Abstract
Fusarium spp. may cause invasive disseminated infections in immunocompromised patients, associated with significant morbidity and mortality. We describe a case of disseminated fusariosis with fungemia and skin localization caused by Fusarium musae in a patient with acute myeloid leukemia successfully treated using liposomal amphotericin B and voriconazole.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case Report
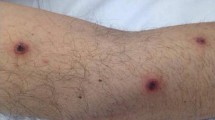
A 29-year-old Iraqi male without significant medical history was admitted because of severe B-symptoms, in particular night sweats and anorexia. The hematologic evaluation revealed a blood leukocyte count of 45,300 cells/µl with 91% blastic forms. Morphological examination and immunophenotyping on bone marrow coagulum confirmed the suspicion of AML with minimal maturation (AML-M1). Classical AML induction chemotherapy with daunorubicin and cytarabine was started. Oral ciprofloxacin 500 mg bid was administered for selective intestinal decontamination and fluconazole 200 mg bid as antifungal prophylaxis. Fever and hemodynamic instability shortly after the induction therapy motivated his transfer to the intensive care unit (ICU), and meropenem 1 g tid IV empirically started together with vancomycin in continuous infusion (recommended serum concentration: 20–25 mg/l). Blood cultures showed methicillin-sensitive Staphylococcus aureus, and antibiotic treatment continued for 14 days. Considering suspected tumor lysis syndrome, adding solumedrol 1 mg/kg yielded a positive clinical and biochemical evolution. A repeat bone marrow biopsy on day 14 of induction revealed refractory disease with 63% blasts. A second induction with idarubicin and high-dose cytarabine was started immediately, leading to prolonged myelosuppression. After 20 days of profound neutropenia, the patient redeveloped fever up to 40 °C without identifiable clinical signs (except for 3 small skin lesions on both legs), or computed tomography (CT) graphical abnormalities. Biochemically, high C-reactive protein (CRP) values up to 328 mg/l were noted. Meropenem and vancomycin were restarted empirically after obtaining blood cultures. These blood cultures turned positive on day 16 post-second induction. Microscopical examination detected septate hyphae suggestive for Fusarium spp. and confirmed with culture (Fig. 1). Macroscopically, the culture on Sabouraud agar grew a cottony colony with white to purple aerial mycelium. MALDI-TOF (Bruker, Kontich, Belgium) with mass spectrometry identification [1] identified the spectra matching the level of Fusarium musae species, belonging to the Fusarium fujikuroi species complex (FFSC). The three above-mentioned lesions at the lower extremities were possibly the port of entry. The patient’s thrombopenia did not allow a biopsy of these skin lesions. Therapy with fluconazole was discontinued, and liposomal amphotericin B (5 mg/kg/d) and voriconazole IV (recommended trough serum concentration: 2–5.5 µg/ml) were started. Furthermore, granulocyte colony-stimulating factor (G-CSF) was added to shorten the neutropenic phase. Because of significant clinical deterioration (hypotension, extravascular fluid overload, and desaturation), the patient was readmitted to the ICU. CT scan of brain–thorax–abdomen showed no abnormalities. Blood culture vials remained positive for Fusarium spp. until removal of the Hickman catheter on day 19 post-second induction. The catheter was cultured according to the semiquantitative roll plate method, but remained negative. No sonification was performed. Antifungal susceptibility testing was performed at the National Reference Centre. Briefly, minimum inhibitory concentrations (MICs) for the isolate were obtained by the broth microdilution methodology, according to EUCAST [2]. The MICs for amphotericin B, itraconazole, voriconazole, and posaconazole were 1 mg/L, 16 mg/L, 2 mg/L, and 1 mg/L, respectively. The fever persisted for 14 days despite adequate combination therapy. The drug monitoring of voriconazole twice a week showed therapeutic levels. Daily chest radiography excluded pulmonary involvement, transesophageal echocardiography showed no arguments for endocarditis, and CRP values stagnated between 200 and 300 mg/L. On day 25 post-second induction, the patient recovered from neutropenia with an absolute neutrophilic count of 1000/µl. Three days later, CT thorax showed overwhelming bilateral pneumonia. Because Pneumocystis jirovecii pneumonia was suspected, high-dose cotrimoxazole was added (1600/320 mg tid). Broncho-alveolar lavage culture and molecular testing remained negative for viruses, bacteria (among others Mycobacteria spp., Nocardia spp.), and fungi (pan-fungal PCR); Aspergillus galactomannan (GM) index (Bio-Rad, Marne La Coquette, France) was 0.05 (negative). Serial serum GM testing during the post-induction period remained negative. The patient was definitively discharged 8 days later from ICU, hence a positive clinical and biochemical evolution. Vancomycin and meropenem were stopped. Two days post-discharge (10 days post-neutrophilic recovery), several skin lesions appeared, starting from the legs and spreading to the upper extremities. The lesions exhibited necrotic centers surrounded by spreading erythema (Fig. 2). Amplification and sequencing of the internal transcribed spacer 1 and 2 fungal rDNA region was performed on one of these abscesses (primer sets ITS1-ITS4: forward 5’-TCCGTAGGTGAACCTGCGG-3’ and reverse 5’-TCCTCCGCTTATTGATATGC-3’; ITS86-ITS4: forward 5’-TCC TCC GCT TAT TGA TAT GC 3’ and reverse 5’-TCCTCCGCTTATTGATATGC-3’). The sequence obtained was analyzed by using the alignment program of the CBS Filamentous Fungi Database (Utrecht, the Netherlands). A 99.6% homology was found for SH1610157.08FU KX683427 Fusarium fujikuroi. Local debridement resulted in healing. After 26 days of combination antifungal therapy, oral voriconazole was continued in monotherapy. The patient was discharged home for 2 weeks before the start of the first consolidation.
Discussion
Immunocompromised patients are at high risk for invasive or disseminated fungal infections. The most commonly encountered genera are Candida and Aspergillus. However, other molds, like Fusarium spp. and Scedosporium spp., are emerging as opportunistic pathogens. Fusarium, a saprophytic filamentous fungus, is widely distributed in the environment including in soil, plants, air, and organic debris. Notwithstanding its ubiquitous presence, the prevalence of invasive infection is quite low, estimated at only 0.06–0.13% of patients with hematologic malignancies. The most common Fusarium spp. causing invasive and disseminated infections in humans are species from the Fusarium solani complex (∼ 50% of reported cases), Fusarium oxysporum complex (∼ 20%), and Fusarium verticillioides which is part of the FFSC complex and is responsible for 20% of infections [3]. Immunocompromised patients with hematologic malignancies, especially acute leukemia and allogeneic stem cell transplantation, are at highest risk for infection. The most common presentation in neutropenic patients is persistent fever, despite adequate antibacterial and antifungal therapy, with the development of cutaneous lesions and positive blood cultures. The transmission of Fusarium is airborne or by inoculation through a defective skin barrier, as was likely the case in our report. Skin lesions predominantly involve the extremities. The infections have an initial presentation of subcutaneous lesions, evolving to erythematous indurations, followed by target-like central necrotic lesions. The ecthyma gangrenosum-like appearance is due to thrombosis and infarction caused by angioinvasion of Fusarium hyphae. The lesions appear rapidly within one to 5 days, at various stages of evolution, with occasional myalgias [4]. Up to 70% of immunocompromised patients with invasive fusariosis have pulmonary, sinus, cutaneous, and hematologic involvement. Pulmonary involvement varies from patchy interstitial disease to nodules and cavities on radiography, similar to an Aspergillus pneumonia [5]. The Aspergillus GM enzyme-linked immunosorbent assay (EIA) may not be useful for distinguishing Fusarium from Aspergillus as Fusarium exoantigens may cross-react. However, Fusarium musae shows no cross-reactivity in the Platelia Aspergillus GM EIA, which explains the negativity of our GM determinations [6]. The definitive diagnosis of disseminated Fusarium infection requires isolation from blood and tissue culture (such as the skin, lungs, and sinuses) [3]. Fungemia is likely to develop in a median of 5 days (range 1–10 days) following the appearance of skin lesions [4], as was the case in our report (5-day interval).
Currently, there are no guidelines for the treatment of invasive fusariosis because of the paucity of data. However, early aggressive management is critical, given the rapid progression and high mortality rates, ranging up to 50-80% in the case of fungemia [4, 7]. Treatment of fusariosis involves a combination of antifungal therapy, surgical debridement of infected tissue, and removal of foreign material such as central catheters. However, neutrophil recovery is the crucial element for patient survival and may warrant the use of G-CSF. Currently, high-dose amphotericin B is considered as the primary treatment for invasive fusariosis [8]. Experts also advise expanded-spectrum triazoles, particularly voriconazole and posaconazole. The CLSI epidemiological cutoff values (ECVs) of antifungal agents are available for the more prevalent Fusarium spp. but not for Fusarium musae. These values may aid in the detection of strains with acquired mechanisms of resistance (non-wild type); however, ECVs cannot predict clinical response to therapy. Knowledge regarding molecular mechanisms of resistance and their relationship with MICs is needed [9]. In our case, given the prolonged severe neutropenia after two induction courses, prophylaxis with posaconazole or voriconazole could have been a better strategy against invasive mold infections [10]. As invasive fusariosis has a high mortality rate under monotherapy in immunocompromised patients, there is a growing interest in combination therapy. Nowadays, there are only isolated case reports of successful treatment with an amphotericin B and voriconazole combination for at least 12 weeks and until immune recovery [11]. Combination antifungal therapy is well-tolerated with acceptable minor toxicity and may theoretically have benefits stabilizing the infection and preventing fatal progression [12]. However, a case series and literature review of 97 cases reported the superiority of medical monotherapy with voriconazole or amphotericin B. Briefly, the evidence remains inconclusive. Large prospective randomized controlled clinical trials are needed to prove the superiority of combination therapy, but impossible to produce owing to the rareness of infection [13].
In conclusion, although the incidence of systemic fusariosis is significantly lower in comparison with Candida or Aspergillus infections, its occurrence is highly significant in patients with hematologic malignancies. In this context, fusariosis usually evolves as a severe invasive fungal disease, which is associated with a high mortality rate. The description of the present case underlines the importance of early identification, thorough skin evaluation, aggressive antifungal therapy, and early immune recovery to achieve a successful outcome.
References
Normand AC, Becker P, Gabriel F, Cassagne C, Accoceberry I, Gari-Toussaint M, Hasseine L, De Geyter D, Pierard D, Surmont I, Djenad F, Donnadieu JL, Piarroux M, Ranque S, Hendrickx M, Piarroux R. Validation of a new web application for identification of fungi by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2017;55:2661–70.
EUCAST. Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds—subcommittee on antifungal susceptibility testing (AFST) of the ESCMID European committee for antimicrobial susceptibility testing (EUCAST). Clin Microbiol Infect. 2008;14:982–4.
Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695–704.
Dignani MC, Anaissie E. Human fusariosis. Clin Microbiol Infect. 2004;10:67–75.
Fanci R, Pini G, Bartolesi AM, Pecile P. Refractory disseminated fusariosis by Fusarium verticillioides in a patient with acute myeloid leukaemia relapsed after allogeneic hematopoietic stem cell transplantation: a case report and literature review. Rev Iberoam Micol. 2013;30:51–3.
Tortorano AM, Esposto MC, Prigitano A, et al. Cross-reactivity of Fusarium spp. in the Aspergillus Galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol. 2012;50:1051–3.
Tezcan G, Ozhak-Baysan B, Alastruey-Izquierdo A, et al. Disseminated fusariosis caused by Fusarium verticillioides in an acute lymphoblastic leukemia patient after allogeneic hematopoietic stem cell transplantation. J Clin Microbiol. 2009;47:278–81.
Bhatti Z, Shaukat A, Almyroudis NG, Segal BH. Review of epidemiology, diagnosis, and treatment of invasive mould infections in allogeneic hematopoietic stem cell transplant recipients. Mycopathologia. 2006;162:1–15.
Espinel-Ingroff A, Colombo AL, Cordoba S, et al. International evaluation of mic distributions and epidemiological cutoff value (ecv) definitions for fusarium species identified by molecular methods for the clsi broth microdilution method. Antimicrob Agents Chemother. 2016;60:1079–84.
Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, Langston AA, Nastoupil LJ, Rajotte M, Rolston KV, Strasfeld L, Flowers CR. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol. 2018;36:3043.
Stanzani M, Vianelli N, Bandini G, et al. Successful treatment of disseminated Fusariosis after allogeneic hematopoietic stem cell transplantation with the combination of voriconazole and liposomal amphotericin B. J Infect. 2006;53:E243–6.
Rojas R, Molina JR, Jarque I, et al. Outcome of antifungal combination therapy for invasive mold infections in hematological patients is independent of the chosen combination. Mediterr J Hematol Infect Dis. 2012;4:e2012011.
Nucci M, Marr KA, Vehreschild MJGT, et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect. 2014;20:580–5.
Funding
No funding was received for this case report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare not to have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Ferry Hagen.
Rights and permissions
About this article
Cite this article
Verbeke, V., Bourgeois, T., Lodewyck, T. et al. Successful Outcome of Disseminated Fusarium musae Fungemia with Skin Localization Treated with Liposomal Amphotericin B and Voriconazole in a Patient with Acute Myeloid Leukemia. Mycopathologia 185, 1085–1089 (2020). https://doi.org/10.1007/s11046-020-00499-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-020-00499-w