Abstract
Phaeohyphomycosis is caused by a heterogeneous group of mycelial dematiaceous (phaeoid) fungi, which produce melanin pigment. This condition is often confused with chromoblastomycosis. Rhytidhysteron is a dematiaceous fungus, which has been recently found to be causing human infections. Till date only three cases of infection with Rhytidhysteron rufulum have been reported in the literature. All three cases have been from North India. Hereby, we present another two cases where Rhytidhysteron was isolated. Both the patients belonged to Chandigarh (India) and presented with subcutaneous lesions. The isolates were confirmed by ITS sequencing. Both the patients were immunocompetent and gave no history of trauma or any other predisposing factor. Phaeohyphomycosis are often missed due to lack of knowledge regarding the fungi causing the infections and there is need for clinical, pathological and microbiological correlation for effective diagnosis and treatment in these cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phaeoid fungi are heterogeneous group of organisms which are unified due to production of melanin pigment. The term phaeohyphomycosis has been coined to describe superficial, cutaneous, subcutaneous or systemic infections caused by melanized fungi. The term phaeo has been derived from the Greek word phaios, which means grey and refers to the colour of these fungi in vivo and/or in vitro [1]. About 100 genera have been attributed to cause phaeohyphomycosis. Most common genera causing phaeohyphomycosis are Curvularia, Alternaria, Bipolaris, Exophiala, Cladophialophora, etc. Phaeohyphomycosis need to be differentiated from chromoblastomycosis, which is fungal infection involving skin and subcutaneous tissues in tropical and sub-tropical regions. The main difference between the two conditions is presence of sclerotic bodies in tissues in chromoblastomycosis whereas presence of hyphal forms clinches the diagnosis of phaeohyphomycosis [1].
The first case of phaeohyphomycosis was reported by De Beurman and Gougerot in France in 1907. Isolated cases are being reported regularly from time to time. Phaeohyphomycosis can be classified into four basic forms: superficial, cutaneous and corneal, subcutaneous and systemic rapidly fulminant illness [2]. Mostly the infections are opportunistic in nature, presenting in immunocompromised individuals with granulocytopenia, leukemia, chemotherapy or transplant patients. Recently many rare melanized fungi as etiologic agents of phaeohyphomycosis have been identified primarily attributed to the availability of molecular techniques. Rhytidhysteron spp. are rare melanized fungi causing chromoblastomycosis or phaeohyphomycosis primarily reported from India. Rhytidhysteron was traditionally classified into phylum Ascomycota, family Patellariaceae [3]. However, recent molecular analyses of multiple nuclear loci considers it to be closely related to the saprophyte Hysterium pulicare [4]. Although there are some basic differences in the character of ascomata, peridium and ascospores, Rhytidhysteron has been finally placed in Hysteriaceae rather than Patellariaceae based on aminoacid and genetic sequence data [4]. Recently we identified two cases of phaeohyphomycosis caused by R. rufulum.
Clinical Presentations
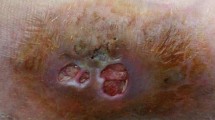
Case 1 was a 45-year-old male resident of Chandigarh, a landlord by profession, owned agriculture fields but he was not actively involved in farming. Patient came to the fine needle aspiration cytology clinic in January 2012 with swelling on dorsal aspect of right foot clinically suspected to be a lipoma. The swelling was 3 × 3 cm in size, non-tender and mobile over the underlying bone. The overlying skin was intact but slightly tense and the mobility was restricted. The patient could not recollect any history of trauma. Fine needle aspiration cytology was performed from the swelling and 5 mL hemorrhagic fluid was aspirated which was sent to department of Pathology and Microbiology for examination.
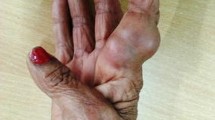
Case 2 was a 50-year-old male patient, in a private office job, resident of Chandigarh who presented with swelling on anterolateral aspect of left knee (Fig. 1a) and gave history of swelling being present for the last 8–10 years. For the past 2 years, he observed increase in the size. It was a painless and asymptomatic swelling. Patient did not reveal any history of prior trauma. On palpation, it was a small nodule, soft, movable, free from the overlying skin and underlying tissues, non-tender with no signs of inflammation, discharge or draining sinuses. A fungal etiology was suspected after fine needle aspiration cytology at another centre thereby the patient was referred to our institution. The excision biopsy was done and the sample was sent to the Departments of Pathology as well as Microbiology for further evaluation.
a Subcutaneous swelling on anterolateral aspect of left knee. b Post-treatment complete resolution of the nodular swelling. c Fine needle aspirate smear showing septate hyphae (arrow) in the background of inflammation (MGG, ×400). d Direct KOH mount from the aspirate showing dark brown branching septate hyphae and no sclerotic bodies (KOH, ×400). e Smear showing fungal profiles highlighted on Periodic Acid Schiff staining (PAS, ×400). f Lactophenol cotton blue mount showing septate dematiaceous tortuous fungal hyphae with irregular branching (LCB, ×400). g Growth on SDA showing dark, velvety, olivaceous fungal colonies—obverse. h Growth on SDA—reverse. (Color figure online)
In both the patients, routine laboratory investigations; haemogram, complete blood counts, fasting and random blood glucose levels, renal and liver function tests, were done and reported to be within normal limits. The HIV status of both the patients was non-reactive. No significant findings were seen on chest X-rays. There was no history of diabetes, tuberculosis or any other chronic illness.
Pathological Examination
The aspirate smears were stained with conventional stainings; haematoxylin and eosin (H&E) and May-Grunwald Giemsa (MGG). Special staining, periodic acid Schiff (PAS) was also done. Smears showed numerous neutrophils, few lymphocytes and macrophages in a background of amorphous debris. Few septate fungal hyphae were noted (Fig. 1c). The impression was of an inflammatory lesion with presence of fungal profiles. The septate fungal hyphae seen in both the cases, were more highlighted on PAS staining (Fig. 1e). Acid fast bacilli staining was also done which was negative in both the cases.
Mycological Examination
The potassium hydroxide (KOH) wet mounts made from the samples obtained in both the cases showed long, thick, septate, tortuous, dark brown hyphae and no sclerotic bodies (Fig. 1d). The material was inoculated onto the blood agar, MacConkey agar and Sabouraud dextrose agar (SDA) with and without actidione and incubated at both 37 °C and 25 °C. SDA showed growth of dematiaceous mycelial fungi after 15–20 days as multiple colonies. Colonies were light grey to green in colour at first and later on became dark green, velvety and olivaceous at 25 °C on further incubation (Fig. 1g, h). Lactophenol cotton blue mount showed septate, irregularly branched dematiaceous hyphae which were tortuous in nature (Fig. 1f). The samples were inoculated on potato dextrose agar, malt extract agar and cornmeal agar to induce sporulation but no sporulation could be induced.
As isolates obtained from both patients did not sporulate despite repeated attempts, they were subjected to molecular identification by ITS sequencing at Department of Medical Mycology Vallabhbhai Patel Chest Institute, Delhi, India and were confirmed to be R. rufulum. Genomic DNA was extracted from isolates and was amplified using the ITS-1 (5=-TCCGTAGGTGAACCTTGCGG-3=) and ITS-4 (5=-TCCTCCGCTTATTGATATGC-3=) primers, which amplify the internal transcribed spacer (ITS) region of the ribosomal subunit Amplified DNA was sequenced in both strands on an ABI 3130XL Genetic Analyzer (Applied Biosystems, Foster City, CA) using a Big Dye Terminator kit, v3.1 (Applied Biosystems). Sequences were aligned using Sequencing Analysis 5.3.1 software (Applied Biosystems). GenBank basic local alignment search tool (BLAST) searches (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) were performed for species identification. BLAST searches confirmed the isolates as R. rufulum (GenBank accession no. KU236375 and KU236376). The sequences showed 99–100 % homology (query coverage 100 %) to R. rufulum isolates in GenBank (accession no.JX868651.1, KP162180.1).
Treatment and Follow Up
The first patient did not agree for any surgical intervention despite counselling the benefit of it. He was put on oral itraconazole therapy, 200 mg three times a day. The patient also never turned up for any follow up visits. Second patient underwent excision biopsy with complete removal of the lesion and also started on oral itraconazole therapy, 200 mg two times a day after confirmation of fungal pathology on report. Patient is on regular follow up and has not shown any recurrence or relapse.
Discussion
Rhytidhysteron species are saprophytes capable of utilizing different substrata and occupying diverse habitats. They have been traditionally associated with plant disease and occur on the wood of living or dead dicotyledonous plants including citrus and especially mangroves [5]. Genus Rhytidhysteron has four species: R. rufulum, R. hysterinum, R. opuntiae and R. dissimile. The most conspicuous and determined morphological characteristic to differentiate species in Rhytidhysteron is ascospore septation. R. hysterinum differs in having one-septate ascospores [6], R. rufulum has three septations whereas the ascospores in R. dissimile are 5-septate [7]. Rhytidhysteron opuntiae has 1–3 (4–5) septate ascospores, with one longitudinal septum in mid cells [8].
In the literature, only three cases of infection due to Rhytidhysteron rufulum have been described. First case was described by Chowdhary et al. [9] 2008, in a 50 year male patient of renal transplant. He had two noduloulcerative lesions on his left leg diagnosed as chromoblastomycosis and could not survive despite itraconazole treatment. Another case was recently reported by Mahajan et al. [10] 2014, in a 72 years diabetic male with soft painless swelling on right foot. His lesion was excised and itraconazole was instituted. However, the surgical wound developed multiple discharging sinuses and ultimately the cure was achieved with intralesional liposomal amphotericin B. Mishra et al. [11] reported a case of subcutaneous phaeohyphomycosis in a 65 year immunocompetent male with subcutaneous nodule on left foot where patient was treated with oral terbinafine and itraconazole.
Skin and subcutaneous phaeohyphomycosis is the most common manifestation and usually presents as localized infection following deep inoculation of fungus into site. The lesions may be seen as nodular, cystic, abscess or granulomatous, commonly seen on exposed part of the body such as feet, legs, hands, arms and back. In our case, one patient presented with subcutaneous cystic lesion and other with a nodular swelling.
In both of our patients, no history of known trauma could be elicited. Most of the cases of phaeohyphomycosis give history of trauma but the reports without trauma are also not unknown. No explicit history of trauma was given by any patient suffering of Rhytidhysteron infection in cases reported before. Our first patient was a landlord and owned agriculture fields but he was not actively involved in farming and could not remember any history of trauma. Second patient was in desk job and even he was unable to give any history of trauma. Both the patients were immunocompetent. There was no history of metabolic disorders like diabetes or any other immunosuppressive disease or drug intake. Previously, Mishra et al. [11] also have reported the infection by Rhytidhysteron in an immunocompetent person.
Histopathology has been the mainstay of diagnosis especially when the fungi are rare, slow growing or difficult to grow. However, positive growth in fungal cultures is invaluable from epidemiological point of view. Further, in case of non-sporulating fungi, it is the molecular techniques especially the genetic sequencing, which clinches the exact diagnosis.
There is also need to establish ecological niche for this fungus. All the cases reported till date (including present ones) for this fungus are from north to west India only (2 from Delhi; 2 Chandigarh; 1 Tanda, Himachal Pradesh). Both of our patients were from Chandigarh and any association with the specific environmental exposure could not be ascertained.
As such no management protocols have been decided for newer fungal agents and treatment is usually subjective. Recently, the European Society of Clinical Microbiology and Infectious Diseases and European Confederation of Medical Mycology released joint guidelines for the diagnosis and management of systemic phaeohyphomycosis [12]. However, for subcutaneous phaeohyphomycosis, surgical excision is the treatment of choice. Cryotherapy, laser, heat, photodynamic therapies are other modalities tried successfully. As far as medical treatment is concerned, amphotericin B, itraconazole, terbinafine have been tried previously in cases of Rhytidhysteron. In first ever case of human infection by Rhytidhysteron, the role of itraconazole treatment could not be ascertained as the patient was of renal transplant and died 2 weeks after itraconazole was initiated [9]. Mishra et al. [11] treated the patient with terbinafine and oral itraconazole. Mahajan et al. 10] successfully treated the patient with surgery and intralesional amphotericin B. In both of our patients, oral itraconazole was given. First patient was lost on follow up while second patient was not willing to continue on antifungal medication for prolonged time period and left the medication in between, after 2 weeks. However, he is on follow up and does not have any relapse or recurrence.
In conclusion, the fungi belonging to genus Rhytidhysteron are establishing themselves as human pathogens, not only in immunocompromised but also in immunocompetent individuals and molecular diagnosis is indispensable in identification of these non-sporulating fungi.
References
Chander J. Textbook of medical mycology. 3rd ed. New Delhi: Mehta Publishers; 2009.
Kumarguru BN, Srinivas T, Jagadish MH. A case of subcutaneous phaeohyphomycosis in a diabetic patient: a cryptic entity. J Glob Infect Dis. 2014;6:45–6.
Chowdhary A, Perfect J, de Hoog GS. Black molds and melanized yeasts pathogenic to humans. Cold Spring Harb Perspect Med. 2014;5:a019570.
Boehm EWA, Mugambi GK, Miller AN, Huhndorf SM, Marincowitz S, Spatafora JW, et al. A molecular phylogenetic reappraisal of the Hysteriaceae, Mytilinidiaceae and Gloniaceae (Pleosporomycetidae, Dothideomycetes) with keys to world species. Stud Mycol. 2009;64:49–84.
Pudhom K, Teerawatananond T, Chookpaiboon S. Spirobisnaphthalenes from the mangrove-derived fungus Rhytidhysteron sp. AS21B. Drugs. 2014;12:1271–80.
Barr ME. Rhytidhysteron opuntiae. Mem N Y Bot Gard. 1990;62:72.
Magnes M. Weltmonographie der Triblidiaceae. Bibl Mycol. 1997;165:127–30.
Murillo C, Albertazzi FJ, Carranzab J, Lumbsch HT, Tamayo G. Molecular data indicate that Rhytidhysteron rufulum (Ascomycetes, Patellariales) in Costa Rica consists of four distinct lineages corroborated by morphological and chemical characters. Mycol Res. 2009;113:405–16.
Chowdhary A, Guarro J, Randhawa HS, Gene J, Cano J, Jain RK, et al. A rare case of chromoblastomycosis in a renal transplant recipient caused by a non-sporulating species of Rhytidhysteron. Med Mycol. 2008;46:163–6.
Mahajan VK, Sharma V, Prabha N, Thakur K, Sharma NL, Rudramurthy SM, et al. A rare case of subcutaneous phaeohyphomycosis caused by Rhytidhysteron species: a clinico-therapeutic experience. Int J Dermatol. 2014;53:1485–9.
Mishra K, Das S, Goyal S, Gupta C, Rai G, Ansari MA, et al. Subcutaneous mycoses caused by Rhytidhysteron species in an immunocompetent patient. Med Mycol Case Rep. 2014;5:32–4.
Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, et al. ESCMID and ECMM joint guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect. 2014;20(Suppl. 3):47–75.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Chander, J., Singla, N., Kundu, R. et al. Phaeohyphomycosis Caused by Rhytidhysteron rufulum and Review of Literature. Mycopathologia 182, 403–407 (2017). https://doi.org/10.1007/s11046-016-0064-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-016-0064-x