species causing invasive Candida infection in patients with hematologic malignancies has shifted from C. albicans to non-albicans Candida species over the past three decades. A 2009 study at a large cancer center in the US found that rates of candidemia from C. albicans declined from 34% in 1988–1992 to 24% in 2001–2007 [2]. During these time periods, rates of C. parapsilosis infections increased from 14 to 24% [2]. Overall, 76% of candidemia infections in the study were caused by non-albicans Candida species [2]. A multicenter registry study published in 2014 found that nearly 50% of all invasive fungal infections in general patient population were from non-albicans Candida species [10, 11], while 55.8% of patients with invasive C. krusei infections had hematologic malignancy [10].
Registry studies are useful for understanding large shifts in epidemiology, but the predominant non-albicans Candida species causing invasive candidiasis is often specific to a region or institution [10]. This pattern is well demonstrated in several single center and regional multicenter studies that identified different non-albicans Candida species as the leading cause of invasive candidiasis. A 2008 study of patients at a large cancer center in Texas found that C. glabrata and C. krusei caused 31% and 24% of invasive Candida infections respectively [14] and fluconazole prophylaxis was an independent risk factor for infection with these two species. In contrast, a large, single center study of Chinese patients with history of hematologic malignancy or allogeneic stem cell transplant, more than 80% of whom had prior azole exposure, found that Candida tropicalis accounted for 69% of candidemia [16]. A 2006 single center study in Brazil, where azole use was relatively low at the time, found that 78.9% of candidemia in patients with hematologic malignancy was caused by non-albicans Candida, especially C. tropicalis and C. parapsilosis [5]. C. albicans (33%) and C. parapsilosis (22%) were the predominant species in a multicenter study conducted in Italy of patients with hematologic malignancy and candidemia [17]. Regional patterns proposed by Kullberg, et al., suggest that C. glabrata is a major problem in northern Europe, the United States, and Canada, while C. parapsilosis is more predominant in southern Europe, Asia, and South America [13].
Epidemiological trends of invasive disease due to C. auris in patients with hematological malignancy has yet to be specifically described. There is suggestion that neutropenia and gut mucositis, traditional risk factors for invasive candidiasis, play lesser roles. Unlike other Candida species, C. auris evades neutrophils and thereby prevents the production of neutrophil extracellular traps responsible for the immune response against Candida [18]. Further, it is not a commensal gut organism because it grows poorly in anaerobic conditions. Evaluating for emerging trends of C. auris infections in patients with hematological malignancy, a population heavily exposed to anti-fungals and at high risk of infections transmitted in healthcare settings, can provide insight into the nuanced interplay of institutional practices, regional Candida patterns, and host immune factors.
Risk Factors for Invasive Candida Infections in Patients with Hematologic Malignancy
Despite the widespread adoption of antifungal prophylaxis in patients with hematologic malignancy, this population remains at risk for invasive Candida infections. Some of the traditional risk factors for invasive candidiasis, such as critical illness, indwelling central venous catheters (CVCs), mechanical ventilation, parenteral nutrition, broad spectrum antibiotic exposure, and glucocorticoid use are more common in patients with hematologic malignancy or HSCT [4, 12,13,14]. However, hematologic malignancy itself is an independent risk factor for invasive Candida infections in hospitalized patients [4, 13], reflecting disease or treatment associated neutropenia or immunocompromise and disturbances in mucosal barrier integrity. Although some studies in this patient population have identified CVCs as a major risk factor for candidemia [14, 17], it is important to note that gastrointestinal translocation in neutropenic patients is significant source of invasive Candida infection. Thus, CVC removal should be considered on an individualized basis [12].
The type of hematologic malignancy, oncologic therapeutic history, and immune status also contribute to risk for invasive Candida infections. While invasive fungal disease is most commonly seen in patients with acute myeloid leukemia (AML) undergoing intensive induction chemotherapy and allogeneic SCT recipients, invasive Candida infections in patients with acute lymphoproliferative leukemia (ALL) and patients with chronic lymphoproliferative disease being treated with ibrutinib are also increasingly reported [19]. HSCT recipients are at greatest risk of invasive Candida infections during the first 4 months after transplant [20].
Intestinal integrity plays a major role in risk for invasive Candida infection. Just as recent abdominal surgery is a traditional risk factor for candidemia, a compromise in the gut mucosal barrier due to neutropenia, cytotoxic chemotherapy, or gut graft versus host disease (GVHD) increases the risk for invasive Candida infections in patients with hematologic malignancy [5, 12, 21]. The use of antibiotics for fever or prophylaxis during periods of neutropenia also affects gut microbiota and enables fungal overgrowth [21].
Risk factors for species-specific invasive Candida infections are largely related to prior antifungal exposure and presence of neutropenia in patients with hematologic malignancies. For instance, antifungal prophylaxis with fluconazole has been identified as a risk factor for C krusei and C glabrata candidemia [14]. Additionally, the widespread use of antifungal prophylaxis has led to changes in the most common Candida species causing infections in patients with hematologic malignancies [22].
Evidence for Antifungal Prophylaxis in Hematologic Malignancy
Invasive candidiasis, specifically from C. albicans, was a major cause of morbidity and mortality for patients in the early years of hematopoietic stem cell transplantation [4, 6, 23]. In fact, before the introduction of antifungal prophylaxis as standard of care in this patient population, Candida species caused the majority of invasive fungal infections [7]. Invasive aspergillosis has now replaced candidiasis as the major cause of morbidity and mortality in HSCT recipients [19, 23]. To understand how the epidemiology of invasive Candida infections has changed over time, it is helpful to review how antifungal management evolved in patients with hematologic malignancies.
Several studies over the past three decades have shown significant benefit in using antifungal prophylaxis to prevent invasive fungal infections in patients undergoing HSCT, thereby improving rates of infection-related outcomes. The landmark 1992 trial by Goodman et al. showed the benefits of using systemic antifungal prophylaxis in preventing invasive candidiasis in HSCT recipients [24], especially during the pre-engraftment period of neutropenia. Fluconazole became the standard, guideline-recommended fungal prophylaxis after HSCT throughout 1990s. Subsequent studies by Slavin et al. and Marr et al. showed long term mortality benefit and prevention of candidiasis-related death when fluconazole was continued in HSCT recipients beyond the initial pre-engraftment period [25, 26]. In 2004, a study demonstrated that micafungin prophylaxis during pre-engraftment neutropenia was superior to fluconazole at preventing invasive fungal infections, with fewer adverse drug events in the echinocandin arm [15]. In 2007, a randomized trial showed improved infectious outcomes in HSCT recipients treated with posaconazole prophylaxis compared to fluconazole prophylaxis, reflecting the increasing burden of invasive Aspergillus and other mold infections in this patient population [27].
With this data and the increasing availability of more tolerable, well-absorbed oral antifungal agents, prophylaxis for HSCT recipients and neutropenic patients with acute leukemia or myelodysplastic syndrome has shifted to predominantly mold-active agents. Currently, major guidelines recommend posaconazole prophylaxis for patients undergoing intensive induction chemotherapy for AML or MDS, allogeneic HSCT during pre-engraftment (neutropenic) period, and GVHD treatment with high dose steroids [28,29,30]. Candida-active prophylaxis with fluconazole or micafungin may be considered in autologous HSCT recipients or in areas with low risk for invasive mold infections [28,29,30]. Interestingly, although these guidelines frequently distinguish risk for invasive fungal infections in hematologic malignancy treatment or HSCT by expected duration of neutropenia, the same cannot be said for treatment with chimeric antigen receptor therapy (CAR-T). In a recent single center study of nearly 300 adults undergoing CD-19 directed CAR-T therapy without antifungal prophylaxis, the cumulative incidence of invasive fungal infections was only 1.8%, despite more than 40% of study participants experiencing prolonged neutropenia [31]. This demonstrates that gut mucosal injury caused by cytotoxic chemotherapy significantly contributes to the overall risk for invasive candidiasis. To date, the available data on risk of invasive fungal infections in patients undergoing novel oncologic treatment with bispecific T cell engagers, checkpoint inhibitors, and immunotherapies are not sufficient to recommend routine antifungal prophylaxis in these patients [32].
Reasons for a Changing Candidemia Epidemiology
The emergence of non-albicans Candida species in patients with hematologic malignancy, especially species that exhibit azole resistance, is partially a product of antifungal exposure. In contrast, C. albicans remains the leading cause of candidemia in patients with solid tumor malignancies [33], a population that does not receive routine antifungal prophylaxis and whose chemotherapeutic regimens have different immunological and gut mucosal barrier consequences. As antifungal drugs with efficacy against C. albicans increased in patients with hematologic malignancies, there was a corresponding rise in the incidence of invasive infections from non-albicans Candida [7]. Extensive use of fluconazole likely places selective pressure on yeast such that fluconazole-non-susceptible Candida species become predominant [11, 15]. In a study of 71 patients, of whom 78.9% received triazole prophylaxis and 21.1% received echinocandin prophylaxis, there were 33 breakthrough episodes of candidemia [16]. The Candida isolates identified in the breakthrough infections had voriconazole and caspofungin resistance rates reaching nearly 50% and 24% respectively [16].
However, the use of fluconazole prophylaxis does not fully explain the shift toward non-albicans Candida infections. In a small multicenter study of cancer patients in Brazil, Nucci et al. found that non-albicans Candida species accounted for 84% of invasive candidiasis although only 18.2% of the study population received azole prophylaxis [34].
Selection pressure related to non-azole antifungal use, such as echinocandins, and a general increase in the number of complex patients living with prolonged cytopenias, may be playing a role in the distribution of invasive candidiasis [22]. The rise in invasive infections by Candida parapsilosis, an organism with predilection for adhering to indwelling prosthetics [2, 8, 13] and elevated minimum inhibitory concentrations to echinocandins, is correlated with the increased prevalence of hematologic malignancy patients living with CVCs and increased use of echinocandins [2]. Interestingly, despite a decline in fluconazole use in favor of mold-active antifungals such as posaconazole in patients with hematologic malignancy, incidence rates of non-albicans candidemia remain relatively unchanged [2, 20].
In addition to selective pressure from antifungal use and changes in patient risk factors for invasive Candida infections, advances in diagnostic techniques have changed our ability to detect Candida species. Current formulations of culture media, venting and aerobic preparation of liquid media, and lysis centrifugation technique have all increased the yield of fungal growth in standard blood cultures [35]. Novel molecular diagnostic platforms and mass spectrometry are now capable of rapidly and accurately identifying Candida species in the blood [36]. Despite these innovative diagnostics, many cases of invasive Candida infections in patients with hematologic malignancies are missed with blood cultures alone, especially given low rates of autopsy in these patient populations [35, 37, 38].
Antifungal Resistance and Empiric Treatment for Invasive Candida Infections
With changes in the epidemiology of invasive Candida infections in patients with hematologic malignancy, antifungal resistance testing has become vital. While older methods such as broth dilution and disc diffusion are still commonly used, newer methods of antifungal susceptibility testing such as gradient diffusion strips, YeastOne colorimetric antifungal panel, and the automated Vitek2 yeast susceptibility panel are increasingly available [39]. As previously noted, antifungal exposure in the form of prophylaxis in the neutropenic host likely contributes to colonization by resistant fungal species. This is supported by numerous studies showing the rise in infections by Candida species with intrinsic antifungal resistance, such as C. krusei. In addition to inherent resistance to fluconazole, C. krusei is also capable of rapidly acquiring resistance to other antifungals, making this a formidable pathogen in the hematologic malignancy patient population [9]. With rising rates of non-albicans Candida infections, antifungal susceptibility testing has appropriately increased [39]. Susceptibility testing is especially important for C. glabrata, which has higher incidence of echinocandin resistance [39, 40]. One study found that 10% of Candida glabrata isolates in the US were resistant to fluconazole, and that 9% of isolates were resistant to echinocandins and fluconazole [40].
Guidelines recommend echinocandins as first-line empiric treatment for invasive Candida infections in both neutropenic and non-neutropenic patients, with the caveat that lipid formulation amphotericin may also be used as a “less attractive” alternative first-line agent in neutropenic patients and that echinocandins can achieve high concentrations at the infection site in question [12]. These recommendations were based on multiple studies showing improved survival with use of echinocandins for invasive candidiasis compared to other antifungal agents. However, both the guidelines and commentary response note that neutropenic patients may be at higher risk for antifungal resistance due to prior exposure, and that neutropenic patients represent only a small subset of the study population in randomized trials of antifungal treatment drugs [12, 41, 42]. Thus, as previously noted, antifungal resistance testing is paramount, and decisions on empiric treatment of invasive Candida infection should be individualized in patients with hematologic malignancy.
As routine susceptibility testing is more commonly performed for invasive fungal infections, it is important that hospitals and regional networks take time to periodically review Candida epidemiology and resistance patterns. The inclusion of Candida species in institutional antibiograms can assist in identifying shifts in the local distribution of Candida species causing infection and thereby guide optimal clinical management and antifungal stewardship [39].
Outcomes of Invasive Candida Infections in Patients with Hematologic Malignancy
Invasive Candida infections in patients with hematologic malignancy confer substantial risk of morbidity and mortality. In the literature, mortality rates for invasive Candida infections ranges between 5–71% [2, 3, 5, 11, 20]. This broad range reflects the difficulty attributing the cause of death in complex patients who are often critically ill and have concomitant bacterial infection [12] and the clinical heterogeneity of this population. A multicenter North American registry study of invasive fungal disease have reported overall mortality of 35% at 12 weeks for candidemia [11], while a prospective study of US cancer centers found a 1-year mortality rate of 76% among hematopoietic stem cell recipients with invasive candidiasis [19]. Bloodstream infections from Candida krusei, which are more common in patients with hematologic malignancy due to prior azole exposure [10, 13], had the highest mortality rate (52.9%) of all Candida species identified in the study by Horn, et al. A similar 31% cumulative mortality rate associated with invasive Candida infections was seen in a single center study of patients with hematologic malignancy and HSCT [16]. The widespread use of antifungal prophylaxis has not changed the recent incidence of candidemia or the mortality rates associated with invasive Candida infections in this patient population [2]. The stable incidence suggests that patient specific factors such as compromise of the mucocutaneous barrier, CVC use, and prolonged neutropenia contribute to the development of invasive candidiasis. Time to effective antifungal therapy and source control have been correlated with survival in several reports [12], but the continued mortality burden is an overall consequence of the patients’ comorbidity index.
In patients with hematologic malignancy, invasive Candida infections may also contribute to delay in oncologic therapy and prolonged hospitalizations. While the majority of candidemia are reported in the first few months after HSCT [20], this does not capture the downstream effects of these serious infections. Invasive Candida infections during neutropenia may require longer courses of therapy, as treatment is often continued until recovery of neutrophil count, and evaluation for metastatic disease such as endophthalmitis may be delayed or suboptimal [12]. Thus, high mortality rates reported at 6 or 12 months after invasive Candida infections more likely represent the poor prognostic implications of candidiasis in this patient population.
Conclusion
The epidemiology of invasive Candida infections in patients with hematologic malignancy has changed over the past three decades with the majority of infections now caused by non-albicans Candida species. Variability in Candida species distribution and antifungal susceptibility is driven by local patient populations, oncologic therapy, and antifungal use. Institution-level antibiograms describing the prevalence of Candida species and antifungal susceptibility patterns should be implemented in such a rapidly changing field. In addition to this epidemiological data, a stronger understanding of the clinical parameters that drive the predominance of a specific non-albicans Candida species or resistance pattern is crucial to optimizing the clinical management of invasive candidiasis in patients with hematologic malignancy.
References
Centers for Disease Control and Prevention. Hematologic Cancer Incidence, Survival, and Prevalence. USCS Data Brief, no. 30. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2022.
Sipsas NV, Lewis RE, Tarrand J, Hachem R, Rolston KV, Raad II, Kontoyiannis DP. Candidemia in patients with haematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer. 2009;115:4745–52. https://doi.org/10.1002/cncr.24507.
Gamaletsou MN, Walsh TJ, Zaoutis T, Pagoni M, Kotsopoulou M, Voulgarelis M, et al. A prospective cohort multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect. 2014;20:O50–7. https://doi.org/10.1111/1469-0691.12312.
Wingard JR, Merz WG, Rinaldi MG, Johnson TR, Karp JE, Saral R, et al. Increase in C krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;326:1274–7. https://doi.org/10.1111/1469-0691.12312.
Pasqualotto AC, Rosa DD, Medeiros LR, Severo LC. Candidemia and cancer: patients are not all the same. BMC Infect Dis. 2006;6:1–7. https://doi.org/10.1186/1471-2334-6-50.
Solberg CO, Meuwissen HJ, Needham RN, Good RA, Matsen JM. Infectious complications in bone marrow transplant patients. Br Med J. 1971;1(5739):18–23. https://doi.org/10.1136/bmj.1.5739.18.
Bays DJ, Thompson GR. Fungal infections of the stem cell transplant recipient and hematologic malignancy patients. Infect Dis Clin N Am. 2019;33:545–66. https://doi.org/10.1016/j.idc.2019.02.006.
Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Int Med. 2016;34:21–8. https://doi.org/10.1016/j.ejim.2016.06.029.
Jaimu AT, Albertyn J, Sebolai OM, Pohl CH. Update on Candida krusei, a potential multidrug-resistant pathogen. Med Mycol. 2021;59:14–30. https://doi.org/10.1093/mmy/myaa031.
Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2496 patients: data from the prospective antifungal therapy (PATH) registry 2004–2008. PLoS ONE. 2014;9(7):e101510. https://doi.org/10.1371/journal.pone.0101510.
Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–703. https://doi.org/10.1086/599039.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):e1-50. https://doi.org/10.1093/cid/civ933.
Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373:1445–56. https://doi.org/10.1056/NEJMra1315399.
Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I. The Changing epidemiology of invasive Candidiasis. Cancer. 2008;112(11):2493–9. https://doi.org/10.1002/cncr.23466.
Van Burik JH, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, et al. Micafungin versus fluconazole for prophylaxis against IFIs during neutropenia in patients undergoing hematologic stem cell transplantation. Clin Infect Dis. 2004;39(10):1407–16. https://doi.org/10.1086/422312.
Chen X-C, Xu J, Wu D-P. Clinical characteristics and outcomes of breakthrough candidemia in 71 hematological malignancy and/or allogeneic hematopoietic stem cell transplant recipients: a single center retrospective study from China, 2011–2018. Clin Infect Dis. 2020;71(suppl 4):S394–9. https://doi.org/10.1093/cid/ciaa1523.
Criscuolo M, Marchesi F, Candoin A, Cattaneo C, Nosari A, Veggia B, et al. Fungaemia in haematological malignancies: SEIFEM-2015 survey. Eur J Clin Invest. 2019;49(5):e13083. https://doi.org/10.1111/eci.13083.
El Baradei A. A decade after the emergence of Candida auris: what do we know? Eur J Clin Microbiol Infect Dis. 2020;39(9):1617–27. https://doi.org/10.1007/s10096-020-03886-9.
Bergamasco MD, Pereira CA, Arrais-Rodrigues C, Ferreira DB, Baiocchi O, Kerbauy F, et al. Epidemiology of invasive fungal diseases in patients with hematologic malignancies and hematopoietic cell transplantation recipients managed with an antifungal diagnostic driven approach. J Fungi (Basel). 2021;7:588. https://doi.org/10.3390/jof7080588.
Kontoyiannis DP, Marr KA, Park BJ, Alexander BJ, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis. 2010;50:1091–100. https://doi.org/10.1086/651263.
Wirk B, Wingard JR. Current approaches in antifungal prophylaxis in high risk hematologic malignancy and hematopoietic stem cell transplant patients. Mycopathologia. 2009;168:299–311. https://doi.org/10.1007/s11046-009-9188-6.
Colombo AL, Agnelli C, Kontoyiannis DP. Knowledge gaps in candidaemia/invasive candidiasis in haematological cancer patients. J Antimicrob Chemother. 2021;76:543–6. https://doi.org/10.1093/jac/dkaa446.
Bhatti Z, Shaukat A, Almyroudis NG, Segal BH. Review of epidemiology, diagnosis, and treatment of invasive mould infections in allogeneic hematopoietic stem cell transplant recipients. Mycopathologia. 2006;162:1–15. https://doi.org/10.1007/s11046-006-0025-x.
Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–55. https://doi.org/10.1056/NEJM199203263261301.
Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospection, randomized, double-blind study. J Infect Dis. 1995;171:1545–52. https://doi.org/10.1093/infdis/171.6.1545.
Marr K, Seidel K, Slavin M, Bowden RA, Schoch HG, Flowers ME, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96:2055–61.
Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–59. https://doi.org/10.1056/NEJMoa061094.
Maertens JA, Girmenia C, Brüggemann RJ, Duarte RF, Kibbler CC, Ljungman P, et al; European Conference on Infections in Leukaemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and; European Conference on Infections in Leukaemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and the European LeukemiaNet (ELN). European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 2018;73(12):3221–3230. doi: https://doi.org/10.1093/jac/dky286.
Baden LR, Swaminathan S, Angarone M, Blouin G, Camins BC, Casper C, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882–913. https://doi.org/10.6004/jnccn.2016.0093.
Fleming S, Yannakou CK, Haeusler GM, Clark J, Grigg A, Heath CH, et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J. 2014;44(12b):1283–97. https://doi.org/10.1111/imj.12595.
Little JS, Aleissa MM, Beluch K, Gonzalez-Bocco IH, Marty FM, Manne-Goehler J, et al. Low incidence of invasive fungal disease following CD19 chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma. Blood Adv. 2022;6(16):4821–30. https://doi.org/10.1182/bloodadvances.2022007474.
Lindsay J, Teh BW, Micklethwaite K, Slavin M. Azole antifungals and new targeted therapies for hematological malignancy. Curr Opin Infect Dis. 2019;32(6):538–45. https://doi.org/10.1097/QCO.0000000000000611.
Cornely OA, Gachot B, Akan H, Bassetti M, Uzun O, Kibbler C, et al. EORTC infectious diseases group. Epidemiology and outcome of fungemia in a cancer cohort of the infectious diseases group (IDG) of the European organization for research and treatment of cancer (EORTC 65031). Clin Infect Dis. 2015;61(3):324–31. https://doi.org/10.1093/cid/civ293.
Nucci M, Silveira MI, Spector N, Silveira F, Velasco E, Martins CA, et al. Fungemia in cancer patients in Brazil: predominance of non-albicans species. Mycopathologia. 1998;141(2):65–8. https://doi.org/10.1023/a:1006951619245.
Reimer LG, Wilson ML, Weinstein MP. Update on detection of bacteremia and fungemia. Clin Microbiol Rev. 1997;10:444–65. https://doi.org/10.1128/CMR.10.3.444.
Marinach-Patrice C, Fekkar A, Atanasova R, Gomes J, Djamdjian L, Brossas JY, et al. Rapid species diagnosis for invasive candidiasis using mass spectrometry. PLoS ONE. 2010;5(1):e8862. https://doi.org/10.1371/journal.pone.0008862.
Seftel MD, Ho M, Pruthi D, Orbanski S, Rubinger M, Schacter B, et al. High rate of discordance between clinical and autopsy diagnoses in blood and marrow transplantation. Bone Marrow Transpl. 2007;40(11):1049–53. https://doi.org/10.1038/sj.bmt.1705855.
Chamilos G, Luna M, Lewis RE, Bodey GP, Chemaly R, Tarrand JJ, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica. 2006;91:986–9.
Berkow EL, Lockhart SR, Ostrosky-Zeichner L. Antifungal susceptibility testing: current approaches. Clin Microbiol Rev. 2020;33(3):e00069-e119. https://doi.org/10.1128/CMR.00069-19.
Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from, et al. to 2011. J Clin Microbiol. 2008;2012(50):3435–42. https://doi.org/10.1128/JCM.01283-12.
Kontoyiannis DP (2009) Echinocandin-based initial therapy in fungemic patients with cancer: a focus on recent guidelines of the infectious diseases society of America. Clin Infect Dis. 49(4):638–9; author reply 639–40. doi: https://doi.org/10.1086/603585
Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356(24):2472–82. https://doi.org/10.1056/NEJMoa066906.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Ferry Hagen
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McCort, M.E., Tsai, H. Epidemiology of Invasive Candidiasis in Patients with Hematologic Malignancy on Antifungal Prophylaxis. Mycopathologia 188, 885–892 (2023). https://doi.org/10.1007/s11046-023-00754-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-023-00754-w