Abstract
Introduction
Pneumocystis jirovecii pneumonia (PCP) is a major cause of disease in immunocompromised individuals. Diagnosis is typically obtained by microscopy and/or PCR. For ambiguous PCR results, we evaluated the new biomarker 1,3-Beta-d-Glucan (BDG).
Methods
BDG serum levels were assessed and correlated to PCR results in immunosuppressed patients with ARDS.
Results
11 (22%) out of 50 patients had suspected PCP. APACHE II (26 vs. 24; p < 0.002), SOFA score (16 vs. 14; p < 0.010) and mortality rate (34 vs. 69% p < 0.004; 34 vs. 80% p < 0.003) were significantly altered in patients with positive (pPCR) and slightly positive (spPCR) PCJ PCR as compared to patients with no-PCP (nPCP). BDG levels were significantly lower in patients with nPCP (86; 30–315 pg/ml) than in patients with pPCR (589; 356–1000 pg/ml; p < 0.001) and spPCP (398; 297–516 pg/ml; p < 0.004) referring to the cutoff in this study for PCP of 275 pg/ml. An overall sensitivity (S) of 92% (95% CI 86–96%) and specificity (SP) of 84% (95% CI 79–85%) for PCP were found for the BDG Fungitell assay. In detail, S of 98% (95% CI 94–100%) and SP of 86% (95% CI 82–92%) for pPCP and S of 98% (95% CI 96–100%) and SP of 88% (95% CI 86–96%) for spPCO were found.
Conclusion
Serum BDG levels were strongly elevated in PCP, and the negative predictive value is high. BDG could be used as a preliminary test for patients with suspected PCP, especially in patients with slightly positive PCR results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunocompromised patients, particularly critically ill, are at increased risk for Pneumocystis jirovecii pneumonia (PCP). PCP still remains a serious and potentially life threatening infection linked with a high morbidity and mortality [1, 2]. Incidence of PCP is increasing due to wide spread use of immunosuppressants [1].
In immunosuppressed critically ill patients, PCP presents in nearly 10% as acute respiratory distress syndrome (ARDS) [3]. Without appropriate antibiotic therapy, the mortality from PCP in non-HIV-infected patients rises up to 90% [1, 3].
However, early identification of PCP is still challenging, especially in critically ill. Not only the patients’ complexity, moreover, accompanying signs and symptoms are in most cases nonspecific and may not be present until the disease is advanced or disseminated [4, 5].
Diagnosis of PCP is typically obtained by microscopically staining and polymerase chain reaction (PCR) of bronchoalveolar lavage (BAL) fluids which is the gold standard [4, 5]. However, as reported in former studies lack of sensitivity of these techniques in non-HIV than in HIV infected patients may be a problem in early identification [4]. Moreover, ambiguous results regarding PCP in critically ill patients with ARDS may be fatal not only because of increasing morbidity but also considering side effects of therapy with an inappropriate indication.
The noninvasive serum biomarker 1,3-Beta-d-Glucan (BDG), a typical cell wall component of most pathogenic fungi, including P. jirovecii, could be a potentially useful marker in early detection of PCP [6, 7]. The so-called Fungitell BDG assay (Associates of Cape Cod, East Falmouth, MA) is approved by the FDA for the diagnosis of invasive fungal diseases [8, 9]. Up to now, data about the performance characteristics of the Fungitell BDG test for the diagnosis of PCP have been scarce, especially in critically ill patients [9, 10]. Therefore, we examined in this observational study the use of the 1,3 Beta-d-Glucan Fungitell assay for early detection of PCP and potentially discrimination in not clearly stated diagnostic results in immunocompromised critically ill patients with ARDS.
Materials and Methods
Setting and Patients
This observational study was conducted at the medical ICU of the University Hospital Technische Universität München, Germany. All immunocompromised patients (18 years of age or older) without HIV with ARDS and suspected PCP between December 2014 and December 2015 were included. All patients with respiratory failure received bronchoalveolar lavage (BAL) and computed tomography of the chest.
This study was reviewed and approved by the ethics committee of our university hospital, and all data were processed anonymously. Need for informed consent was waived for this analysis.
Definitions and Microbiological Testings
ARDS was defined following the ARDS Definition Task Force conducted Berlin definition [11]. For definition of fungal disease, we used the criteria proposed by the EORTC/MSG Consensus Group [12]. Sepsis was defined following the International Guidelines for Management of Severe Sepsis and Septic Shock from 2012 [13].
Standard Microbiological Testing
BAL Fungal Testing
Microbiological specimens were tested at the Institute of Microbiology, by direct examination, culture and with an automated microbiology growth and detection system.
1,3-Beta-d-Glucan
BDG was detected with the Fungitell assay as recommended by the manufacturer (Associates of Cape Cod). BDG levels of ≥80 pg/ml were considered positive results. BDG levels of >275 pg/ml were considered as positive in patients with PCP.
PCJ Testing
PCJ diagnostic was performed in all patients by microscopy with direct fluorescent antibody staining and real-time polymerase chain reaction (PCR) assay targeting mitochondrial rRNA from P. jirovecii [14]. PCR was set as positive over 104 copies/ml (pPCP), slightly positive between 103 and 104 copies/ml (spPCP) and negative below 103 copies/ml (nPCP) in BAL fluid.
Statistics
We used IBM SPSS Statistics 23 (SPSS inc., Chicago, IL, USA) for all statistical analyses in this study. A p value below a significance level of 5% (p < 0.05) indicates statistical significance. The diagnostic performance of BDG Fungitell assay test was evaluated as sensitivity and specificity, with 95% confidence intervals (CI). The optimal BDG cutoff was determined with the maximum Youden index.
Results
Patients’ Characteristics
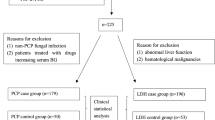
We analyzed the medical records of all patients with ARDS and suspected PCP that were treated at the ICU between December 2014 and December 2015. Out of these 97 patients, we identified 50 (51%) immunocompromised patients without HIV. The demographic and clinical characteristics of the patients are presented in Table 1.
A high overall APACHE II and SOFA score could be observed in this study population which expresses the severity of the underlying diseases. APACHE II and SOFA score were significantly higher in patients with positive and slightly positive PCJ PCR as compared to patients with no-PCP (26 vs. 24; p < 0.002 and 16 vs. 14; p < 0.010).
All patients were admitted because of ARDS and/or sepsis. 40% needed renal replacement therapy. Thus, an overall mean ICU stay of 20 days and mortality rate of 66% could be observed (see Table 1).
Comparing patients with no and with positive and slightly positive PCJ PCR, some significant differences could be observed: Positive and slightly positive PCJ PCR were significantly more frequently observed in patients with hematological malignancies (40 vs. 50% p < 0.044; 40 vs. 64% p < 0.035) and after chemotherapy of solid tumors (34 vs. 5%; p < 0.035). Moreover, neutropenia was significantly more often in patients with positive and slightly positive PCJ PCR (17 vs. 41% p < 0.042; 17 vs. 40% p < 0.045).
The Horowitz Index (paO2/FiO2) as expression of ARDS severity was significantly lower in patients with positive and with slightly positive PCJ PCR, which means worse ARDS, as compared to patients with no-PCP (97 vs. 79 p < 0.002; 97 vs. 71 p < 0.001).
Mortality rate was significantly higher in patients with positive and slightly positive PCJ PCR as compared to patients with no-PCP (34 vs. 69% p < 0.004; 34 vs. 80% p < 0.003).
1,3-Beta-d-Glucan Results and Microbiological Findings
The results of 1,3-Beta-d-Glucan (BDG) levels are presented in Table 2. All PCP patients presented elevated BDG concentrations.
The BDG cutoff for invasive fungal diseases was set beyond 80 pg/m as recommended by the manufacturer. For others such as PCP, a clearly defined cutoff is not available; therefore, we performed a ROC analysis on our data. Following these data, the optimal BDG cutoff for the diagnosis of PCP, determined with the maximum Youden index, would be >90 pg/ml.
However, to optimize specificity and sensitivity we tripled the BDG cutoff for PCP up to 275 pg/ml.
The BDG levels measured in patients with no-PCP ranged from 30 to 1000 pg/ml, with a mean concentration of 186 pg/ml. After exclusion of two patients with invasive aspergillosis (IA), the BDG levels ranged from 30 to 315 pg/ml with a mean concentration of 86 pg/ml which was only slightly elevated beyond the overall cutoff of 80 pg/ml and below the cutoff for PCP of 275 pg/ml.
In patients with positive and slightly positive PCJ PCR, significantly higher BDG mean levels of 589 pg/ml (range 356–1000 pg/ml; p < 0.001) and 398 pg/ml (range 297–516 pg/ml; p < 0.004) could be observed as compared to patients without PCP. Even after exclusion of one patient with IA in each group, the BDG concentrations were significantly beyond the cutoff of 275 pg/ml (mean 563 pg/ml, range 356–893, p < 0.001 and 344 pg/ml; range 297–489 pg/ml, p < 0.001) compared to the PCP negative group. Microscopic staining was in two patients positive (4%, BDG level 893 and 489 pg/ml, respectively).
Leukocyte count, C-reactive protein, procalcitonin levels and especially LDH were not statistical significant different between patients with elevated BDG levels and positive/slightly positive PCJ PCR (p = 0.138; p = 0.433; p = 0.323; p = 0.123 and p = 0.244; p = 0.267; p = 0.344; p = 0.455) compared to PCP negative patients.
The fungal findings of Candida spp. in BAL were interpreted as colonization in most cases.
Four patients with elevated BDG levels had findings of Aspergillus spp. in BAL and standard microbiological testing (see Table 2). Following the EORTC/MSG Consensus Group, these cases were interpreted as probable invasive fungal disease. These patients were excluded, and the BDG levels were adjusted.
Trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxes and the other used antibiotics are listed in Table 2.
An overall sensitivity of 92% (95% CI 86–96%) and specificity of 84% (95% CI 79–85%) for PCP were found for the BDG Fungitell assay. In detail, a sensitivity of 98% (95% CI 94–100%) and specificity of 86% (95% CI 82–92%) for PCJ positive PCR and a sensitivity of 98% (95% CI 96–100%) and specificity of 88% (95% CI 86–96%) PCJ slightly positive PCR was found. The negative predictive value was 96%.
Discussion
In our observational study, we evaluated the usefulness of 1,3 Beta-d-Glucan (BDG) detection in non-HIV immunocompromised critically ill patients with ARDS and suspected P. jirovecii pneumonia (PCP). Our results presented a highly reliable diagnostic value for the BDG Fungitell assay with an overall sensitivity of 92% and a specificity of 84%.
The measurement of BDG levels is based on the Limulus test. The activity of this reaction can be measured with the use of colorimetric or turbidimetric methods, in which optical density is converted to beta-d-glucan concentrations [8, 9, 15].
According to the interpretation criteria of the manufacturer, BDG concentrations higher than 80 pg/ml are considered to be a positive result [8]. However, this cutoff value is used for all findings and not specific for PCP [16–18]. Our results confirm that elevated BDG levels are detected in sera from patients with PCP and also illustrate that generally very high levels are measured.
Therefore, we set a high cutoff of 275 pg/ml in our study, especially to discriminate between slightly positive PCJ PCR results. Thus, we found for positive PCJ PCR a sensitivity of 98% and specificity of 86% and for slightly positive PCJ PCR a sensitivity of 98% and specificity of 88%. Our data are too limited to validate a cutoff value but suggest that a higher cutoff value for the diagnosis of PCP than for the diagnosis of invasive aspergillosis or candidiasis could be used safely. BDG levels alone cannot prove the existence of PCP, and they must be interpreted considering clinical findings. However, with a negative predictive value of 96%, a BDG level <80 pg/ml almost rules out PCP.
The development of PCR techniques for the diagnosis of PCP improved the reproducibility and the sensitivity of the laboratory diagnosis of PCP, but differentiation between colonization and infection by these techniques remains challenging [4, 19].
However, differentiation is vital especially in critically ill patients with ARDS, because early detection and effective therapy is the cornerstone in reducing mortality in PCP.
As presented in our study, high BDG values can confirm a positive PCJ PCR. However, much more important is detection of a high BDG value in patients with slightly positive PCJ PCR to confirm suspected PCP even though microscopic staining was positive in only 4%. Thus, BDG measurement can reduce time delay and early therapy of PCP.
As reported in our study, patients with ARDS and with suspected PCP are severely ill. Moreover, this can also be observed regarding the mortality rates which are significantly increased in patients with positive PCJ PCR but are highest in the group with slightly positive PCJ PCR (34 vs. 69% p < 0.004; 34 vs. 80% p < 0.003). This underlines the necessity of definitely diagnosis.
A limitation of the BDG test is its panfungal character [8, 20]. As reported in this study, four patients were detected with invasive aspergillosis. However, as a standard procedure in critically ill patients, different diagnostic approaches such as galactomannan detection in serum and BAL fluid samples and culture of respiratory samples are used to different microbiological and fungal findings [20, 21].
Beyond these possible disruptive factors, the BDG assay can be safely used for detecting PCP early in the course of infection, prior to the onset of overt clinical findings as reported in this study. Therefore, following our results we described a possible diagnostic approach using 1,3-Beta-d-Glucan in managing suspected PCP as presented in Fig. 1.
Conclusion
To conclude, using the BDG assay appeared to shorten the time interval between suspected infection and established diagnosis, when compared with waiting for clinical signs and symptoms of disease. However, this strategy has not been validated in critically ill patients, but as presented in our study combining PCR results with BDG levels could be used to initiate early therapy and aware suspicion for PCJ pneumonia.
References
Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–98.
Fujii T, Nakamura T, Iwamoto A. Pneumocystis pneumonia in patients with HIV infection: clinical manifestations, laboratory findings, and radiological features. J Infect Chemother. 2007;13:1–7.
Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med. 2003;31:S276–84.
Azoulay E, Bergeron A, Chevret S, et al. Polymerase chain reaction for diagnosing pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009;135:655–61.
Morris A, Wei K, Afshar K, Huang L. Epidemiology and clinical significance of pneumocystis colonization. J Infect Dis. 2008;197:10–7.
Desmet S, Van Wijngaerden E, Maertens J, et al. Serum (1-3)-beta-d-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J Clin Microbiol. 2009;47:3871–4.
Marty FM, Koo S, Bryar J, Baden LR. (1, 3)-beta-d-glucan assay positivity in patients with Pneumocystis (carinii) jiroveci pneumonia. Ann Intern Med. 2007;147:70–2.
Held J, Wagner D. β-d-Glucan kinetics for the assessment of treatment response in Pneumocystis jirovecii pneumonia. Clin Microbiol Infect. 2011;17(7):1118–22.
Held J, Koch MS, Reischl U, et al. Serum (1→3)-β-d-glucan measurement as an early indicator of Pneumocystis jirovecii pneumonia and evaluation of its prognostic value. Clin Microbiol Infect. 2011;17(4):595–602.
Pisculli ML, Sax PE. Use of a serum beta-glucan assay for diagnosis of HIV-related Pneumocystis jiroveci pneumonia in patients with negative microscopic examination results. Clin Infect Dis. 2008;46:1928–30.
Ranieri VM, Rubenfeld GD, Thompson BT, ARDS Definition Task Force, et al. Acute respiratory distress syndrome. JAMA. 2012;307(23):2526–33.
De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–21.
Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Intensive Care Med. 2013;39(2):165–228.
Larsen HH, Kovacs JA, Stock F, Vestereng VH, et al. Development of a rapid real-time PCR assay for quantitation of Pneumocystis carinii f. sp. carinii. J Clin Microbiol. 2002;40(8):2989–93.
Cuetara MS, Alhambra A, Chaves F, Moragues MD, Ponton J, del Palacio A. Use of a serum (1,3)Beta-d-glucan assay for diagnosis and follow-up of Pneumocystis jiroveci pneumonia. Clin Infect Dis. 2008;47:1364–6.
Watanabe T, Yasuoka A, Tanuma J, et al. Serum 1,3-beta-d-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin Infect Dis. 2009;49:1128–31.
Yasuoka A, Tachikawa N, Shimada K, et al. 1,3-beta-d-glucan as a quantitative serological marker for Pneumocystis carinii pneumonia. Clin Diagn Lab Immunol. 1996;3:197–9.
Cuétara MS, Alhambra A, Chaves F, et al. Use of a serum (1,3)-beta-d-glucan assay for diagnosis and follow-up of Pneumocystis jiroveci pneumonia. Clin Infect Dis. 2008;47:1364–6.
Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-β-d-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50(1):7–15.
Ogawa M, Hori H, Niiguchi S, et al. False positive plasma (1,3)-b-d-glucan following immunoglobulin product replacement in adult bone marrow recipient. Int J Hematol. 2004;80:97–8.
Usami M, Ohata A, Horiuchi T, et al. Positive (1,3)-beta-d-glucan in blood components and release of (1 fi 3)-beta-d-glucan from depth-type membrane filters for blood processing. Transfusion. 2002;42:1189–95.
Author information
Authors and Affiliations
Contributions
Tobias Lahmer (TL), Clarissa Prazeres da Costa (DC), Jürgen Held (JH), Sebastian Rasch (SR), Ursula Ehmer (UE) and Roland M Schmid (RMS), Wolfgang Huber (WH) were involved in study design. Tobias Lahmer (TL), Sebastian Rasch (SR) and Ursula Ehmer (UE) collected the data. Tobias Lahmer (TL), Clarissa Prazeres da Costa (DC), Jürgen Held (JH), Sebastian Rasch (SR), Ursula Ehmer (UE) and Roland M Schmid (RMS), Wolfgang Huber (WH) analyzed the data. Tobias Lahmer (TL), Clarissa Prazeres da Costa (DC), Jürgen Held (JH), Sebastian Rasch (SR), Ursula Ehmer (UE) and Roland M Schmid (RMS), Wolfgang Huber (WH) wrote the paper
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential financial or non-financial conflicts of interest related to this manuscript.
Rights and permissions
About this article
Cite this article
Lahmer, T., da Costa, C.P., Held, J. et al. Usefulness of 1,3 Beta-d-Glucan Detection in non-HIV Immunocompromised Mechanical Ventilated Critically Ill Patients with ARDS and Suspected Pneumocystis jirovecii Pneumonia. Mycopathologia 182, 701–708 (2017). https://doi.org/10.1007/s11046-017-0132-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-017-0132-x