Abstract
Paracoccidioidomycosis (PCM) is an endemic disease of humans from Latin America that is caused by Paracoccidioides brasiliensis and P. lutzii, with most cases of PCM in domestic animals being associated with P. brasiliensis. This study presents the clinical, cytological, mycological, serological, and molecular findings associated with P. brasiliensis in a dog from Southern Brazil. Fine needle biopsies were collected from the skin and several lymph nodes of a 5-year-old female Labrador dog that had enlargement of most superficial lymph nodes. Cytology of the skin and lymph nodes revealed pyogranulomatous dermatitis and lymphadenitis associated with fine-necked, budding fungal structures consistent with the Paracoccidioides genus of organisms; mycological culture derived from the lymph node aspirate demonstrated similar budding structures. Serological assays using exoantigens obtained from the fungal culture demonstrated that the fungal organisms derived from the lymph node were antigenically similar to P. brasiliensis by immunodiffusion and Western blot. A PCR assay, using the fungal culture as input, amplified a partial segment of the internal transcribed spacer 1 and 2 regions of P. brasiliensis; direct sequencing and phylogenetic analyses confirmed the PCR product as P. brasiliensis. The combined cytological, mycological, serological, and molecular findings confirmed a diagnosis of fungal dermatitis and lymphadenitis due to P. brasiliensis in this dog. This case represents the third description of clinical PCM in dogs and the first confirmation of mycotic dermatitis associated with P. brasiliensis in this species. The participation of dogs in the possible dissemination of PCM is reviewed, and it is proposed that dogs are probable accidental hosts in the epidemiological cycle associated with P. brasiliensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paracoccidioidomycosis (PCM) is an endemic mycotic disease predominantly of humans in Latin America, caused by members of the dimorphic fungi of the Paracoccidioides complex [1, 2]. Molecular investigations have demonstrated that PCM can be caused by P. brasiliensis and P. lutzii [1, 3]. P. brasiliensis is divided into four cryptic species (S1, PS2, PS3, and PS4) and occurs in several Latin American countries [2, 3], while P. lutzii is associated with infections in human populations from the central, northern, and southern regions of Brazil [1, 3] and Ecuador [3]. Moreover, the incidence of human PCM is more elevated in south, southeast, and central-west Brazil [4].
Although disease due to P. brasiliensis infection occurs primarily in humans, within endemic areas there is serological evidence of paracoccidioidomycosis in several animal species; these include the dog [5–7], cat [8], sheep [9], domestic chicken [10], cattle [11], pig [12], wild monkeys [13], rabbit [14], armadillo [15], horse [16, 17], and the goat [18]. Although the exact animal host of P. brasiliensis has not been fully elucidated, the armadillo was considered a natural reservoir of P. brasiliensis [19], and several studies [20–24] have since demonstrated the occurrence of the Paracoccidioides complex in this animal species.
However, the role of the dog in the epidemiological cycle of P. brasiliensis remains obscure [25], even though dogs experimentally infected with P. brasiliensis developed PCM [26, 27] or had an immune response to this fungal organism [28], and there are serological evidences of this fungal infection in dogs [5–7, 29–32]. Moreover, reports of spontaneous cases of PCM in dogs are scarce; two were described in female Doberman dogs, one, originated from the State of São Paulo, had cervical lymphadenomegaly [33], and the other, from the State of Paraná, was emaciated with lymphadenomegaly and hepatosplenomegaly [34]. This study presents the clinical, cytological, mycological, serological, and molecular findings associated with PCM in a dog from Southern Brazil and examines the existing evidence relative to the participation of dogs in the development and maintenance of P. brasiliensis.
Materials and Methods
Clinical History
In late January 2015, a 5-year-old female Labrador dog was attended at the Veterinary Teaching Hospital, Universidade Estadual de Londrina (VTH-UEL), Southern Brazil; this region is considered as having an elevated risk for human PCM [4]. The owner reported that the dog demonstrated apathy with reduced appetite during the last 30 days prior to this visit, and that there was fever within the last 24 h. In addition, the owner indicated that this dog shared the same accommodations and yard space with another dog that was diagnosed with toxoplasmosis and neosporosis by serology. A clinical evaluation revealed hyperemic mucosa membranes and an enlarged, 1 cm in diameter, soft nodule that was adhered to the erythematous skin at the left side of the upper lip. Serological and hematological evaluations were solicited; meanwhile, the animal did not permit a biopsy without sedation and the attending clinician decided to await the laboratory findings. The dog was serologically negative for Toxoplasma gondii and Neospora caninum; hematological analyses revealed normocytic normochromic non-regenerative anemia and thrombocytopenia. Consequently, canine ehrlichiosis was suspected and the dog was placed on medication that initiated with doxycycline (10 mg/kg, oral, 12/12 h for 4 weeks) and imidocarb hydrochloride (5 mg/kg, subcutaneous, two administrations with 2 weeks interval).
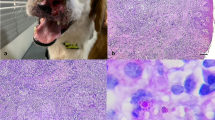
However, 2 weeks later the dog returned to the VTH-UEL without anorexia and apathy, but with a clinical complaint of lameness of both pelvic members. At this time, a clinical evaluation revealed hyperthermia (39.7 °C), pain at both hip joints with enlargement of most superficial lymph nodes (submandibular 3.5 cm diameter; prescapular and popliteal, 2.5 cm diameter) and a cutaneous nodule (1 × 2 × 7 cm) adjacent to the fifth left mammary gland (Fig. 1a, b). Lymphoma, mammary tumor, a possible infectious disease, and hip dysplasia were included as differential diagnosis. Fine needle biopsy aspirates of all enlarged lymph nodes and the cutaneous mass close to the mammary gland were collected and submitted for cytological evaluation; a part of the submandibular lymph node aspirate was submitted for microbiological evaluations.
Clinical, cytological, and mycological findings associated with P. brasiliensis in a dog. Observe the enlarged lymph nodes before therapy (a) and the absence of swelling after therapy (b). Cytological demonstration of mycotic pyogranulomatous lymphadenitis (c, d): observe the intralesional fungal organisms demonstrating the Mickey Mouse head pattern (arrowhead) and within (arrow) a multinucleated giant cell (MGN); several fugal organisms are also shown (arrowheads). Cytology of mycotic pyogranulomatous dermatitis, with the mariner wheel (e) and Mickey Mouse head (f) presentations of the fungal pathogen. Mycological culture demonstration typical budding characteristic associated with P. brasiliensis; observe the forming of the marine wheel (g) and Mickey Mouse head (g) patterns. c–f Giemsa stain. g, h lactophenol cotton blue stain. Bars c–h 10 µm
Mycological Culture and Isolation
The sample from the fine needle aspirate derived from the enlarged lymph node was plated in duplicate in Sabouraud Dextrose Agar with and without chloramphenicol, and then incubated at 25 and 37 °C for 30 days. The micromorphological characteristics of emerging colonies were observed with the lactophenol cotton blue stain. Samples of the pure fungal colonies were submitted for molecular confirmation and for the preparation of exoantigen to be used in an immunodiffusion assay and Western blot.
Molecular Characterization of P. brasiliensis
Pure fungal DNA was extracted from fungal cultures by using the commercial kit Qiagen DNeasy blood & tissue kit (Qiagen Sample & Assay Technologies, Hilden, Germany) and then subjected to a PCR assay that targeted the internal transcribed spacer (ITS) 1 and 2 regions by using the universal ITS 1 and 2 primers [35]. Positive controls consisted of fungal DNA from a previous study [36]. Nuclease-free water (Invitrogen Corp., Carlsbad, CA, USA) was used as negative controls in all PCR assays. All PCR products were separated by electrophoresis in 2 % agarose gels, stained with ethidium bromide, and examined under ultraviolet light. The amplified PCR products were then purified (illustra GFX PCR DNA and Gel Band Purification Kit, GE Healthcare, Little Chalfont, Buckinghamshire, UK) and submitted for direct sequencing using the forward and reverse primers.
The obtained sequences were examined for quality analysis of chromatogram readings by using the PHRED software (http://asparagin.cenargen.embrapa.br/phph); sequences were only accepted if base quality was equal to or greater than 20. Consensus sequences were then generated by the CAP3 program (http://asparagin.cenargen.embrapa.br/cgi-bin/phph/cap3.pl), after which the partial nucleotide sequences were initially compared by the Basic Local Alignment Search Tool (BLAST) program (http://www.ncbi.nlm.nih.gov/BLAST) with similar sequences deposited in GenBank. Phylogenetic tree and sequence alignments based on the ITS 1 and 2 regions of P. brasiliensis were then created by using MEGA 6 [37]; model selection indicated the Jukes–Cantor model as being the most appropriate for determination of the phylogenetic relationship with the maximum likelihood method. The initial tree for the heuristic search was obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood approach and then selecting the topology with superior log likelihood value. A sequence identity matrix was then generated by using BioEdit software version 7.2. [38], to identify possible differences between isolates of the PCM complex. Microsporum canis was used as the out-group to provide stability to the generated tree.
Preparation of P. brasiliensis Exoantigens
Exoantigen derived from the known P. brasiliensis isolate (B-339) and from the fungal culture of this dog (LLGS-1) was obtained as previously described [39].
Immunodiffusion Test
The reactivity between a serum sample from a human patient diagnosed with paracoccidioidomycosis was evaluated with the exoantigen derived from P. brasiliensis isolates B-339 and LLGS1 as previously described [7].
Western Blot
After electrophoresis of exoantigens from P. brasiliensis isolates B-339 and LLGS-1 in sodium dodecyl sulfate–polyacrylamide gel, the proteins were transferred to the nitrocellulose membrane and blocked with 5 % skim milk in PBS. The membrane was then incubated with a serum sample (1:100 dilution) from a human patient with paracoccidioidomycosis for 1 h at room temperature and after washing and was incubated with anti-human IgG-peroxidase conjugate for 1 h at room temperature. The reaction was revealed with substrate/chromogen solution (H2O2/diaminobenzidine) and stopped with distilled water.
Results
Cytological, Clinical, and Mycological Findings
The cytological evaluations of all lymph nodes were similar, with discrete cytological difference, and revealed pyogranulomatous lymphadenitis associated with numerous budding, intracytoplasmic (within macrophages and multinucleated giant cells) and extracellular fungal cells consistent with the Paracoccidioides complex of organisms [4, 40, 41]; similar inflammatory reaction associated with intralesional fungal structures was observed in the cutaneous sample, resulting in a cytological diagnosis of fungal dermatitis due to Paracoccidioides spp. These fungal organisms varied between 10 and 20 µm in diameter were oval to elongated in shape with a thickened cell wall. Budding organisms consisted of two or more yeast cells arranged in chains. Further, there were numerous fungal organisms that presented the classical “Mickey-Mouse head” pattern (one large centrally located fungi with two offside smaller buds) and the “mariner pilot-wheel” morphology, formed by a mother cell surrounded by several peripheral daughter yeasts (Fig. 1c–f).
During the second clinical visit, a radiographical evaluation revealed discrete bilateral hip dysplasia and due to the confirmation of a Paracoccidioides genus-induced infection by cytology, the therapy with doxycycline and imidocarb hydrochloride was suspended. The dog was then placed on a protocol that consisted of itraconazole (10 mg/kg, oral, 12/12 h), meloxicam (0.1 mg/kg, oral, daily for 3 days), and tramadol (3 mg/kg, oral, 8/8 h for 7 days) and returned for clinical evaluation 9 days later when hyperthermia (39.8 °C), discrete dehydration, and persistence of lymphadenopathy of superficial lymph nodes were identified. At this time, the dog was hospitalized at the VTH-UEL, doxycycline was included in the clinical therapeutic protocol and the animal remained as an inpatient for an additional 3 days after which she was released.
The dog was in and out of the VTH-UEL repeatedly for clinical evaluation and associated therapy until late June 2015, 5 months after the first visit, when lymphadenopathy had regressed completely. Additional clinical conditions presented and resolved (therapeutically and/or surgically) during this period included anemia, intermittent anorexia, widespread dermatitis, and purulent lymphadenitis of the right submandibular node. Since then, this dog has been visiting the VTH-UEL regularly as an out-patient and has been receiving appropriate therapy based on the clinical and laboratory findings presented when examined. At the time of preparation of this manuscript (almost 18 months after the initial visit), the dog is alive, without enlarged lymph nodes, in good state of health and maintained on oral administration of itraconazole (10 mg/kg, 12/12 h). Further, due to animal health concerns and the general wellbeing of the dog, additional incisional biopsies were not taken to evaluate the presence of fungal organisms.
Mycological culture yielded slow-growing, fine-necked, multipolar budding fungal organisms that were whitish, globose to ovoid in shape, 2–30 µm in diameter; the Mickey Mouse head and “mariner pilot-wheel” morphological presentations were identified (Fig. 1g, h).
Molecular Characterization of Paracoccidioides brasiliensis
The PCR assay amplified a partial 239-bp fragment of the ITS1 and 2TS2 regions of the fungal culture derived from the lymph node of this dog. The sequence from this study has been deposited in GenBank (accession # KX417771) and was named P. brasiliensis isolate LLGS1. The BLAST analysis revealed that the strain identified during this investigation had 97–99 % nucleotide sequence identity with similar isolates of the Paracoccidioides complex of organisms deposited in GenBank. However, the nucleotide identity matrix (not shown) demonstrated that the isolate P. brasiliensis strain LLGS1 had 99 % similarity with several strains (GenBank accession # AB304436.1; AB304434.1; AF322389.1; AB304448.1; and AF416745.1) of P. brasiliensis. In addition, the sequence identity was 98 % with other isolates (AY618999.1; AY374339.1; and EU870316.1) of P. brasiliensis, but with 97 % identity with P. lutzii (KJ540979.1).
The generated phylogenetic tree revealed two distinct clusters of the Paracoccidioides complex of organisms: one formed by isolates of the P. brasiliensis group, and the other by P. lutzii; the isolate from this study clustered with other strains of the P. brasiliensis group deposited in GenBank. Further, all members of the Paracoccidioides complex clustered together, being distinct from Microsporum canis (Fig. 2).
Phylogenetic tree based on the ITS 1 and 2 regions of selected strains of the Paracoccidioides complex of organisms generated by MEGA 7. The tree was constructed by the maximum likelihood method, based on 1000 bootstrapped data sets; distances values were calculated by using the Jukes–Cantor parameter model. The GenBank accession numbers of the sequences used are given; the isolate derived from this study is highlighted (black dot)
Analysis of P. brasiliensis exoantigens by Immunodiffusion Assay and Western Blot
The analysis of the reactivity of a serum sample from a patient with paracoccidioidomycosis with exoantigens from P. brasiliensis B-339 and LLGS1 by immunodiffusion assay showed a pattern of complete identity, indicating that both exoantigens are antigenically identical (Fig. 3a). Further, the Western blot analysis revealed that the P. brasiliensis LLGS1 isolate produced gp43 antigen, as observed with the P. brasiliensis reference strain B-339 (Fig. 3b).
Discussion
The combined results of the cytological, serological, and molecular investigations confirmed the fungal agent associated with lymphadenopathy in this dog as P. brasiliensis and represent only the third spontaneous case of PCM in dogs; two cases were previously described [33, 34]. The cytological findings of the lymph nodes and the skin from this dog are consistent with a diagnosis of the Paracoccidioides genus of organisms [4, 40–42]; mycological culture of the lymph node aspirate confirmed the cytological diagnosis, while the serological (immunodiffusion and Western blot) and molecular investigations (PCR with subsequent sequencing and phylogenetic analysis) were fundamental to effectively characterize this fungal pathogen as P. brasiliensis [1, 3, 41].
Cutaneous dermatitis associated with P. brasiliensis, as observed in the skin biopsy from this dog, was not previously described in canine PCM; other lesions already associated with canine PCM include fungal pneumonia, hepatitis, splenitis [26], and epididymitis [32]. However, these pathological alterations were observed in experimentally induced cases of canine PCM; alternatively, spontaneous cutaneous manifestations of PCM in humans are frequently described [3, 4, 40, 43].
The severe lymphadenopathy and the associated findings observed in this dog were also described in the other two spontaneous cases [33, 34] of canine PCM. However, in an experimental study done by our group with four puppies [26], lymphadenopathy was not identified. In addition, 41 dogs that were euthanized during a serological investigation did not demonstrate lymphadenopathy or histological evidence of P. brasiliensis [5]. A striking difference in the possible pathogenesis of canine PCM cannot be underscored: All previous cases of spontaneous canine PCM with lymphadenopathy were diagnosed in adult dogs, while puppies experimental infected with P. brasiliensis did not develop this clinical manifestation and some of these died. This might suggest that there is a possible age-related susceptibility associated with canine PCM [26], where puppies are more susceptible, due to the immature immunological system, to develop fatal PCM resulting in organ dissemination, while older dogs are more resistant, developed an adequate immune response resulting in clinical PCM that is restricted to superficial lymph nodes. We have also demonstrated that older dogs that were seropositive to P. brasiliensis did not demonstrate clinical or histopathological manifestation of PCM [6]. However, age did not influence the serological occurrence of P. brasiliensis in dogs in an epidemiological study done in Rio Grande do Sul, Southern Brazil [29], but there was no mention of clinical manifestation of PCM in that study population. Further, dogs that were inoculated via the testicles demonstrated localized testicular lesions with spontaneous regression [32], but clinical manifestations of PCM were not described. Collectively, these results might indicate that dogs in a general manner are somewhat resistant to infection by P. brasiliensis, considering the few spontaneous cases in dogs that have been published even in areas where the disease is endemic in human populations. However, additional cases of canine PCM are needed to fully understand the role of dogs in the epidemiology of P. brasiliensis.
Nevertheless, clinical cases of canine PCM in Brazil, and probably Latin America, might not be adequately identified because of a lack of diagnosis in veterinary services, especially those that are distant from urban centers and/or not located within veterinary teaching hospitals. Another issue that cannot be overlooked and must be highlighted is the submission and conditioning of suitable samples for laboratory diagnosis, which would definitely affect the expected result. Consequently, canine PCM might be underdiagnosed within the epidemiological regions with elevated indices of human PCM.
Alternatively, it cannot be ignored that PCM might not be a disease of dogs in Brazil, and probably Latin America, and that this species might not be threatened or seriously affected by P. brasiliensis, as is the case of human PCM [2–4, 40]. This theory can be justified and appreciated if the following are considered: (1) the relatively few cases of canine PCM diagnosed with regards to the large volume of canine submissions for necropsy and/or histopathology evaluated annually by veterinary pathologists throughout Latin America, and (2) the frustrated attempts to reproduce this disease in dogs [26]. Further, the pathologists involved in this study had no problem with the cytological identification of the fungal organisms and confirmed the diagnosis by molecular and serological assays. Therefore, the identification of canine PCM should not be a diagnostic challenge to trained veterinary pathologists, since the organism is readily identified in routine histological and/or cytological preparations [42], even though these are not highly sensitive diagnostic methods [44]. Collectively, the proposed theory and the reduced number of cases of PCM identified in dogs would then, at least in part, explain the paucity of cases of canine PCM; therefore, dogs might simply be the incidental (dead-end or accidental) hosts in the epidemiological cycle of this organism and be resistant [5] to the development of clinical disease associated with P. brasiliensis, even though they can be serologically reactive. Further, the possibility of canine PCM being transmitted to other dogs and humans has never been documented; in the case herein described, as was well as in the other two descriptions of canine PCM [33, 34], other dogs and humans were in contact with the animals that developed the disease, but these were asymptomatic. Moreover, transmission of PCM between an infected individual to contact persons was never demonstrated [4].
During this investigation, the dog was administered a therapeutic protocol that consisted of itraconazole (10 mg/kg, 12/12 h) and other supportive therapy, which resulted in reduction in lymph node enlargement after a period of 5 months; this fungal drug was also efficient in the therapy of another case of canine PCM [34]; both of these dogs recovered after therapy. However, the use of ketoconazole in canine PCM controlled the clinical manifestations but resulted in remission after 18 months, with subsequent euthanasia [33]. Therefore, when PCM is suspected in dogs, the treatment of choice seems to be itraconazole; this is also the preferred drug for human PCM [4].
In conclusion, P. brasiliensis was identified by a combination of cytological, mycological, serological, and molecular diagnostic techniques in a dog that had lymphadenopathy. This case represents the third confirmation of clinical PCM in dogs. The role of dogs in the maintenance of P. brasiliensis is poorly understood. However, this specie of domestic animal seems to be resistant to infection by this fungal pathogen or might be a terminal or accidental host that does not easily demonstrate clinical manifestation of PCM even when there is serological evidence of the disease agent.
References
Teixeira Mde M, Theodoro RC, Oliveira FF, Machado GC, Hahn RC, Bagagli E, et al. Paracoccidioides lutzii sp. nov.: biological and clinical implications. Med Mycol. 2014;52(1):19–28.
Teixeira MM, Theodoro RC, Nino-Vega G, Bagagli E, Felipe MSS. Paracoccidioides species complex: ecology, phylogeny, sexual reproduction, and virulence. PLoS Pathog. 2014;10(10):e1004397.
Bocca AL, Amaral AC, Teixeira MM, Sato PK, Shikanai-Yasuda MA, Soares Felipe MS. Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol. 2013;8(9):1177–91.
Shikanai-Yasuda MA, Telles Filho F, Mendes RP, Colombo AL, Moretti ML. Group of Consultants on the Guidelines in Paracoccidioidomycosis. Rev Soc Bras Med Trop. 2006;39:297–310.
Fontana FF, dos Santos CT, Esteves FM, Rocha A, Fernandes GF, do Amaral CC, et al. Seroepidemiological survey of paracoccidioidomycosis infection among urban and rural dogs from Uberaba, Minas Gerais, Brazil. Mycopathologia. 2010;169(3):159–65.
Ono MA, Bracarense AP, Morais HS, Trapp SM, Belitardo DR, Camargo ZP. Canine paracoccidioidomycosis: a seroepidemiologic study. Med Mycol. 2001;39(3):277–82.
Silveira LH, Domingos IH, Kouchi K, Itano EN, Silva EA, Landgraf VO, et al. Serological detection of antibodies against Paracoccidioides brasiliensis in dogs with leishmaniasis. Mycopathologia. 2006;162(5):325–9.
Oliveira GG, Belitardo DR, Balarin MR, Freire RL, Camargo ZP, Ono MA. Serological survey of paracoccidioidomycosis in cats. Mycopathologia. 2013;176(3–4):299–302.
Oliveira GG, Navarro IT, Freire RL, Belitardo DR, Silveira LH, Camargo ZP, et al. Serological survey of paracoccidioidomycosis in sheep. Mycopathologia. 2012;173(1):63–8.
Oliveira GG, Silveira LH, Itano EN, Soares RM, Freire RL, Watanabe MA, et al. Serological evidence of Paracoccidioides brasiliensis infection in chickens from Parana and Mato Grosso do Sul States, Brazil. Mycopathologia. 2011;171(3):197–202.
Silveira LH, Paes RC, Medeiros EV, Itano EN, Camargo ZP, Ono MA. Occurrence of antibodies to Paracoccidioides brasiliensis in dairy cattle from Mato Grosso do Sul, Brazil. Mycopathologia. 2008;165(6):367–71.
Belitardo DR, Calefi AS, Borges IK, de Oliveira GG, Sbeghen MR, Itano EN, et al. Detection of antibodies against Paracoccidioides brasiliensis in free-range domestic pigs (Sus scrofa). Mycopathologia. 2014;177(1–2):91–5.
Corte AC, Svoboda WK, Navarro IT, Freire RL, Malanski LS, Shiozawa MM, et al. Paracoccidioidomycosis in wild monkeys from Parana State, Brazil. Mycopathologia. 2007;164(5):225–8.
Belitardo DR, Calefi AS, Sbeghen MR, de Oliveira GG, Watanabe MA, de Camargo ZP, et al. Paracoccidioides brasiliensis infection in domestic rabbits (Oryctolagus cuniculus). Mycoses. 2014;57(4):222–7.
Fernandes GF, Deps P, Tomimori-Yamashita J, Camargo ZP. IgM and IgG antibody response to Paracoccidioides brasiliensis in naturally infected wild armadillos (Dasypus novemcinctus). Med Mycol. 2004;42(4):363–8.
Albano APN, Klafke GB, Brandolt TM, Da Hora VP, Nogueira CEW, Xavier MO, et al. Seroepidemiology of Paracoccidioides brasiliensis infection in horses from Rio Grande do Sul, Brazil. Braz J Microbiol. 2015;46(2):513–7.
Corte AC, Itano EN, Freire RL, de Camargo ZP, Ono MA. Detection of antibodies to Paracoccidioides brasiliensis in horses from northern region of Paraná State. Semin-Cienc Agrar. 2009;30(2):441–6.
Ferreira JB, Navarro IT, Freire RL, Oliveira GG, Omori AM, Belitardo DR, et al. Evaluation of Paracoccidioides brasiliensis infection in dairy goats. Mycopathologia. 2013;176(1–2):95–9.
Bagagli E, Sano A, Coelho KI, Alquati S, Miyaji M, de Camargo ZP, et al. Isolation of Paracoccidioides brasiliensis from armadillos (Dasypus novemcinctus) captured in an endemic area of paracoccidioidomycosis. Am J Trop Med Hyg. 1998;58(4):505–12.
Silva-Vergara ML, Martinez R, Camargo ZP, Malta MH, Maffei CM, Chadu JB. Isolation of Paracoccidioides brasiliensis from armadillos (Dasypus novemcinctus) in an area where the fungus was recently isolated from soil. Med Mycol. 2000;38(3):193–9.
Vergara ML, Martinez R. Role of the armadillo Dasypus novemcinctus in the epidemiology of paracoccidioidomycosis. Mycopathologia. 1998;144(3):131–3.
Bagagli E, Franco M, Bosco Sde M, Hebeler-Barbosa F, Trinca LA, Montenegro MR. High frequency of Paracoccidioides brasiliensis infection in armadillos (Dasypus novemcinctus): an ecological study. Med Mycol. 2003;41(3):217–23.
Corredor GG, Peralta LA, Castano JH, Zuluaga JS, Henao B, Arango M, et al. The naked-tailed armadillo Cabassous centralis (Miller 1899): a new host to Paracoccidioides brasiliensis. Molecular identification of the isolate. Med Mycol. 2005;43(3):275–80.
Bagagli E, Bosco SM, Theodoro RC, Franco M. Phylogenetic and evolutionary aspects of Paracoccidioides brasiliensis reveal a long coexistence with animal hosts that explain several biological features of the pathogen. Infect Genet Evol. 2006;6(5):344–51.
Neves LN, Petroni TF, Fedatto PF, Ono MA. Paracoccidioidomycosis in wild and domestic animals. Semin-Cienc Agrar. 2006;27(3):481–8.
Ono MA, Kishima MO, Itano EN, Bracarense AP, Camargo ZP. Experimental paracoccidioidomycosis in dogs. Med Mycol. 2003;41(3):265–8.
Mós EN, Fava Netto C. Contribuição ao estudo da paracoccidiodomicose. I. Possível papel epidemiológico dos cães. Estudo sorológico e anatomo-patológico. Rev Inst Med Trop São Paulo. 1974;16(3):154–9.
Eisele RC, Juliani LC, Belitardo DR, Itano EN, Estevao D, Bracarense AP, et al. Immune response in dogs experimentally infected with Paracoccidioides brasiliensis. Med Mycol. 2004;42(6):549–53.
Teles AJ, Klafke GB, Cabana AL, Albano AP, Xavier MO, Meireles MC. Serological investigation into Paracoccidioides brasiliensis infection in dogs from Southern Rio Grande do Sul, Brazil. Mycopathologia. 2016;181(3–4):323–8.
Canteros CE, Madariaga MJ, Lee W, Rivas MC, Davel G, Iachini R. Endemic fungal pathogens in a rural setting of Argentina: seroepidemiological study in dogs. Rev Iberoam Micol. 2010;27(1):14–9.
Corte AC, Gennari SM, Labruna MB, Camargo LMA, Itano EN, Freire RL, et al. Paracoccidioides brasiliensis infection in dogs from Western Brazilian Amazon. Pesq Vet Bras. 2012;32:649–52.
Mós EN, Fava Netto C, Saliba AM, Brito T. Contribuição ao estudo da paracoccidiodomicose. II. Infecção experimental do cão. Rev Inst Med Trop São Paulo. 1974;16(4):232–7.
Ricci G, Mota FT, Wakamatsu A, Serafim RC, Borra RC, Franco M. Canine paracoccidioidomycosis. Med Mycol. 2004;42(4):379–83.
Farias MR, Condas LA, Ribeiro MG, Bosco Sde M, Muro MD, Werner J, et al. Paracoccidioidomycosis in a dog: case report of generalized lymphadenomegaly. Mycopathologia. 2011;172(2):147–52.
White T, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. p. 315–22.
Headley SA, Di Santis GW, de Alcantara BK, Costa TC, da Silva EO, Pretto-Giordano LG, et al. Cryptococcus gattii-induced infections in dogs from Southern Brazil. Mycopathologia. 2015;180(3–4):265–75.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9.
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–8.
Camargo Z, Unterkircher C, Campoy SP, Travassos LR. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J Clin Microbiol. 1988;26(10):2147–51.
Brummer E, Castaneda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993;6(2):89–117.
Reiss E, Shadomy HJ, Lyon GM. Fundamental medical mycology. Hoboken: Wiley; 2012.
Ramos-e-Silva M, Saraiva LES. Paracoccidioidomycosis. Dermatol Clin. 2008;26(2):257–69.
Bellissimo-Rodrigues F, Bollela VR, Da Fonseca BA, Martinez R. Endemic paracoccidioidomycosis: relationship between clinical presentation and patients’ demographic features. Med Mycol. 2013;51(3):313–8.
Teles FR, Martins ML. Laboratorial diagnosis of paracoccidioidomycosis and new insights for the future of fungal diagnosis. Talanta. 2011;85(5):2254–64.
Acknowledgments
Selwyn A. Headley, Amauri A. Alfieri, and Mario Augusto Ono are recipients of the National Council for Scientific and Technological Development (CNPq; Brazil) fellowships and grants. The authors thank the owners of this dog for the usage during this investigation. We express sincere appreciation and gratitude to Mrs Eliana Celia Pereira, technician Laboratory of Animal Mycology, Universidade Estadual de Londrina, for the preparation of the fungal culture used during this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Rights and permissions
About this article
Cite this article
Headley, S.A., Pretto-Giordano, L.G., Di Santis, G.W. et al. Paracoccidioides brasiliensis-associated dermatitis and lymphadenitis in a dog. Mycopathologia 182, 425–434 (2017). https://doi.org/10.1007/s11046-016-0075-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-016-0075-7