Abstract
Members of the Cryptococcus gattii species complex are notorious causes of cryptococcosis as they often cause severe, life-threatening infections. Here we describe a case of a severe disseminated C. deuterogattii infection in a previously healthy patient who was initially treated with amphotericin B, 5-fluorocytosine and fluconazole, which led to a good neurological response, but the infection in the lungs remained unaltered and was not completely resolved until switching the antifungal therapy to isavuconazole. The infection was likely acquired during a one-month stay at the Azores Islands, Portugal. Environmental sampling did not yield any cryptococcal isolate; therefore, the source of this apparent autochthonous case could not be determined. Molecular typing showed that the cultured C. deuterogattii isolates were closely related to the Vancouver Island outbreak-genotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the Cryptococcus gattii species complex are notorious causes of cryptococcosis as they often cause severe, life-threatening infections. In the past, cryptococcosis was mainly reported from (sub)tropical regions, but it has expanded to temperate areas probably due to climate change, increased travel or anthropogenic activities [1, 2]. Two decades ago, an unprecedented outbreak of human C. gattii sensu lato infections in a non-endemic area was reported from Vancouver Island (British Columbia, Canada) from where it spread to mainland with 218 human cases reported between 1999 and 2007 [3, 4]. In the Pacific Northwest of the U.S.A., C. gattii s.l. infections emerged from 2004 onwards [5].
The C. gattii species complex is composed of five species, namely C. gattii sensu stricto (genotype AFLP4/VGI), C. bacillispora (genotype AFLP5/VGIII), C. deuterogattii (genotype AFLP6/VGII), C. tetragattii (genotype AFLP7/VGIV) and C. decagattii (genotype AFLP10) [6,7,8]. C. gattii sensu stricto and C. deuterogattii are major culprits of infection encountered among immunocompetent patients, whereas C. bacillisporus, C. tetragattii and C. decagattii are commonly associated with immunocompromised subjects [2, 6]. C. deuterogattii cases are rare in Europe and they are all presumably imported [9,10,11,12]. Here, we report a severe case of a disseminated C. deuterogattii infection in an immunocompetent patient from Spain.
Case Report
A previously healthy 67-year-old man presented to our hospital in Madrid, Spain, in August 2016 with a three-week history of progressive and severe headache, asthenia, hyporexia, arthralgia in lower and upper limbs without arthritis and general malaise. The frontotemporal headache radiated to the occipital area and increased during physical activities and in decubitus position, waking him up at night, accompanied with nausea and occasional vomiting; this did not disappear with analgesics. No fever, feeling of dysthermia, dyspnoea, or expectoration was reported. A sudden pleuritic-like pain in the right hemithorax three weeks before his admission was reported during the initial anamnesis. It was attributed to overexertion during a recent one-month holiday trip to the Azores Islands, Portugal. During that trip no cutaneous lesions nor any other relevant symptoms occurred. The previous medical history showed nothing relevant, besides an essential tremor without treatment, and the fact that the patient smoked ~ 6 cigarettes per day. Physical examination yielded a temperature of 37 °C, and no respiratory or neurological symptoms. Skin, ophthalmic, cardiac, abdominal and neurological examinations were normal too.
A chest radiograph showed pulmonary infiltrates within the right pulmonary base and left mid pulmonary field (Fig. 1a–b). However, a computed tomography (CT) revealed patchy areas of alveolar parenchymatous consolidation, some of them with aerial bronchogram. They were observed in both lungs, in the middle right lobe and both lower lobes, mainly showing subpleural and peribronchovascular location without swollen lymph nodes, which was suggestive of infectious aetiology and/or vasculitis (Fig. 1c–d). Spirometry was normal.
A–B Chest radiograph before antifungal therapy. X-ray reveals increased patched density with a rounded contour in the left mid-lung zone and right pulmonary base. C–D Computed tomography of the chest. E–F Chest radiograph after completion of the antifungal treatment (22 days post admission). X-ray reveals a resolution of the cryptococcal infiltrate with minimal scarring (a nodular image is observed in the middle lung lobe)
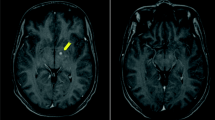
Central nervous system (CNS) CT scans, with and without contrast, were normal. Nevertheless, a cranial magnetic resonance imaging (MRI) showed multiple small round or oval cysts with high signal in T2, low signal in Fluid Attenuated Inversion Recovery (FLAIR) and low or intermediate signal in T1. No restriction in diffusion following a perivascular pattern was observed that may correspond to dilated Virchow Robin spaces and/or gelatinous pseudocysts in basal ganglia, anterior perforated substance, thalamus, periaqueductal grey substance, and cerebellar dentate nuclei adjacent to the IV ventricle. The larger ones were detected in the head of the left caudate nucleus. No hydrocephalus, meningeal enhancement or oedema was detected. Likewise, no cysts, a sign of putative cryptococcomas, were seen (Fig. 2A–D).
A–D Basal MR. A axial T2 images show dilated perivascular spaces and gelatinous pseudocysts in the basal ganglia and thalamus and enlargement of the subarachnoid spaces in the cerebellum. B Vasogenic oedema is not present. C–D Axial T1 C + (Gd) images show neither enhancement of these lesions nor leptomeningeal enhancement. E Follow-up MR after 4 weeks of treatment. F MR axial FLAIR shows diffuse biparietal and bifrontal sulcal hyperintensity. G MR axial T1 C + (Gd) shows enhancement extending into the subarachnoid space between the cerebellar folia-pial-arachnoid leptomeningeal enhancement and H enhancement in the basal ganglia lesions. I MR axial T1 C + (Gd) demonstrates disappearance of the previous leptomeningeal enhancement in the cerebellum. J MR axial FLAIR shows little enhancement in the basal regions. K MR axial FLAIR shows no sulcal hyperintensity
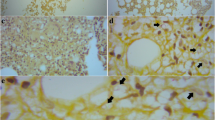
The first lumbar puncture was performed at admission and the cerebrospinal fluid (CSF) opening pressure was 25.5 cm of water. The CSF analysis yielded: leukocytes 112 cells/mm3 with mononuclear predominance; protein content 80 mg/dL; glucose 48 mg/dL (blood glucose 117 mg/dL) and adenosine deaminase 7 U/L. A CSF India ink stain was positive (Fig. 3A) with 300 cells/µL and cryptococcal antigen (CrAg) titers (CryptoPS, Biosynex, Illkirch-Graffenstaden, France) were remarkably high in CSF (1/10,000), as well as in blood (1/100,000) (Fig. 4).
Laboratory blood testing (hemogram, coagulation, hepatic and renal function, erythrocyte sedimentation rate and C-reactive protein) was within normal ranges. The immunological study showed normal values of complement C3 and C4, immunoglobulin (Ig) A, IgG and IgM, with slightly increased levels of IgE (128 IU/L; normal values 0–100 IU/L). Antinuclear antibodies (ANA) (ELISA), Extractable Nuclear Antigen antibodies (ENA) and antineutrophil cytoplasmic antibodies (ANCA) tests were negative. Serological tests for Borrelia, Brucella, syphilis and HIV infections were negative. Lymphocyte subpopulations, including CD4 + and CD8 + T cells, subsets of naïve and memory CD4 + and CD8 + T cells, B cells and NK cells were found to be normal. However, slightly decreased values of Th1-like lymphocytes, with normal values of Th2-like, Th17-like and follicular T lymphocytes were observed. We also analysed the IL-12/IL-23- IFN-ϒ axis as previously reported, and no anomalies were observed [13,14,15]. In brief, we analysed the expression of IFN-ϒR1, the production of IFN-ϒ in response to phytohemagglutinin and Bacille Calmette-Guerin (BCG) with and without IL-12, and the production of TNF-α and IL-12p70 in response to BCG with and without IFN-ϒ, as well as to lipopolysaccharide from Salmonella enteritidis with increasing doses of IFN-ϒ. Additionally, autoantibodies against granulocyte/macrophage colony stimulating factor (Anti GM-CSF) were tested in order to rule out whether these anti-cytokine autoantibodies, as previously reported [16], were causing susceptibility to infection in our patient. An in-house ELISA test was performed following the standardized procedure [17], using serial dilutions of the patient's serum starting from 1/101, with negative results in all of them.
Bronchoscopy with bronchoalveolar lavage (BAL) and brushing was performed as we suspected a lymphocyte meningitis with a cryptococcal aetiology with pulmonary involvement and no respiratory symptoms. Respiratory samples were sent for routine anatomopathological and microbiological investigations (bacteria, mycobacteria, viruses and fungi). CSF and BAL samples were seeded onto Sabouraud dextrose agar supplemented with chloramphenicol and incubated in a normal atmosphere at 35 °C. The respiratory samples were also inoculated onto Sabouraud with and without caffeic acid and in Brain Heart Infusion (BHI) supplemented with 10% sheep blood. These were also incubated in a normal atmosphere at 25 °C and 35 °C.
All samples, including blood cultures, remained negative for bacteria, mycobacteria and viruses. Cultures of CSF and respiratory samples onto Sabouraud dextrose (Fig. 3B) and BHI yielded colonies initially smooth and translucent, becoming mucoid and cream-coloured with age and turned dark brown onto Sabouraud supplemented with caffeic acid.
Isolated yeasts were further identified by means of conventional methods that included microscopic examination, the ability to grow at 37 °C, urease activity, melanin production onto l-DOPA agar and growth onto canavanine-glycine-bromothymol blue agar (CGB). These tests revealed that the identity of the yeast was Cryptococcus gattii sensu lato (Fig. 3C–D). Furthermore, molecular typing using URA5-RFLP was performed with isolates from BAL and CSF, resulting in a profile corresponding to molecular type VGII [2]. After that, detailed molecular typing was carried out by applying amplified fingerprinting length polymorphism (AFLP) and multi-locus sequence typing (MLST) by partly sequencing CAP59, GPD1, IGS1, LAC1, PLB1, SOD1 and URA5 loci [11, 18]. These analyses identified the two cryptococcal isolates as being Cryptococcus deuterogattii (genotype AFLP6/VGII). A phylogenetic analysis with reference strains and isolates from different geographical regions was carried out as previously described [11, 19] showing that the two isolates were highly similar to the Vancouver Island outbreak lineage (Fig. 5). Isolates were deposited in the CBS culture collection (CBS14685 and CBS14686) and sequences are available via Genbank (MW246067-MW246080).
Antifungal susceptibility testing was performed by using the commercial colorimetric microplate system Sensititre YeastOne (ThermoFisher Scientific, Waltham, MA, U.S.A.). This showed MICs to amphotericin B (AMB) of 0.5 µg/mL, 5-fluorocytosine (5FC) of 2 µg/mL, fluconazole (FLC) of 2 µg/mL, posaconazole of 0.25 µg/mL, voriconazole (VOR) of 0.5 µg/mL and itraconazole of 0.25 µg/mL. Retrospectively, isavuconazole (ISA) susceptibility was tested using the new Sensititre YTAMYUCC panel (ThermoFisher Scientific) and isolates were observed to be sensitive (0.03–0.06 µg/mL).
Treatment with liposomal AMB (AmBisome) 4 mg/kg/d i.v. and 5FC 100 mg/kg/d v.o. was immediately initiated after obtaining the first CSF, blood and BAL samples (Fig. 4). As an adjunct to antifungal therapy, serial lumbar punctures were performed to decrease intracranial pressure below 25 cm H2O (Fig. 4). On day 12 of treatment (September 6th, 2016) the pressure was normal (11 cm H2O), even though Indian ink staining, culture and CrAg in CSF (1/8,000) and high-positive antigenemia (1/100,000) showed that Cryptococcus cells persisted.
After 4 weeks of treatment, the follow-up MRI remained unchanged, with gelatinous pseudocysts, mainly at the level of the left caudate without hydrocephalus (Fig. 2E), and the fourth lumbar puncture was performed with normal pressure (11 cm H2O). CSF culture was negative, but the India ink stain was positive, and the same high antigen titer was detected in CSF (1/8000). Nevertheless, the antigenemia decreased drastically (from 1/100,000 to 1/10,000) (Fig. 4). The patient was discharged, and the induction treatment was daily administered at the hospital.
During his stay at home, a progressive deterioration of his general condition occurred, with progressive drowsiness, gait instability, frequent falls, bradipsychia and presyncopal conditions. Neurological examination confirmed gait instability, paraesthesias and decreased vibratory sensitivity in the feet, with weak Achilles reflexes. Cranial CT scan was normal, which ruled out the presence of stroke, haemorrhage or hydrocephalus. Brain MR was performed with skull protocol (Fig. 2F–H) which showed enhancement along the folia of the cerebellum and the basal ganglia lesions and diffuse sulcal hyperintensity. The radiological features were characteristic of Immune Reconstitution Inflammatory Syndrome (IRIS), considering the clinical context.
Repeated CSF checks showed the appearance of oligoclonal bands and an increase in Tibbling I, both of which involved an intrathecal synthesis of IgG, maintaining CSF at normal pressure. As IRIS was suspected, treatment with prednisone was initiated at a dose of 1 mg/kg/day (50 mg/d) for 1 month, followed by a progressively descending pattern for 7 months. Simultaneously, an intolerance and/or toxicity for 5FC (4 weeks and 3 days) and AMB (after 5 weeks and 2 days) resulted in a change in antifungal treatment to FLC (800 mg/d for the first 2 months and then 400 mg/d).
After a 20-day FLC treatment, the patient underwent a check-up as he felt afebrile, with no headache but with some episodes of orthostatic dizziness and tiredness when trying to concentrate. The rest of the neurological examination (gait and cranial nerves) was normal. Additionally, an improvement in the CSF analysis and a marked decrease in Tibbling I were observed, and cultures were negative (Fig. 4).
After 2 months of treatment (October 22nd, 2016) the patient continued improving from a neurological perspective as he no longer experienced dizziness, and walked with a walker up to 2 km/d. India ink staining remained negative and CSF CrAg decreased, while CrAg in serum remained at a high level (1/10,000).
After 7 months of treatment an MRI was performed, with T1 sequences, sagittal CUBE FLAIR, Axial T2, diffusion and gadolinium T1; compared with previous ones it showed a good evolution of the lesions with a reduction in the size and in the captation of the basal ganglia and the captation of the leptomeninges (Fig. 2I–K).
In June 2017, after 10 months of treatment, abnormal radiology of the thorax remained just as it was at the beginning of the disease. Therefore, new respiratory samples (bronchoaspirate, BAL and transbronchial biopsy) were obtained to rule out any other pathogens. India ink and microbiological cultures were all negative; however, PCR of BAL and transbronchial biopsy were positive for C. gattii s.l. and the plasma CrAg remained high (1/1000) (Fig. 4). On August 1st, 2017, antifungal treatment with FLC (400 mg/day) was changed to ISA (200 mg/d). This new therapeutic protocol was maintained for another 10 months, completing a total of 22 months since the patient’s first admission.
Under ISA therapy, serial chest X-rays showed a gradual disappearance of the bilateral lung lesions. In July 2018, a small increase in density projected on the right lung base persisted. With respect to the lateral plate, it seemed to correspond to the right middle lobe and was interpreted as a residual lesion. Given the good clinical progress of the patient it was decided to discontinue the ISA. The patient remained asymptomatic with that persisting small radiological infiltrate in the right lower lobe and a very low CrAg in plasma (1:2) until the last evaluation carried out during the follow-up taking place 26 months after the commencement of the antifungal treatment.
Discussion
Here we described a case of disseminated cryptococcosis due to Cryptococcus deuterogattii (AFLP6/VGII) in an apparently immunocompetent autochthonous patient from a non-endemic area. The overwhelmingly high fungal load and the appearance of the acute symptomatology suggest it was acquired after massive exposure, most probably during a recent trip to the Azores Islands, Portugal.
Different studies carried out in Europe (including Spain) show that infections caused by C. deuterogattii are quite rare and in the majority of the cases described so far, the origin of the pathogen could be related to exposure during trips to endemic areas [10,11,12, 20,21,22]. During a prospective environmental study in the Mediterranean basin conducted from 2012 to 2015 [10], 47 samples from Spain tested positive for Cryptococcus: 40 yielded C. neoformans sensu stricto (AFLP1/VNI), 1 for C. deneoformans (AFLP2/VNIV), 3 for C. gattii s.s. (AFLP/VGI) and 1 for C. tetragattii (AFLP7/VGIV). Hagen et al. [11] investigated 40 European cases of C. gattii infections: 24 patients most likely acquired the disease outside Europe, while 16 individuals (26 isolates) could have acquired the infection within Europe. Among the latter, twelve patients (75%) had an infection caused by C. gattii s.s. (AFLP4/VGI) and 4 patients (25%) had a C. deuterogattii (AFLP6 /VGII) infection. The presence of C. gattii s.s. in the European environment has been demonstrated [9, 10, 12, 18, 23, 24] but the environmental source of C. deuterogattii has never been found in Europe and the clinical cases described were most likely acquired elsewhere.
Regarding the current case, the acute symptomatology of costal pain and a 3-week headache after a month-long visit to the Portuguese Azores Islands, made us suspect that the infection was acquired during the patient’ stay in these islands. However, there are no reports on the presence of the highly virulent C. deuterogattii (AFLP6/VGII) in this area. During this trip, the patient took part in regular tourist activities, including a visit to an abandoned hotel in a wooded and humid area, littered with bird excreta and decayed vegetation. In order to investigate that environment as a possible source of infection, environmental samples were collected there and subsequently studied. Only one strain of Papiliotrema laurentii (formerly Cryptococcus laurentii) was obtained. The sampling was not representative as only 4 samples were taken.
Cryptococcus species isolated from a respiratory sample should always be studied. Full workup is required, ideally to the species level, which may influence management and be relevant for public health surveillance [25]. The identification of the members of the genus Cryptococcus at species level cannot be achieved by means of conventional clinical laboratory methods. Confirmation of C. gattii s.l. and the identification of the species requires molecular methods or MALDI-TOF MS analysis [6]. In this case, complete phenotypic and molecular typing of the isolates from CSF and lung samples allowed for the identification of C. deuterogattii (AFLP6/VGII). As the patient was HIV-negative and did not show any evident deficit, a specific study of the immune status was performed, including GM-CSF detection. GM-CSF is a key regulator of pulmonary alveolar phagocytes and macrophages, critical elements for cryptococcal control. This cytokine is neutralized by high-titer anti-GM-CSF antibodies in the autoimmune form of pulmonary alveolar proteinosis (PAP). Recently, Rosen et al. [16] evaluated whether anti-GM-CSF autoantibodies were specifically associated with cryptococcal meningitis in a large historical cohort (1955–1984) of one hundred and three patients. They examined archived sera from previously healthy, HIV-negative adults with cryptococcal meningitis and identified three of 67 archived patient specimens, with no recognized immunodeficiency or comorbidities, as positive for anti-GM-CSF autoantibodies. According to this and other articles, a fraction of the cases of susceptibility to cryptococcal meningitis in immunocompetent patients may be associated with these antibodies, but given that the anti-GM CSF test was negative, this does not seem to be the cause in the case of our patient, where no immune defects were eventually identified.
The clinical features of C. neoformans/C. gattii s.l. infections are often indistinguishable [25]. Nevertheless, C. neoformans s.l. classically causes meningitis, whereas C. gattii s.l. often affects either the lung or the central nervous system and can lead to cryptococcoma formation [26,27,28]. In these cases, the infection requires a longer treatment with antifungal therapy as compared with infections caused by C. neoformans s.l. In HIV-negative subjects, C. gattii s.l. infection presents itself with simultaneous lung and brain involvement more often than C. neoformans s.l. cryptococcosis does [28]. Our patient was clinically healthy before progressive and severe headache (neurological symptomatology of intracranial hypertension) appeared as the main symptom. The absence of fever is not unusual in cryptococcal infections [25]; in fact, some asymptomatic cryptococcosis have been reported in patients that presented a mass lesion(s) that was discovered on a chest radiograph during investigations related to non-CNS manifestations, particularly in immunocompromised patients [25].
Detection of CrAg in CSF was used as it has replaced India ink stain for a rapid diagnosis of meningitis because of its higher sensitivity, specificity, and positive and negative predictive values (99, 97, 87, and 100%, respectively) [29, 30]. Detection by means of serum CrAg testing is considered useful, specially to determine whether infections remain confined to the lung, in which case it is usually negative, while positive results suggest likely dissemination and the importance of ruling out a possible CNS disease [25]. Besides, in HIV patients with a baseline CD4 < 100cells/µL a blood CrAg titer of > 1:8 may allow for the implementation of targeted pre-emptive treatment strategy [31]. In our case, the persistence of high antigen detection in blood after 10 months of treatment, even when the patient showed clinical improvement, was a deciding factor in for the decision of switching the therapy from fluconazole to isavuconazole, which subsequently led to the solution of the disease.
The management of the patient was based on the international guidelines for cryptococcosis. Therefore, the antifungal treatment consisting of an induction regimen with AMB plus 5FC and longer consolidation and maintenance therapy with FLC was established as recommended [29, 32,33,34,35]. Optimal duration of induction therapy for C. gattii s.l. infection is uncertain, but it is thought that it needs to be applied longer than in the case of C. neoformans s.l. infections. This due to the tendency of C. gattii s.l. to form multiple or large cryptococcomas that are more difficult to penetrate with antifungal agents. This could explain the delayed response to antifungal treatment, more frequent clinical relapses, and more neurological sequelae for severe C. gattii s.l. infection. In the case of our patient, we applied the longer recommended antifungal treatment with a long induction therapy with liposomal AMB and 5FC (6 weeks). After using a conventional treatment for almost a year, the initial good clinical response stopped, and the patient’s status did not change for more than 6 months. During that time, CrAg decreased dramatically in CSF but persisted with high titers in blood (1/1000) and the respiratory involvement remained almost unaltered as compared with the initial stage of the process (according to the radiological abnormalities). It seems that the infection was somehow cantoned in the lungs. The PCR analysis of BAL and transbronchial biopsy showed the persistence of Cryptococcus deuterogattii in these lesions.
Isavuconazole has been proven to have in vitro activity against C. neoformans/C. gattii species complexes, with MICs ranging between 0.008 and 0.5 µg/mL [36]. Currently there is no standardized clinical breakpoint to predict clinical success or failure when using ISA for the treatment of cryptococcal infections, but there is some evidence of successful treatment of cryptococcosis using this drug [37]. Although in the current case ISA was empirically introduced, the cultured C. deuterogattii isolates were retrospectively tested and were found to show low MICs for this antifungal. Moreover, the decision to adopt the ISA treatment was based on different aspects of the patient management. On the one hand, the patient refused further admission for a new induction treatment and did not tolerate higher doses of FLC. Therefore, we had to choose an outpatient oral treatment. In this case, we chose ISA instead of VOR due to the advantages described for this drug, such as its remarkably high bioavailability with very high distribution to the lung (which was our main goal at the moment), no need for the monitoring of the plasmatic concentration, low interaction with other drugs, and general good tolerance [38, 39]. The patient improved with no neurological sequelae, remaining currently asymptomatic and without recurrence. This success may be related to the high concentration ISA reaches in the lung, together with the apparent high sensitivity of this specific strain to the drug.
Optimal therapeutic regimens are yet to be determined. The WHO guidelines only apply to HIV population [29], and the guidelines published by the Infectious Diseases Society of America [33] were primarily based on data from C. neoformans s.l. infections in HIV and solid organ transplant patients with limited number of C. gattii s.l.-specific recommendations, and even in these latter cases only based on data from endemic infections.
As it was described in the literature for immunocompetent patients with C. gattii s.l. infection after initiation of antifungal therapy [25], after 5 weeks of treatment our patient was diagnosed with cryptococcal IRIS and responded well to oral prednisone treatment. It is important to distinguish a relapse from late onset cryptococcal IRIS, as it requires a different treatment approach. Cryptococcosis relapse may require re-induction antifungal therapy, while cryptococcal IRIS may require anti-inflammatory drugs like non-steroidal anti-inflammatory drugs or corticosteroids.
Despite being an immunocompetent host, the patient had several well-known poor prognosis factors [40], having a disseminated infection; a high CSF opening pressure; CSF glucose < 40 mg/dL and > 20 leukocytes/mm3; high number of cryptococcal cells observed by means of India ink stain and high CrAg in CSF and serum; and absence of leptomeningeal enhancement on brain images. Other indicators of a poor prognosis were intracranial hypertension (papilledema, deafness, altered mental state), extraneural affectation, and low levels of proteins in pre-treatment CSF; cryptococcemia were absent in our patient [41]. The latter indicator is notably associated with a high mortality rate [41]. Therefore, clinical suspicion and blood culture request soon after admission are essential for a better outcome.
In summary, we reported a severe case of disseminated cryptococcal infection in an otherwise healthy European patient living in Spain. The culprit of the disease was identified as C. deuterogattii (AFLP6/VGII) genetically closely related to the Vancouver Island outbreak-lineage. Despite having several poor prognostic factors, the patient responded well to antifungal therapy that resulted in a good outcome. The present case stresses the importance of performing species identification testing beyond the two species complexes, especially in travellers and migrants. No autochthonous C. deuterogattii cases have ever been reported in Spain, the Azores Islands (Portugal), or elsewhere in Europe. We want to highlight the successful application of ISA in the antifungal treatment, even though it is not currently recommended and should therefore be used with caution. At 26 months post-treatment follow-up the patient was fully recovered with no neurological sequelae.
References
Springer DJ, Chaturvedi V. Projecting global occurrence of Cryptococcus gattii. Emerg Infect Dis. 2010;16(1):14–20. https://doi.org/10.3201/eid1601.090369.
Herkert PF, Hagen F, Pinheiro RL, Muro MD, Meis JF, Queiroz-Telles F. Ecoepidemiology of Cryptococcus gattii in developing countries. J Fungi (Basel). 2017;3(4):62. https://doi.org/10.3390/jof3040062.
Galanis E, MacDougall L, Kidd S, Morshed M. British Columbia Cryptococcus gattii working group epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis. 2010;16(2):251–7. https://doi.org/10.3201/eid1602.090900.
Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A. 2004;101(49):17258–63. https://doi.org/10.1073/pnas.0402981101.
Smith RM, Mba-Jonas A, Tourdjman M, Schimek T, DeBess E, Marsden-Haug N, Harris JR. Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLoS ONE. 2014;9(2):e88875. https://doi.org/10.1371/journal.pone.0088875.
Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16–48. https://doi.org/10.1016/j.fgb.2015.02.009.
Hagen F, Lumbsch HT, Arsic Arsenijevic V, Badali H, Bertout S, Billmyre RB, et al. 2017 Importance of resolving fungal nomenclature: the case of multiple pathogenic species in the Cryptococcus genus. mSphere. 2(4): 00238–17. Doi: https://doi.org/10.1128/mSphere.00238-17
Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. 2017 The case for adopting the "species complex" nomenclature for the etiologic agents of cryptococcosis. mSphere. 2(1): 00357–16. doi: https://doi.org/10.1128/mSphere.00357-16.
Cogliati M, Desnos-Ollivier M, McCormick-Smith I, Rickerts V, Ferreira-Paim K, Meyer W, et al. Genotypes and population genetics of Cryptococcus neoformans and Cryptococcus gattii species complexes in Europe and the Mediterranean area. Fungal Genet Biol. 2019;129:16–29. https://doi.org/10.1016/j.fgb.2019.04.001.
Cogliati M, D’Amicis R, Zani A, Montagna MT, Caggiano G, De Giglio O, et al. Environmental distribution of Cryptococcus neoformans and C. gattii around the Mediterranean basin. FEMS Yeast Res. 2016. https://doi.org/10.1093/femsyr/fow086.
Hagen F, Colom MF, Swinne D, Tintelnot K, Iatta R, Montagna MT, et al. Autochthonous and dormant Cryptococcus gattii infections in Europe. Emerg Infect Dis. 2012;18(10):1618–24. https://doi.org/10.3201/eid1810.120068.
Smith IM, Stephan C, Hogardt M, Klawe C, Tintelnot K, Rickerts V. Cryptococcosis due to Cryptococcus gattii in Germany from 2004–2013. Int J Med Microbiol. 2015;305(7):719–23. https://doi.org/10.1016/j.ijmm.2015.08.023.
Cárdenes M, Angel-Moreno A, Fieschi C, Sologuren I, Colino E, Molinés A, et al. Oesophageal squamous cell carcinoma in a young adult with IL-12R beta 1 deficiency. J Med Genet. 2010;47(9):635–7. https://doi.org/10.1136/jmg.2009.071910.
Esteve-Solé A, Sologuren I, Martínez-Saavedra MT, Deyà-Martínez À, Oleaga-Quintas C, Martinez-Barricarte R, et al. Laboratory evaluation of the IFN-γ circuit for the molecular diagnosis of Mendelian susceptibility to mycobacterial disease. Crit Rev Clin Lab Sci. 2018;55(3):184–204. https://doi.org/10.1080/10408363.2018.1444580.
Sologuren I, Boisson-Dupuis S, Pestano J, Vincent QB, Fernández-Pérez L, Chapgier A, et al. Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum Mol Genet. 2011;20(8):1509–23. https://doi.org/10.1093/hmg/ddr029.
Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti–GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013;190(8):3959–66.
Uchida K, Nakata K, Carey B, Chalk C, Suzuki T, Sakagami T, et al. Standardized serum GM-CSF autoantibody testing for the routine clinical diagnosis of autoimmune pulmonary alveolar proteinosis. J Immunol Methods. 2014;402(1–2):57–70. https://doi.org/10.1016/j.jim.2013.11.011.
Tomazin R, Matos T, Meis JF, Hagen F. Molecular characterization and antifungal susceptibility testing of sequentially obtained clinical Cryptococcus deneoformans and Cryptococcus neoformans isolates from Ljubljana. Slovenia Mycopathologia. 2018;183(2):371–80. https://doi.org/10.1007/s11046-017-0214-9.
Kinne J, Joseph M, Wernery U, Nogradi N, Hagen F. Disseminated Cryptococcus deuterogattii (AFLP6/VGII) infection in an Arabian horse from Dubai. United Arab Emirates Rev Iberoam Micol. 2017;34(4):229–32. https://doi.org/10.1016/j.riam.2017.02.007.
Bauer M, Wickenhauser C, Haak A, Pazaitis N, Siebolts U, Mawrin C, et al. Case report: a fatal case of cryptococcosis in an immunocompetent patient due to Cryptococcus deuterogattii (AFLP6/VGII). JMM Case Rep. 2018;5(10):e005168. https://doi.org/10.1099/jmmcr.0.005168.
Georgi A, Schneemann M, Tintelnot K, Calligaris-Maibach RC, Meyer S, Weber R, Bosshard PP. Cryptococcus gattii meningoencephalitis in an immunocompetent person 13 months after exposure. Infection. 2009;37(4):370–3. https://doi.org/10.1007/s15010-008-8211-z.
Hagen F, van Assen S, Luijckx GJ, Boekhout T, Kampinga GA. Activated dormant Cryptococcus gattii infection in a Dutch tourist who visited Vancouver Island (Canada): a molecular epidemiological approach. Med Mycol. 2010;48(3):528–31. https://doi.org/10.3109/13693780903300319.
Colom MF, Hagen F, Gonzalez A, Mellado A, Morera N, Linares C, et al. Ceratonia siliqua (carob) trees as natural habitat and source of infection by Cryptococcus gattii in the Mediterranean environment. Med Mycol. 2012;50(1):67–73. https://doi.org/10.3109/13693786.2011.574239.
Chowdhary A, Randhawa HS, Boekhout T, Hagen F, Klaassen CH, Meis JF. Temperate climate niche for Cryptococcus gattii in Northern Europe. Emerg Infect Dis. 2012;18(1):172–4. https://doi.org/10.3201/eid1801.111190.
Hoang L, Philips P, Galanis E. Cryptococcus gattii: a review of the epidemiology, clinical presentation, diagnosis and management of this endemic yeast in the Pacific Northwest. Clin Microbiol Lett. 2011;33(24):187–95. https://doi.org/10.1016/j.clinmicnews.2011.11.003.
Ulett KB, Cockburn JW, Jeffree R, Woods ML. Cerebral cryptococcoma mimicking glioblastoma. BMJ Case Rep. 2017. https://doi.org/10.1136/bcr-2016-218824.
Chen SC, Slavin MA, Heath CH, Playford EG, Byth K, Marriott D, et al. Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis. 2012;55(6):789–98. https://doi.org/10.1093/cid/cis529.
Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis. 2000;31(2):499–508. https://doi.org/10.1086/31399229.
World Health Organization (WHO). Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva, World Health Organization, 2018.
Bloch KC, Bailin SS. Update on fungal infections of the central nervous system: emerging pathogens and emerging diagnostics. Curr Opin Infect Dis. 2019;32(3):277–84. https://doi.org/10.1097/QCO.0000000000000541.
Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48(7):856–62. https://doi.org/10.1086/597262.
Bruner KT, Franco-Paredes C, Henao-Martínez AF, Steele GM, Chastain DB. Cryptococcus gattii complex infections in HIV-infected patients Southeastern United States. Emerg Infect Dis. 2018;24(11):1998–2002. https://doi.org/10.3201/eid2411.180787.
Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50(3):291–322. https://doi.org/10.1086/649858.
Chen SC, Korman TM, Slavin MA, Marriott D, Byth K, Bak N, et al. Antifungal therapy and management of complications of cryptococcosis due to Cryptococcus gattii. Clin Infect Dis. 2013;57(4):543–51. https://doi.org/10.1093/cid/cit341.
Lawrence DS, Boyer-Chammard T, Jarvis JN. Emerging concepts in HIV-associated cyptococcal meningitis. Curr Opin Infect Dis. 2019;32(1):16–23. https://doi.org/10.1097/QCO.0000000000000514.
Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Guinea J, Hagen F, Meis JF, et al. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for the Cryptococcus neoformans-Cryptococcus gattii species complex using the CLSI M27–A3 broth microdilution method. Antimicrob Agents Chemother. 2015;59(1):666–8. https://doi.org/10.1128/AAC.04055-14.
Thompson GR, Rendon A, Ribeiro dos Santos R, Queiroz-Telles F, Ostrosky-Zeichner L, Azie N, et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis. 2016;63:356–62. https://doi.org/10.1093/cid/ciw305.
Garcia-Vidal C. Current therapeutic options in invasive mycosis and potential therapeutic role of isavuconazole. Rev Iberoam Micol. 2018;35(4):192–7. https://doi.org/10.1016/j.riam.2018.07.003.
Ellsworth M, Ostrosky-Zeichner L. Isavuconazole: Mechanism of action, clinical efficacy, and resistance. J Fungi. 2020;6:324. https://doi.org/10.3390/jof6040324.
Galanis E, Hoang L, Kibsey P, Morshed M, Phillips P. Clinical presentation, diagnosis and management of Cryptococcus gattii cases: Lessons learned from British Columbia. Can J Infect Dis Med Microbiol. 2009;20(1):23–8. https://doi.org/10.1155/2009/719659.
Tsujisaki RA, Paniago AM, Lima Júnior MS, Alencar Dde S, Spositto FL, de Nunes MO, et al. First molecular typing of cryptococcemia-causing Cryptococcus in central-west Brazil. Mycopathologia. 2013;176(3–4):267–72. https://doi.org/10.1007/s11046-013-9676-6.
Acknowledgements
The authors thank the patient for consenting to the publication of this case. We are grateful to Ms Isabel Martinez-Hervás Librarian of Severo Ochoa University Hospital for searching literature and to Ms Elisa Parrilla for her assistance in environmental sample collection in Azores Islands. We also want to thank Pablo Flores for his professional review of the English language and Amanda S. Cuétara for her assistance in the design of Fig. 4. Finally, we thank Master Labor SL Company for the donation of the Biosynex CryptoPS kit.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in this work.
Ethical Approval
The Ethics Committee of the Severo Ochoa Hospital approved the publication of the case.
Informed Consent
Informed consent was also obtained from the patient for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Patrick CY Woo.
Rights and permissions
About this article
Cite this article
Cuetara, M.S., Jusdado Ruiz-Capillas, J.J., Nuñez-Valentin, M.P. et al. Successful Isavuconazole Salvage Therapy for a Cryptococcus deuterogattii (AFLP6/VGII) Disseminated Infection in a European Immunocompetent Patient. Mycopathologia 186, 507–518 (2021). https://doi.org/10.1007/s11046-021-00566-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-021-00566-w